Short-Term Standard Diet Consumption Prior to the Oral Fat Tolerance Test Modulates the Postprandial Triglyceride Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometric Measurements
2.3. Dietary Assessments
2.4. Oral Fat Tolerance Test
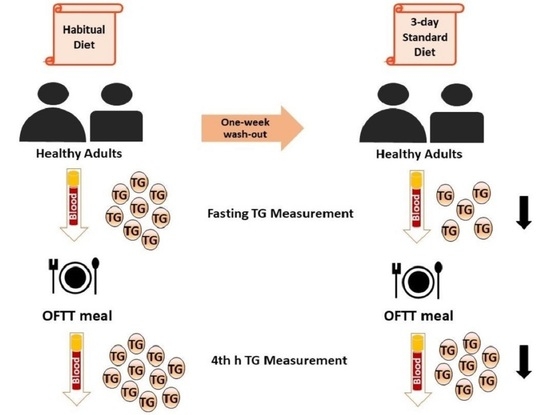
2.5. Study Design
2.6. Biochemical Tests
2.7. Statistical Analyses
3. Results
3.1. Findings concerning the Macronutrient Content of Habitual and Standard Diets
3.2. The Effects of the Standard Diet on Anthropometric and Biochemical Variables
3.3. The Effects of the Standard Diet on OFTT Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Liu, L.; Yang, S.; Liu, G.; Pan, L.; Gu, C.; Wang, Y.; Li, D.; Zhao, R.; Wu, M. Mechanisms of atherosclerosis induced by postprandial lipemia. Front. Cardiovasc. Med. 2021, 8, 636947. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Miyoshi, T.; Yunoki, K.; Ito, H. Postprandial hyperlipidemia as a potential residual risk factor. J. Cardiol. 2016, 67, 35–39. [Google Scholar] [CrossRef]
- Nakajima, K.; Nakano, T.; Tokita, Y.; Nagamine, T.; Inazu, A.; Kobayashi, J.; Mabuchi, H.; Stanhope, K.L.; Havel, P.J.; Okazaki, M.; et al. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin. Chim. Acta 2011, 412, 1306–1308. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G. A test in context: Lipid profile, fasting versus nonfasting. J. Am. Coll. Cardiol. 2017, 70, 1637–1646. [Google Scholar] [CrossRef]
- Langsted, A.; Nordestgaard, B.G. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology 2019, 51, 131–141. [Google Scholar] [CrossRef]
- Keirns, B.H.; Sciarrillo, C.M.; Koemel, N.A.; Emerson, S.R. Fasting, non-fasting and postprandial triglycerides for screening cardiometabolic risk. J. Nutr. Sci. 2021, 10, 75. [Google Scholar] [CrossRef]
- Lindman, A.S.; Veierød, M.B.; Tverdal, A.; Pedersen, J.I.; Selmer, R. Nonfasting triglycerides and risk of cardiovascular death in men and women from the Norwegian Counties Study. Eur. J. Epidemiol. 2010, 25, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 99–308. [Google Scholar] [CrossRef]
- Bansal, S.; Buring, J.E.; Rifai, N.; Mora, S.; Sacks, F.M.; Ridker, P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA 2007, 298, 309–316. [Google Scholar] [CrossRef]
- Kats, D.; Sharrett, A.R.; Ginsberg, H.N.; Nambi, V.; Ballantyne, C.M.; Hoogeveen, R.C.; Gerardo, H. Postprandial lipemia and the risk of coronary heart disease and stroke: The Atherosclerosis Risk in Communities (ARIC) Study. BMJ Open Diabetes Res. Care 2017, 5, e000335. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Mikhailidis, D.P.; Kovar, J.; Lairon, D.; Nordestgaard, B.G.; Ooi, T.C.; Pérez-Martínez, P.; Bilianou, H.; Anagnostopoulou, K.; Panotopouloset, G. Assessment and clinical relevance of non-fasting and postprandial triglycerides: An expert panel statement. Curr. Vasc. Pharmacol. 2011, 9, 258–270. [Google Scholar] [CrossRef]
