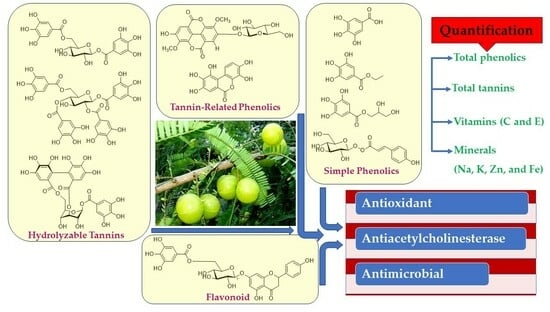
Nutritional, Antioxidant, Antimicrobial, and Anticholinesterase Properties of Phyllanthus emblica: A Study Supported by Spectroscopic and Computational Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Isolation
2.4. Spectroscopic Data of the Isolated Compounds
2.5. Metabolite Quantification
2.5.1. Total Phenolic Content
2.5.2. Total Flavonoid Content
2.5.3. Determination of Tannins Content
2.6. Nutritional Values
2.6.1. Vitamin C Content
2.6.2. Vitamin E Content
2.6.3. Mineral Content
2.7. Biological Investigations
2.7.1. Antioxidant Assay
2.7.2. Antimicrobial Assay
2.8. Investigations of Anti-Acetylcholinesterase by Molecular Dockng
3. Results
3.1. Identification of Isolated Compounds
3.2. Total Phenolic, Flavonoid, and Tannin Contents
3.3. The Nutritional Values
| Content (mg/100 g Fresh Sample) | |||
|---|---|---|---|
| Vitamin | Leaves | Fruits | RAD for Adults (Amount/Day) |
| Vitamin C | 19 ± 1 | 282 ± 6 | 60 mg |
| Vitamin E | 10 ± 0.9 | 0.34 ± 0.001 | 13 mg |
3.4. Biological Properties
3.4.1. The Antioxidant Activity
3.4.2. Antimicrobial Activity
3.5. Computatioal Investigation Antiacetylcholinesterase Properties of Isolated Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.; Uniyal, A.; Rawat, J.; Rana, D. Estimation of genetic variability in Phyllanthus emblica L.—Towards a contribution in sustainable rural development. J. Hortic. For. 2012, 4, 92–95. [Google Scholar] [CrossRef]
- Barthakur, N.; Arnold, N. Chemical analysis of the emblic (Phyllanthus emblica L.) and its potential as a food source. Sci. Hortic. 1991, 47, 99–105. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef] [PubMed]
- Mal, A.; Meena, D.S. Phyllanthus emblica: A Herbal Remedy for Healthy Life. ECS Trans. 2022, 107, 3199. [Google Scholar] [CrossRef]
- Saini, R.; Sharma, N.; Oladeji, O.S.; Sourirajan, A.; Dev, K.; Zengin, G.; El-Shazly, M.; Kumar, V. Traditional uses, bioactive composition, pharmacology, and toxicology of Phyllanthus emblica fruits: A comprehensive review. J. Ethnopharmacol. 2022, 282, 114570. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yokozawa, T.; Kim, H.Y.; Tohda, C.; Rao, T.P.; Juneja, L.R. Influence of amla (Emblica officinalis Gaertn.) on hypercholesterolemia and lipid peroxidation in cholesterol-fed rats. J. Nutr. Sci. Vitaminol. 2005, 51, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Talreja, S.; Kumari, S.; Srivastava, P.; Pandey, S. A complete pharmacognostic review on amla. World. J. Pharm. Pharm. Sci. 2021, 8, 622–637. [Google Scholar] [CrossRef]
- Gul, M.; Liu, Z.W.; Rabail, R.; Faheem, F.; Walayat, N.; Nawaz, A.; Shabbir, M.A.; Munekata, P.E.; Lorenzo, J.M.; Aadil, R.M. Functional and nutraceutical significance of Amla (Phyllanthus emblica L.): A Review. Antioxidants 2022, 11, 816. [Google Scholar] [CrossRef]
- Kudva, A.K.; Baliga, M.S.; Raghu, S.V. Pharmacological application of Phyllanthus emblica as therapeutics in Alzheimer’s disease. In Functional Foods and Therapeutic Strategies for Neurodegenerative Disorders; Springer Nature: Singapore, 2022; pp. 1–63. [Google Scholar] [CrossRef]
- Sabu, M.; Kuttan, R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J. Ethnopharmacol. 2002, 81, 155–160. [Google Scholar] [CrossRef]
- Mirunalini, S.; Krishnaveni, M. Therapeutic potential of Phyllanthus emblica (amla): The ayurvedic wonder. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 93–105. [Google Scholar] [CrossRef]
- Nain, P.; Saini, V.; Sharma, S.; Nain, J. Antidiabetic and antioxidant potential of Emblica officinalis Gaertn. leaves extract in streptozotocin-induced type-2 diabetes mellitus (T2DM) rats. J. Ethnopharmacol. 2012, 142, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ngamkitidechakul, C.; Jaijoy, K.; Hansakul, P.; Soonthornchareonnon, N.; Sireeratawong, S. Antitumour effects of Phyllanthus emblica L.: Induction of cancer cell apoptosis and inhibition of in vivo tumour promotion and in vitro invasion of human cancer cells. Phytother. Res. 2010, 24, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.-F.; Ju, H.-Q.; Li, S.; Zhang, Y.-J.; Yang, C.-R.; Wang, Y.-F. Effects of 1,2,4,6-tetra-O-galloyl-β-D-glucose from P. emblica on HBsAg and HBeAg secretion in HepG2. 2.15 cell culture. Virol. Sin. 2010, 25, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Jose, J.K.; Kuttan, G.; Kuttan, R. Antitumour activity of Emblica officinalis. J. Ethnopharmacol 2001, 75, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Faried, A.; Kurnia, D.; Faried, L.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Osamudiamen, P.M.; Oluremi, B.B.; Oderinlo, O.O.; Aiyelaagbe, O.O. Trans-resveratrol, piceatannol and gallic acid: Potent polyphenols isolated from Mezoneuron. benthamianum effective as anticaries, antioxidant and cytotoxic agents. Sci. Afr. 2020, 7, e00244. [Google Scholar] [CrossRef]
- Sissi, H.I.E.; Saleh, N.A.M.; El Nnegoumy, S.I.; Wagner, H.; Iyengar, M.A.; Seligmann, O. Prunin-O-6″-gallat, aus Acacia arnesiana. Phytochemistry 1974, 13, 2843–2844. [Google Scholar] [CrossRef]
- Ye, G.; Peng, H.; Fan, M.; Huang, C.G. Ellagic acid derivatives from the stem bark of Dipentodon sinicus. Chem. Nat. Compd. 2007, 43, 125–127. [Google Scholar] [CrossRef]
- Nonaka, G.; Nishioka, I. Tannins and related compounds. X. Rhubarb (2): Isolation and structures of a glycerol gallate, gallic acid glucoside gallates, galloylglucoses and isolindleyin. Chem. Pharm. Bull. 1983, 31, 1652–1658. [Google Scholar] [CrossRef]
- Farag, S.F. Polyphenolic compounds from the leaves of Schinus terebinthifolius Raddi. Bull. Pharm. Sci. Assiut 2008, 31, 319–329. [Google Scholar] [CrossRef]
- Mohieldin, E.A.M.; Muddathir, A.M.; Yamauchi, K.; Mitsunaga, T. Anti-caries activity of selected Sudanese medicinal plants with emphasis on Terminalia laxiflora. Rev. Bras. Farm. 2017, 27, 611–618. [Google Scholar] [CrossRef]
- Thitilertdecha, N.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Identification of major phenolic compounds from Nephelium lappaceum L., their antioxidant activities. Molecules 2010, 15, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Kochumadhavan, A.; Mangal, P.; Kumar, L.S.; Meenakshi, B.M.; Venkanna, B.U.; Muguli, G. Corilagin: First time isolation from the whole plant of Phyllanthus maderaspatensis L. Pharmacogn. Commun. 2019, 9, 135–138. [Google Scholar] [CrossRef]
- Leela, V.; Saraswathy, A. Isolation and phytoconstituents of Acacia leucophloea (Roxeb) willd. Int. Res. J. Pharm. 2013, 4, 107–109. [Google Scholar] [CrossRef]
- Dao, N.T.; Jang, Y.; Kim, M.; Nguyen, H.H.; Pham, D.Q.; Le Dang, Q.; Van Nguyen, M.; Yun, B.-S.; Pham, Q.M.; Kim, J.-C.; et al. Chemical constituents and anti-influenza viral activity of the leaves of Vietnamese plant Elaeocarpus tonkinensis. Rec. Nat. Prod. 2019, 13, 171–180. [Google Scholar] [CrossRef]
- Fons, F.; Rapior, S.; Gueiffier, A.; Roussel, J.L.; Gargadennec, A.; Andary, C. (E)-p-coumaroyl-1-O-β-D-glucopyranoside accumulation in roots of Plantago lanceolata cultures. Acta Bot. Gall. 1998, 145, 249–255. Available online: https://hal.umontpellier.fr/hal-02262396 (accessed on 19 July 2023).