- Kolovou, G.D.; Watts, G.F.; Mikhailidis, D.P.; Pérez-Martínez, P.; Mora, S.; Bilianou, H.; Panotopoulos, G.; Katsiki, N.; Ooi, T.C.; Lopez-Miranda, J.; et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: A 2019 expert panel statement, main text. Curr. Vasc. Pharmacol. 2019, 17, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, N.; Kanellos, P.T.; Siami, E.; Athanasopoulou, E.; Chaviaras, N.; Kolovou, G.; Sfikakis, P.P.; Katsilambros, N. Assessment of the validity and reproducibility of a novel standardized test meal for the study of postprandial triacylglycerol concentrations. Lipids 2017, 52, 675–686. [Google Scholar] [CrossRef]
- Sciarrillo, C.M.; Koemel, N.A.; Kurti, S.P.; Emerson, S.R. Validity of an abbreviated, clinically feasible test for postprandial lipemia in healthy adults: A randomized cross-over study. Nutrients 2019, 11, 80. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.D.; Koemel, N.A.; Sciarrillo, C.M.; Lucas, E.A.; Jenkins, N.D.M.; Emerson, S.R. The reliability of an abbreviated fat tolerance test: A comparison to the oral glucose tolerance test. Clin. Nutr. ESPEN 2021, 43, 428–435. [Google Scholar] [CrossRef]
- Mihas, C.; Kolovou, G.D.; Mikhailidis, D.P.; Kovar, J.; Lairon, D.; Nordestgaard, B.G.; Ooi, T.C.; Pérez-Martínez, P.; Bilianou, H.; Anagnostopoulou, K.; et al. Diagnostic value of postprandial triglyceride testing in healthy subjects: A meta-analysis. Curr. Vasc. Pharmacol. 2011, 9, 271–280. [Google Scholar] [CrossRef]
- Dias, C.B.; Moughan, P.J.; Wood, L.G.; Singh, H.; Garg, M.L. Postprandial lipemia: Factoring in lipemic response for ranking foods for their healthiness. Lipids Health Dis. 2017, 16, 178. [Google Scholar] [CrossRef]
- Sun, L.; Tan, K.W.J.; Lim, J.Z.; Magkos, F.; Henry, C.J. Dietary fat and carbohydrate quality have independent effects on postprandial glucose and lipid responses. Eur. J. Nutr. 2018, 57, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Rivellese, A.A.; Maffettone, A.; Vessby, B.; Uusitupa, M.; Hermansen, K.; Berglund, L.; Louheranta, A.; Meyer, B.J.; Riccardi, G. Effects of dietary saturated, monounsaturated and n-3 fatty acids on fasting lipoproteins, LDL size and post-prandial lipid metabolism in healthy subjects. Atherosclerosis 2003, 167, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.R.; Horner, K.E.; Langdon, M.M.; Zhang, J.Q.; Krul, E.S.; Sun, G.Y.; Cox, R.H. Effect of exercise and medium-chain fatty acids on postprandial lipemia. J. Appl. Physiol. 2001, 90, 1239–1246. [Google Scholar] [CrossRef]
- Orem, A.; Yaman, S.O.; Altinkaynak, B.; Kural, B.V.; Yucesan, F.B.; Altinkaynak, Y.; Orem, C. Relationship between postprandial lipemia and atherogenic factors in healthy subjects by considering gender differences. Clin. Chim. Acta 2018, 480, 34–40. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Emerson, E.M.; Cull, B.J.; Casey, K.; Haub, M.D.; Rosenkranz, S.K. Postprandial metabolic responses differ by age group and physical activity level. J. Nutr. Health Aging 2018, 22, 145–153. [Google Scholar] [CrossRef]
- Lee, D.P.S.; Low, J.H.M.; Chen, J.R.; Zimmermann, D.; Actis-Goretta, L.; Kim, J.E. The Influence of Different Foods and Food Ingredients on Acute Postprandial Triglyceride Response: A Systematic Literature Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2020, 6, 1529–1543. [Google Scholar] [CrossRef]