- Xiang, Y.; Pei, Y.; Qu, C.; Lai, Z.; Ren, Z.; Yang, K.; Xiong, S.; Zhang, Y.; Yang, C.; Wang, D.; et al. In vitro anti-herpes simplex virus activity of 1,2,4,6-Tetra-O-galloyl-β-D-glucose from Phyllanthus emblica L. (Euphorbiaceae). Phytother. Res. 2011, 25, 975–982. [Google Scholar] [CrossRef]
- Velioglu, Y.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef]
- Burden, T.P.; Robinson, W.C. Formation of complexes between protein and tannin acid. J. Agric. Food Chem. 1981, 1, 77–82. [Google Scholar] [CrossRef]
- Kall, M.A.; Andersen, C. Improved method for simultaneous determination of ascorbic acid and dehydroascorbic acid, isoascorbic acid and dehydroisoascorbic acid in food and biological samples. J. Chromatogr. B Biomed. Sci. Appl. 1999, 730, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Woo, S.; Kim, H.S.; Jong, S.K.; Lee, J. Comparison of extraction methods for determining tocopherols in soybeans. Eur. J. Lipid Sci. Technol. 2007, 109, 1124–1127. [Google Scholar] [CrossRef]
- Tokalıoğlu, Ş. Determination of trace elements in commonly consumed medicinal herbs by ICP-MS and multivariate analysis. Food Chem. 2012, 134, 2504–2508. [Google Scholar] [CrossRef] [PubMed]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.D.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Maidment, C.; Dembny, Z.; King, P. Investigations into the anti-bacterial properties of garlic using the disc assay method. J. Biol. Educ. 1998, 32, 162–165. [Google Scholar] [CrossRef]
- Vina, A. Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading Trott, Oleg; Olson, Arthur, J. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Anbukarasi, K.; Xavier, S.; Hasan, A.H.; Er, Y.L.; Jamalis, J.; Sebastian, S.; Periandy, S. DFT and molecular docking analysis of newly synthesized compound (2E)-3-[3-(benzyloxy) phenyl]-1-(4′-chlorophe-Nyl)-2-propen-1-One [Bpclpo]. Curr. Phys. Chem. 2023, 13, 37–74. [Google Scholar] [CrossRef]
- Hussen, N.H.; Hasan, A.H.; Jamalis, J.; Shakya, S.; Chander, S.; Kharkwal, H.; Murugesan, S.; Bastikar, V.A.; Gupta, P.P. Potential inhibitory activity of phytoconstituents against black fungus: In silico ADMET, molecular docking and MD simulation studies. Comput. Toxicol. 2022, 24, 100247. [Google Scholar] [CrossRef]
- Salih, R.H.H.; Hasan, A.H.; Hussein, A.J.; Samad, M.K.; Shakya, S.; Jamalis, J.; Hawaiz, F.E.; Pratama, M.R.F. One-pot synthesis, molecular docking, ADMET, and DFT studies of novel pyrazolines as promising SARS-CoV-2 main protease inhibitors. Res. Chem. Intermed. 2022, 48, 4729–4751. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Hasan, A.H.; Shakya, S.; Hussain, F.H.; Murugesan, S.; Chander, S.; Pratama, M.R.F.; Jamil, S.; Das, B.; Biswas, S.; Jamalis, J. Design, synthesis, anti-acetylcholinesterase evaluation and molecular modelling studies of novel coumarin-chalcone hybrids. J. Biomol. Struct. Dyn. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.H.; Murugesan, S.; Amran, S.I.; Chander, S.; Alanazi, M.M.; Hadda, T.B.; Shakya, S.; Pratama, M.R.F.; Das, B.; Biswas, S. Novel thiophene Chalcones-Coumarin as acetylcholinesterase inhibitors: Design, synthesis, biological evaluation, molecular docking, ADMET prediction and molecular dynamics simulation. Bioorg. Chem. 2022, 119, 105572. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.H.; Yusof, F.S.M.; Kamarudin, N.A.; Murugesan, S.; Shakya, S.; Jamalis, J. Synthesis, anti-acetylcholinesterase evaluation, molecular docking and molecular dynamics simulation of novel Psoralen derivatives. Curr. Org. Synth. 2023, 21, 61–77. [Google Scholar] [CrossRef]
- Salih, R.H.H.; Hasan, A.H.; Hussen, N.H.; Hawaiz, F.E.; Hadda, T.B.; Jamalis, J.; Almalki, F.A.; Adeyinka, A.S.; Coetzee, L.C.C.; Oyebamiji, A.K. Thiazole-pyrazoline hybrids as potential antimicrobial agent: Synthesis, biological evaluation, molecular docking, DFT studies and POM analysis. J. Mol. Struct. 2023, 1282, 135191. [Google Scholar] [CrossRef]
- Chandler, S. The nutritional value of bananas. In Bananas and Plantains; Springer: Dordrecht, The Netherlands, 1995; pp. 468–480. Available online: https://link.springer.com/chapter/10.1007/978-94-011-0737-2_16 (accessed on 19 July 2023).
- Palmer, S. Recommended dietary allowances. Eur. J. Clin. Nutr. 1990, 44 (Suppl. S2), 13–21. [Google Scholar] [PubMed]
- Biswas, K.; Islam, A.; Sharmin, T.; Biswas, P.K. In-vitro cholinesterase inhibitory activity of dry fruit extract of Phyllanthus emblica relevant to the treatment of Alzheimer’s disease. J. Phytopharm. 2015, 4, 5–8. [Google Scholar] [CrossRef]
- Heo, H.J.; Kim, M.J.; Lee, J.M.; Choi, S.J.; Cho, H.Y.; Hong, B.; Kim, H.K.; Kim, E.; Shin, D.H. Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and a mitigating effect on amnesia. Dement. Geriatr. Cogn. Disord. 2004, 17, 151–157. [Google Scholar] [CrossRef]
- Umukoro, S.; Kalejaye, H.A.; Ben-Azu, B.; Ajayi, A.M. Naringenin attenuates behavioral derangements induced by social defeat stress in mice via inhibition of acetylcholinesterase activity, oxidative stress and release of pro-inflammatory cytokines. Biomed. Pharmacother. 2018, 105, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Chtourou, Y.; Slima, A.B.; Gdoura, R.; Fetoui, H. Naringenin mitigates iron-induced anxiety-like behavioral impairment, mitochondrial dysfunctions, ectonucleotidases and acetylcholinesterase alteration activities in rat hippocampus. Neurochem. Res. 2015, 40, 1563–1575. [Google Scholar] [CrossRef]
- Sachdeva, A.K.; Kuhad, A.; Chopra, K. Naringin ameliorates memory deficits in experimental paradigm of Alzheimer’s disease by attenuating mitochondrial dysfunction. Pharmacol. Biochem. Behav. 2014, 127, 101–110. [Google Scholar] [CrossRef]
- Zaki, H.F.; Abd-El-Fattah, M.A.; Attia, A.S. Naringenin protects against scopolamine-induced dementia in rats. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 15–25. [Google Scholar] [CrossRef]
- Haider, S.; Liaquat, L.; Ahmad, S.; Batool, Z.; Siddiqui, R.A.; Tabassum, S.; Shahzad, S.; Rafiq, S.; Naz, N. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PloS ONE 2020, 15, e0227631. [Google Scholar] [CrossRef] [PubMed]
- Khajevand-Khazaei, M.R.; Ziaee, P.; Motevalizadeh, S.A.; Rohani, M.; Afshin-Majd, S.; Baluchnejadmojarad, T.; Roghani, M. Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. Eur. J. Pharmacol. 2018, 826, 114–122. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Tomás-Barberán, F.A.; Iglesias-Aguirre, C.E.; Giménez-Bastida, J.A.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagitannins, urolithins, and neuroprotection: Human evidence and the possible link to the gut microbiota. Mol. Asp. Med. 2023, 89, 101109. [Google Scholar] [CrossRef] [PubMed]
- Hasheminezhad, S.H.; Boozari, M.; Iranshahi, M.; Yazarlu, O.; Sahebkar, A.; Hasanpour, M.; Iranshahy, M. A mechanistic insight into the biological activities of urolithins as gut microbial metabolites of ellagitannins. Phytother. Res. 2022, 36, 112–146. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Cai, J.; Fang, Z.; Li, S.; Huang, Z.; Tang, Z.; Luo, Q.; Chen, H. The composition and anti-aging activities of polyphenol extract from Phyllanthus emblica L. fruit. Nutrients 2022, 14, 857. [Google Scholar] [CrossRef]