- Lairon, D.; Defoort, C. Effects of nutrients on postprandial lipemia. Curr. Vasc. Pharmacol. 2011, 9, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Desmarchelier, C.; Borel, P.; Lairon, D.; Maraninchi, M.; Valéro, R. Effect of nutrient and micronutrient intake on chylomicron production and postprandial lipemia. Nutrients 2019, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Nişancı, F.; Kızıl, M.; Çakır, B.; Çarkçı, M. Nutrients and food groups. In Turkey Dietary Guidelines; Ministry of Health of Turkey: Ankara, Turkey, 2016; pp. 30–50. [Google Scholar]
- Agostoni, C.; Bresson, J.L.; Fairweather-Tait, S.; Pison, L. Panel on dietetic products, nutrition, and allergies (NDA); scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 462. [Google Scholar] [CrossRef]
- Yaman, S.O.; Orem, A.; Yucesan, F.B.; Kural, B.V.; Orem, C. Evaluation of circulating miR-122, miR-30c and miR-33a levels and their association with lipids, lipoproteins in postprandial lipemia. Life Sci. 2021, 264, 118585. [Google Scholar] [CrossRef]
- Culling, K.S.; Neil, H.A.; Gilbert, M.; Frayn, K.N. Effects of short-term low- and high-carbohydrate diets on postprandial metabolism in non-diabetic and diabetic subjects. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 345–351. [Google Scholar] [CrossRef]
- Bozzetto, L.; Della Pepa, G.; Vetrani, C.; Rivellese, A.A. Dietary Impact on Postprandial Lipemia. Front. Endocrinol. 2020, 11, 337. [Google Scholar] [CrossRef]
- Koutsari, C.; Malkova, D.; Hardman, A.E. Postprandial lipemia after short-term variation in dietary fat and carbohydrate. Metabolism 2000, 49, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Koutsari, C.; Karpe, F.; Humphreys, S.M.; Frayn, K.N.; Hardman, A.E. Exercise prevents the accumulation of triglyceride-rich lipoproteins and their remnants seen when changing to a high-carbohydrate diet. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1520–1525. [Google Scholar] [CrossRef]
- Koutsari, C.; Hardman, A.E. Exercise prevents the augmentation of postprandial lipaemia attributable to a low-fat high-carbohydrate diet. Br. J. Nutr. 2001, 86, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J.; Krauss, R.M.; Christiansen, M.P.; Neese, R.A.; Hellerstein, M.K. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J. Clin. Investig. 1999, 104, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J. Effect of dietary carbohydrate on triglyceride metabolism in humans. J. Nutr. 2001, 131, 2772S–2774S. [Google Scholar] [CrossRef] [PubMed]


| Variables | Habitual Diet | Standard Diet | Percent Change a | p |
|---|---|---|---|---|
| Energy, kcal/day | 1342 ± 314 | 1349 ± 316 | 3.79 ± 25.3 | 0.913 |
| Carbohydrate, E% | 43 ± 6.53 | 49 ± 3.23 | 15.0 ± 17.7 | 0.001 |
| Fat, E% | 38 ± 4.66 | 32 ± 3.43 | −14.0 ± 12.4 | 0.001 |
| Protein, E% | 19 ± 3.26 | 19 ± 1.75 | 3.98 ± 20.5 | 0.823 |
| Fiber, g | 15 ± 6.10 | 24 ± 9.22 | 80 ± 93 | 0.001 |
| Cholesterol, mg | 272 ± 99 | 281 ± 79 | 15.4 ± 45.5 | 0.713 |
| Variables | Habitual Diet | Standard Diet | p |
|---|---|---|---|
| Body weight, kg | 71.1 ± 11 | 70.7 ± 10.7 | 0.083 |
| BMI, kg/m2 | 24.7 ± 2.61 | 24.6 ± 2.55 | 0.121 |
| Waist circumference, cm | 84.4 ± 10.1 | 84.5 ± 9.95 | 0.833 |
| Hip circumference, cm | 99.1 ± 6.62 | 99 ± 6.72 | 0.427 |
| SBP, mm/Hg | 112 ± 13.1 | 111 ± 12.5 | 0.329 |