- Wu, M.; Liu, M.; Wang, F.; Cai, J.; Luo, Q.; Li, S.; Zhu, J.; Tang, Z.; Fang, Z.; Wang, C. The inhibition mechanism of polyphenols from Phyllanthus emblica Linn. fruit on acetylcholinesterase: A interaction, kinetic, spectroscopic, and molecular simulation study. Food Res. Int. 2022, 158, 111497. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, P. Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica. J. Agric. Food Chem. 2014, 62, 529–541. [Google Scholar] [CrossRef]
- Hasanein, P.; Shahidi, S. Effects of combined treatment with vitamins C and E on passive avoidance learning and memory in diabetic rats. Neurobiol. Learn. Mem. 2010, 93, 472–478. [Google Scholar] [CrossRef]
- Jodh, R.; Tawar, M.; Mude, G.; Fasate, A.; Sutane, R.; Patanray, P. An updated review on vitamin C—An excellent drug having a great scavenging property. Asian J. Pharm. Clin. Res. 2023, 13, 25–30. [Google Scholar] [CrossRef]
- Juszczyk, G.; Mikulska, J.; Kasperek, K.; Pietrzak, D.; Mrozek, W.; Herbet, M. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and Alzheimer’s disease: The role of antioxidants in prevention and treatment. Antioxidants 2021, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Luo, M.; Fu, Y.J.; Zu, Y.G.; Wang, W.; Zhang, L.; Yao, L.P.; Zhao, C.J.; Sun, Y. Effect of corilagin on membrane permeability of Escherichia coli, Staphylococcus aureus and Candida albicans. Phytother. Res. 2013, 27, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial activity of gallic acid against food-related Pseudomonas strains and its use as biocontrol tool to improve the shelf life of fresh black truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Muddathir, A.M.; Yamauchi, K.; Mitsunaga, T. Anti-acne activity of tannin-related compounds isolated from Terminalia laxiflora. J. Wood Sci. 2013, 59, 426–431. [Google Scholar] [CrossRef]
- Passos, M.R.; Almeida, R.S.; Lima, B.O.; de Souza Rodrigues, J.Z.; de Macêdo Neres, N.S.; Pita, L.S.; Marinho, P.D.O.F.; Santos, I.A.; da Silva, J.P.; Oliveira, M.C.; et al. Anticariogenic activities of Libidibia ferrea, gallic acid and ethyl gallate against Streptococcus mutans in biofilm model. J. Ethnopharmacol. 2021, 274, 114059. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, X.; Bai, Y.; Zhao, Z.-N.; Cao, Y.-F.; Liu, L.-K.; Jiang, T.; Hou, J. Inhibition of Escherichia coli nitroreductase by the constituents in Syzygium aromaticum. Chin. J. Nat. Med. 2022, 20, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Cao, F.; Huang, T.; Tang, Y.S.; Zhao, X.; Shaw, P.C. Urolithin M5 from the leaves of Canarium album (Lour.) DC. inhibits influenza virus by targeting neuraminidase. Molecules 2022, 27, 5724. [Google Scholar] [CrossRef]




| Compound 1 | Gallic Acid (in DMSO-d6) [17] | |||
|---|---|---|---|---|
| Position | δH ppm | δC ppm | δH ppm | δC ppm |
| 1 | – | 121.2 | – | 120.9 |
| 2 | 6.91, s | 109.2 | 6.91, s | 109.2 |
| 3 | – | 145.8 | – | 145.9 |
| 4 | – | 138.4 | – | 138.4 |
| 5 | – | 145.8 | – | 145.8 |
| 6 | 6.91, s | 109.2 | 6.91, s | 109.2 |
| 7 | – | 168.2 | – | 167.9 |
| Compound 2 | Prunin 6″-O-Gallate in (CD3)2CO [18] | ||
|---|---|---|---|
| Position | δH ppm (H, mult., J in Hz) | δCa ppm | δH ppm (H, mult., J in Hz) |
| 2 | 5.38 (1H, dd, J = 12.6, 3.2) | 78.8 | 5.41 (1H, td, J = 12) |
| 3 | 2.77 (H-3 eq. dd, J = 17.1, 3.2) 3.16 (H-3 ax. dd, J = 17.1, 12.6) | 42.4 | 3.0 (2H, m) |
| 4 | – | 198.0 | – |
| 5 | – | 163.1 | – |
| 6 | 6.18 (H, d, J = 2.5) | 96.7 | 6.23 (H, br s) |
| 7 | – | 165.1 | – |
| 8 | 6.18 (H, d, J = 2.5) | 95.4 | 6.23 (H, br s) |
| 9 | – | 163.1 | – |
| 10 | – | 103.4 | – |
| 1′ | – | 129.4 | – |
| 2′, 6′ | 7.28 (2H, d, J = 8.5) | 127.9 | 7.35 (2H, d, J = 9) |
| 3′, 5′ | 6.81 (2H, d, J = 8.5) | 115.1 | 6.87 (2H, d, J = 9) |
| 4′ | – | 157.2 | – |
| 1″ | 5.04 (1H, d, J = 7.2) | 99.3 | 5.18 (1H, d, J = 7) |
| 2″ | 3.79 (1H, m) | 73.1 | 3.3–4.1 (4H, m) |
| 3″ | 3.50 (3H, m) | 76.1 | |
| 4″ | 70.5 | ||
| 5″ | 74.1 | ||
| 6″ | 4.61 (1H, dd, J = 12.0, 2.2) 4.41 (1H, dd, J = 12.0, 5.9) | 63.4 | 4.50 (2H, m) |
| 1″′ | – | 119.7 | – |
| 2‴, 6‴ | 7.06 (2H, s) | 109.1 | 7.07 (2H, s) |
| 3‴, 5‴ | – | 145.0 | – |
| 4‴ | – | 138.6 | – |
| 7‴ | – | 167.2 | – |
| Position | δH ppm (H, mult., J in Hz) | δC ppm |
|---|---|---|
| 1 | – | 111.1 |
| 2 | – | 140.9 |
| 3 | – | 140.1 |
| 4 | – | 151.5 |
| 5 | 7.54 (1H, s) | 111.6 |
| 6 | – | 111.9 |
| 7 | – | 158.3 |
| 1′ | – | 114.1 |
| 2′ | – | 141.6 |
| 3′ | – | 141.8 |
| 4′ | – | 152.8 |
| 5′ | 7.81 (1H, s) | 111.9 |
| 6′ | – | 112.7 |
| 7′ | – | 158.3 |
| 3-O-Me | 4.05 | 61.0 |
| 3′-O-Me | 4.09 | 61.6 |
| 1″ | 5.14 (1H, d, J = 7.8 Hz, H-1″) | 101.3 |
| 2″ | 3.35 (2H, overlapped with solvent signal) | 73.3 |
| 3″ | 76.4 | |
| 4″ | 3.27 (1H, t, J = 9) | 69.5 |
| 5″ | 3.43 (1H, ddd, J = 2.4, 5.4, 12) | 77.2 |
| 6″ | 3. 70 (1H, d, J = 12.6), | 60.5 |
| 3.52 (1H, dd, J = 5.4, 12.6) |
| Position | δH ppm (H, mult., J in Hz) | δC ppm |
|---|---|---|
| 1 | 4.24 (1H, dd, J = 11.4, 4.3) | 66.2 |
| 4.15 (1H, d, J = 11.4) | ||
| 2 | 3.92 (1H, m, H-2) | 70.3 |
| 3 | 3.58 (2H, m, H-3) | 63.2 |
| 1′ | – | 120.8 |
| 2′ | – | 109.7 |
| 3′ | – | 145.6 |
| 4′ | – | 138.8 |
| 5′ | 7.81 (1H, s) | 145.6 |
| 6′ | – | 109.7 |
| 7′ | – | 167.5 |
| Position | Compound 5 | 1,6-Digalloyl-β-d-glucose (CD3OD) [21] |
|---|---|---|
| δH ppm (H, mult., J in Hz) | δH ppm (H, mult., J in Hz) | |
| Glucose 1 | 5.67 (1H, d, J = 7.8) | 5.73 (1H, d, J = 7.8) |
| 2 | 3.55–3.75 (4H, m) | 3.55–3.75 (4H, m) |
| 3 | ||
| 4 | ||
| 5 | ||
| 6 | 4.39 (1H, dd, J = 12.0, 5.4) | 4.44 (1H, dd, J = 12.0, 5.4) |
| 4.55 (1H, dd, J = 12.0, 2.0) | 4.59 (1H, dd, J = 12.0, 1.8) | |
| Galloyl [(H-2/H-6) × 2] | 7.08, 7.13 (each 2H, s) | 7.12, 7.17 (each 2H, s) |
| Compound 6 | Flavogallonic Acid Bislactone in CD3OD [22] | |||
|---|---|---|---|---|
| Position | δH ppm (H, mult., J in Hz) | δC (ppm) | δH ppm (H, mult., J in Hz) | δC ppm |
| 1 | – | 108.8 a | – | 108.1 |
| 2 | – | 136.3 | – | 135.7 |
| 3 | – | 137.3 | – | 136.3 |
| 4 | – | 137.8 | – | 136.5 |
| 5 | – | 111.1 | – | 112.8 |
| 6 | 7.29 (1H, s) | 110.9 | 7.26 (1H, s) | 110.1 |
| 7 | 160.5 | 160.4 | ||
| 1′ | 109.0 a | 108.1 | ||
| 2′ | – | 138.9 | – | 137.8 |
| 3′ | – | 139.8 | – | 139.2 |
| 4′ | – | 144.1 | – | 143.2 |
| 5′ | – | 118.1 | – | 117.5 |
| 6′ | – | 113.9 | – | 114.4 |
| 7′ | – | 158.5 | – | 158.9 |
| 1″ | – | 125.8 | – | 124.9 |
| 2″ | – | 121.3 | – | 120.2 |
| 3″ | – | 144.7 | – | 144.1 |
| 4″ | – | 146.6 | – | 145.9 |
| 5″ | – | 148.4 | – | 147.8 |
| 6″ | 7.57 (1H, s) | 113.2 | 7.50 (1H, s) | 113.3 |
| 7″ | – | 168.2 | – | 168.9 |
| Position | δH ppm (H, mult., J in Hz) | δC ppm |
|---|---|---|
| Glucose 1 | 6.34 (1H, d, J = 2) | 95.84 |
| 2 | 4.00 (1H, brs) | 70.07 |
| 3 | 4.81 (1H, brs) | 72.31 |
| 4 | 4.45 (1H, brs) | 63.22 |
| 5 | 4.52 (1H, br t, J = 8) | 76.89 |
| 6 | 4.15 (1H, dd, J = 11, 8) | 65.76 |
| 4.92 (1H, t, J = 11) | ||
| Galloyl 1 | – | 121.3 |
| 2/6 | 7.05 (2H, s) | 111.8 |
| 3/5 | – | 147.1 |
| 4 | – | 141.2 |
| 7 | – | 167.6 |
| HHDP 1,1′ | – | 117.4, 118.0 |
| 2,2′ | – | 126.2, 126.3 |
| 3,3′ | 6.69, 6.66 (each 1H, s) | 109.1, 111.1 |
| 4,4′ | – | 146.4, 146.8 |
| 5,5′ | – | 138.7, 139.0 |
| 6,6′ | – | 146.0, 146.1 |
| 7,7′ | – | 169.4, 170.9 |
| Position | δH ppm (H, mult., J in Hz) | δC ppm |
|---|---|---|
| Galloyl 1 | – | 121.0 |
| 2/6 | 7.06 (2H, s) | 109.5 |
| 3/5 | – | 145.9 |
| 4 | – | 138.7 |
| 7 | – | 167.2 |
| Ethyl CH2 | 4.21 (2H, q, J = 7.1) | 60.9 |
| Ethyl CH3 | 1.27 (3H, t, J = 7.1) | 14.5 |
| Compound 9 | Urolithin M5 in CD3OD [26] | |||
|---|---|---|---|---|
| Position | δH ppm (H, mult., J in Hz) | δC ppm | δH ppm (H, mult., J in Hz) | δC ppm |