| DBP, mm/Hg | 75.5 ± 8.58 | 74.6 ± 7.39 | 0.162 |
| Glucose, mg/dL | 92 (86.8–95.5) | 92 (86.8–97.3) | 0.810 * |
| Insulin, mIU/L | 6.22 (4.90–10.2) | 6.19 (4.02–9.21) | 0.122 * |
| HOMA-IR | 1.43 (1.02–2.29) | 1.40 (0.79–2.06) | 0.131 * |
| TG, mg/dL | 86 (63–116) | 74 (57–96) | 0.118 * |
| TC, mg/dL | 204 ± 33.3 | 203 ± 37.3 | 0.681 |
| LDL-C, mg/dL | 133 ± 29 | 132 ± 31 | 0.754 |
| HDL-C, mg/dL | 52 (46–57) | 52 (46–56) | 0.519 * |
| Variables | OFTT-HD | OFTT-SD | Percent Change a | p |
|---|---|---|---|---|
| TG-fasting, mg/dL | 86 (63–116) | 74 (57–96) | −10.0 (−21.1–10) | 0.118 |
| TG-4th h, mg/dL | 148 (103–196) | 124 (83–149) | −12.8 (−26.5–7.54) | 0.038 |
| Delta TG | 54 (36–103) | 44 (22–73) | −22.7 (−54–27) | 0.058 |
| TG < 89 mg/dL (n = 11) | TG = 89–180 mg/dL (n = 11) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | OFTT-HD | OFTT-SD | Percent Change a TG < 89 | p * | OFTT-HD | OFTT-SD | Percent Change a TG = 89–180 | p ** |
| TG-fasting, mg/dL | 64 (57–79) | 70 (55–75) | −4.9 (−12.5–28.1) | 0.721 | 115 (92–151) | 94 (72–123) | −19.1 (−23.5–1.78) | 0.062 |
| TG-4th h, mg/dL | 103 (94–146) | 108 (63–140) | −12.2 (−19.4–22.9) | 0.533 | 194 (154–236) | 146 (120–228) | −13.45 (−38.1–0.80) | 0.018 |
| Delta TG | 38 (22–71) | 33 (18–55) | −22.7 (−50–52) | 0.534 | 93 (49–112) | 50 (24–98) | −28.7 (−71.6–13.7) | 0.047 |
| Female | Male | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 11) | (n = 11) | |||||||
| Variables | OFTT-HD | OFTT-SD | Percent Change a Female | p * | OFTT-HD | OFTT-SD | Percent Change a Male | p ** |
| TG-fasting, mg/dL | 79 (61–106) | 72 (55–82) | −10.9 (−21.2–4.92) | 0.028 | 90 (71–151) | 91 (70–123) | −1.78 (−21.1–22.7) | 0.756 |
| TG-4th h, mg/dL | 146 (102–194) | 108 (67–148) | −14.7 (−28.1–5.71) | 0.041 | 154 (103–236) | 129 (123–228) | −3.39 (−16.8–9.65) | 0.374 |
| Delta TG | 54 (38–82) | 43 (19–52) | −26 (−54–13) | 0.142 | 67 (31–123) | 52 (23–98) | −18 (−60–45) | 0.169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaban Yucesan, F.; Demir, E.N.; Ozer Yaman, S.; Orem, A.; Dokuz Murat, B.; Bilgin, B. Short-Term Standard Diet Consumption Prior to the Oral Fat Tolerance Test Modulates the Postprandial Triglyceride Response. Metabolites 2023, 13, 1019. https://doi.org/10.3390/metabo13091019
Balaban Yucesan F, Demir EN, Ozer Yaman S, Orem A, Dokuz Murat B, Bilgin B. Short-Term Standard Diet Consumption Prior to the Oral Fat Tolerance Test Modulates the Postprandial Triglyceride Response. Metabolites. 2023; 13(9):1019. https://doi.org/10.3390/metabo13091019
Chicago/Turabian StyleBalaban Yucesan, Fulya, Eda Nur Demir, Serap Ozer Yaman, Asım Orem, Busra Dokuz Murat, and Busra Bilgin. 2023. "Short-Term Standard Diet Consumption Prior to the Oral Fat Tolerance Test Modulates the Postprandial Triglyceride Response" Metabolites 13, no. 9: 1019. https://doi.org/10.3390/metabo13091019
APA StyleBalaban Yucesan, F., Demir, E. N., Ozer Yaman, S., Orem, A., Dokuz Murat, B., & Bilgin, B. (2023). Short-Term Standard Diet Consumption Prior to the Oral Fat Tolerance Test Modulates the Postprandial Triglyceride Response. Metabolites, 13(9), 1019. https://doi.org/10.3390/metabo13091019