| 1 | 8.4 (1H, d, J = 9) | 118.4 | 8.44 (1H, d, J = 9) | 119.2 |
| 2 | 6.8 (1H, d, J = 9) | 112.0 | 6.77 (1H, d, J = 9) | 112.5 |
| 3 | – | 145.9 | – | 144.0 |
| 4 | – | 133.5 | – | 133.3 |
| 4a | – | 140.3 | – | 140.9 |
| 5 | – | – | – | – |
| 6 | – | 162.1 | – | 163.9 |
| 6a | – | 112.0 | – | 112.0 |
| 7 | 7.40 (1H, s) | 107.9 | 7.37 (1H, s) | 108.2 |
| 8 | – | 146.0 | – | 146.4 |
| 9 | – | 147.8 | – | 146.7 |
| 10 | – | 143.3 | – | 141.9 |
| 10a | – | 112.2 | – | 112.8 |
| Position | δH ppm (H, mult., J in Hz) | δC ppm |
|---|---|---|
| 1 | – | 118.1 |
| 2/6 | 7.42 (2H, dd, J = 8.4, 9.0) | 129.7 |
| 3/5 | 7.68 (2H, dd, J = 8.4, 9.0) | 129.1 |
| 4 | – | 146.8 |
| 7 | 7.76 (1H, d, J = 16) | 135.0 |
| 8 | 6.56 (1H, d, J = 16) | 131.4 |
| 9 | 7.40 (1H, s) | 166.2 |
| Glucose 1 | 5.57 (1H, d, J = 8.1) | 95.4 |
| 2 | 3.39–3.53 (4H, m) | 77.3 |
| 3 | 73.4 | |
| 4 | 70.6 | |
| 5 | 78.2 | |
| 6 | 3.80 (1H, dd, J = 12.1, 2.4) | 62.0 |
| 3.65 (1H, dd, J = 12.1, 5.4) |
| Compound 11 | 1,2,4,6-Tetra-O-galloyl-β-d-glucopyranoside in CD3OD [28] | |
|---|---|---|
| Position | δH ppm (H, mult., J in Hz) | δH ppm (H, mult., J in Hz) |
| Galloyls H-2/H-6 | 7.12, 7.11, 7.07, 7.05 (each 2H, s) | 7.12, 7.11, 7.07, 7.05 (each 2H, s) |
| Glucose 1 | 6.07 (1H, d, J = 8.4) | 6.07 (1H, d, J = 8.4) |
| 2 | 5.36 (1H, dd, J = 8.4, 9.6) | 5.37 (1H, dd, J = 8.4, 9.6) |
| 3 | 4.18 (1H, t, J = 9.6) | 4.16 (1H, t, J = 9.6) |
| 4 | 5.39 (1H, t, J = 9.6) | 5.38 (1H, t, J = 9.6) |
| 5 | 4.22 (1H, m) | 4.19 (1H, m) |
| 6 | 4.48 (1H, dd, J = 1.8, 12.3) | 4.49 (1H, dd, J = 1.8, 12.3) |
| 4.31 (1H, dd, J = 4.2, 12.6) | 4.29 (1H, dd, J = 4.2, 12.6) |
| Condition | Vitamin C | Vitamin E |
|---|---|---|
| Column | RP C-18 Jupiter ODS-2 (5 μm) | RP C-18 Nova Pak ODS-2 (4 μm) |
| Dimensions | 250 × 4.6 nm i.d. | 300 × 3.9 mm i.d. |
| Mobile phase | 2.3 mM Na2EDTA in 66 mM phosphate-20 mM acetate buffer (pH = 4.50) | Isocratic (isopropanol: heptane, 1: 99, v/v) |
| Flow rate | 1.2 mL/min | 2 mL/min |
| Detector | UV spectrophotometer | Photodiode array detector (PDA) |
| Detector UV wavelength | 247 nm | 195–330 nm |
| Injection volume | 20 μL | 10 μL |
| Column temperature | 20 °C | 40 °C |
| Run time | 14 min | 13 min |
| Photodiode array (PDA) measurement frequency | ----- | 1 spectrum/s |
| PDA spectral resolution | ----- | 1.2 nm |
| Nebulizer | Babington Type |
|---|---|
| Spray chamber | Quartz, double pass |
| Radiofrequency (RF) generator Frequency | 10 MHz, power output:1220W |
| Air flow rate (L/min) | 20 |
| Auxiliary gas flow rate (L/min) | 0.9 |
| Nebulizer gas flow rate (L/min) | 1–1.2 |
| Sample uptake (L/min) | 400 |
| Number of replicates | 3 |
| Integration time | 0.1 |
| Internal standards | Bi, Be, Rh, Sc |
| Isotopes | 57Fe, 66Zn, 39K, 22Na |
| UV wavelengths of the determined minerals. | |
| Mineral | Wavelength |
| Sodium | 589.592 nm |
| Potassium | 766.490 nm |
| Zinc | 206.200 nm |
| Iron | 238.204 nm |
| Plant Organ | Total Phenolics (mg GAE/g Dry Extract) | Total Flavonoids (mg QE/g Dry Extract) | Total Tannins (mg GAE/g Dry Extract) |
|---|---|---|---|
| Leaves | 29 ± 1 | 13 ± 0.2 | 2.5 ± 0.1 |
| Fruits | 29 ± 1 | 24 ± 0.4 | 2.2 ± 0.2 |
| Stem branches | 8 ± 1 | 4.5 ± 0.1 | 4.2 ± 0.1 |
| Mineral | Content (mg/kg Dry Weight) | RAD for Adults (Amount/Day) | ||
|---|---|---|---|---|
| Leaves | Stem Branches | Fruits | ||
| Zinc | 17 ± 2 | 20 ± 1 | 4 ± 0.2 | 15 mg |
| Sodium | 443 ± 64 | 807 ± 7 | 194 ± 4 | 23 mg |
| Potassium | 10,725 ± 136 | 8665 ± 11 | 13,510 ± 11 | 90 mg |
| Iron | 1039 ± 10 | 25 ± 4 | NA | 10 mg |
| Extract | Bacterial Species | Fungal Species | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ | B. subtilis | S. faecalis | S. aureus | E. coli | P. aeruginosa | N. gonorrhoeae | C. albicans | A. flavus | |||||||||
| I. Z.a | %b | I. Z.a | % b | I. Z.a | % b | I. Z.a | % b | I. Z.a | % b | I. Z.a | % b | I. Z.a | % b | I. Z.a | % b | ||
| Tot. EtOH | L | 22 ± 2 | 73 | 16 ± 1 | 53 | 21 ± 1 | 75 | 19 ± 1 | 63 | 23 ± 2 | 74 | 18 ± 1 | 62 | 10 ± 1 | 50 | 0 | 0 |
| F | 13 ± 1 | 43 | 14 ± 1 | 47 | 12 ± 1 | 43 | 15 ± 1 | 50 | 14 ± 1 | 45 | 12 ± 1 | 41 | 9 ± 1 | 45 | 0 | 0 | |
| Pet. Ether | L | 16 ± 2 | 53 | 15 ± 1 | 50 | 19 ± 2 | 68 | 15 ± 1 | 50 | 16 ± 1 | 52 | 16 ± 1 | 55 | 0 | 0 | 0 | 0 |
| F | 13 ± 1 | 43 | 13 ± 1 | 43 | 13 ± 1 | 46 | 13 ± 1 | 43 | 13 ± 1 | 42 | 12 ± 1 | 41 | 0 | 0 | 0 | 0 | |
| EtOAc | L | 21 ± 1 | 70 | 19 ± 1 | 63 | 23 ± 1 | 82 | 17 ± 1 | 57 | 20 ± 2 | 65 | 23 ± 1 | 79 | 9 ± 0.2 | 45 | 0 | 0 |
| F | 14 ± 1 | 47 | 13 ± 1 | 43 | 13 ± 1 | 46 | 13 ± 1 | 43 | 12 ± 1 | 39 | 12 ± 1 | 41 | 0 | 0 | 0 | 0 | |
| BuOH | L | 18 ± 11 | 60 | 17 ± 1 | 57 | 23 ± 1 | 82 | 19 ± 1 | 63 | 20 ± 1 | 65 | 23 ± 1 | 79 | 0 | 0 | 0 | 0 |
| F | 11 ± 1 | 37 | 12 ± 1 | 40 | 15 ± 1 | 54 | 11 ± 1 | 37 | 16 ± 1 | 52 | 13 ± 1 | 45 | 10 ± 1 | 50 | 0 | 0 | |
| MeOH | L | 13 ± 1 | 43 | 12 ± 1 | 40 | 10 ± 1 | 36 | 12 ± 1 | 40 | 11 ± 1 | 35 | 10 ± 1 | 34 | 0 | 0 | 0 | 0 |
| F | 1 ± 0.1 | 3 | 11 ± 1 | 37 | 12 ± 1 | 43 | 13 ± 1 | 43 | 11 ± 1 | 35 | 11 ± 1 | 38 | 0 | 0 | 0 | 0 | |
| Tetra. | L | 30 ± 2 | 100 | 30 ± 1 | 100 | 28 ± 1 | 100 | 30 ± 1 | 100 | 31 ± 1 | 100 | 29 ± 2 | 100 | -- | -- | -- | |
| Ampho. | F | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 20 ± 2 | 100 | 17 | 100 |
| No | Compound | Binding Affinity (kcal/mol) |
|---|---|---|
| 1 | gallic acid | −6.6 |
| 2 | Prunin 6″-O-gallate (Naringenin 7-O-(6″-O-galloyl)-β-d-glucopyranoside) | −12.4 |
| 3 | 3,3′-di-O-methyl ellagic acid-4′-O-β-d-glucopyranoside | −9.7 |
| 4 | 1-O-Galloylglycerol | −7.8 |
| 5 | 1,6-di-O-galloyl-β-d-glucopyranoside | −10.7 |
| 6 | flavogallonic acid bislactone | −9.3 |
| 7 | Corilagin | −10.1 |
| 8 | Ethyl gallate | −7.1 |
| 9 | Urolithin M5 | −9.8 |
| 10 | (E)-p-coumaroyl-1-O-β-d-glucopyranoside | −9.2 |
| 11 | 1,2,4,6-tetra-O-galloyl-β-d-glucopyranoside | −11.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orabi, M.A.A.; Hasan, A.H.; AbouZid, S.F.; El Amir, D.; Hetta, M.H.; Awadh, A.A.A.; Alqahtani, O.S.; Hatano, T.; El-Shanawany, M.A. Nutritional, Antioxidant, Antimicrobial, and Anticholinesterase Properties of Phyllanthus emblica: A Study Supported by Spectroscopic and Computational Investigations. Metabolites 2023, 13, 1013. https://doi.org/10.3390/metabo13091013
Orabi MAA, Hasan AH, AbouZid SF, El Amir D, Hetta MH, Awadh AAA, Alqahtani OS, Hatano T, El-Shanawany MA. Nutritional, Antioxidant, Antimicrobial, and Anticholinesterase Properties of Phyllanthus emblica: A Study Supported by Spectroscopic and Computational Investigations. Metabolites. 2023; 13(9):1013. https://doi.org/10.3390/metabo13091013
Chicago/Turabian StyleOrabi, Mohamed A. A., Aso Hameed Hasan, Sameh F. AbouZid, Dalia El Amir, Mona H. Hetta, Ahmed Abdullah Al Awadh, Omaish Salman Alqahtani, Tsutomu Hatano, and Mohamed A. El-Shanawany. 2023. "Nutritional, Antioxidant, Antimicrobial, and Anticholinesterase Properties of Phyllanthus emblica: A Study Supported by Spectroscopic and Computational Investigations" Metabolites 13, no. 9: 1013. https://doi.org/10.3390/metabo13091013
APA StyleOrabi, M. A. A., Hasan, A. H., AbouZid, S. F., El Amir, D., Hetta, M. H., Awadh, A. A. A., Alqahtani, O. S., Hatano, T., & El-Shanawany, M. A. (2023). Nutritional, Antioxidant, Antimicrobial, and Anticholinesterase Properties of Phyllanthus emblica: A Study Supported by Spectroscopic and Computational Investigations. Metabolites, 13(9), 1013. https://doi.org/10.3390/metabo13091013