Brazilian Amazon Red Propolis: Leishmanicidal Activity and Chemical Composition of a New Variety of Red Propolis
Abstract
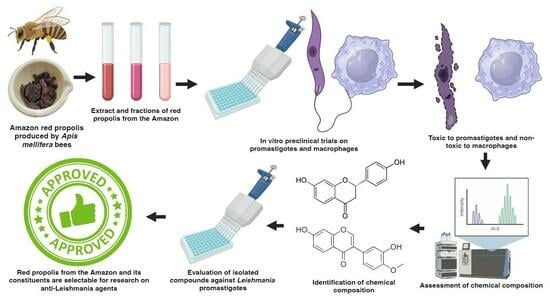
:1. Introduction
2. Materials and Methods
2.1. Collecting Propolis and Obtaining Extracts
2.2. Determining the Total Concentrations of Phenolics and Flavonoids
2.3. Antileishmanial Activity
2.4. Cytotoxicity Assay
2.5. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS)
2.6. In Silico Analysis
2.7. Statistical Analysis
3. Results
3.1. Total Phenolic Content and Total Flavonoids Content
3.2. Leishmanicidal Activity
3.3. Cytotoxicity in Raw 264.7 Cells and Selective Index
3.4. The Chemical Composition of Brazilian Amazon Red Propolis
3.5. In Silico Study
3.6. Antipromastigote Activity of Flavonoids from Amazon Red Propolis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rufatto, L.C.; dos Santos, D.A.; Marinho, F.; Henriques, J.A.P.; Roesch Ely, M.; Moura, S. Red propolis: Chemical composition and pharmacological activity. Asian Pac. J. Trop. Biomed. 2017, 7, 591–598. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How diverse is the chemistry and plant origin of Brazilian propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef]
- Rufatto, L.C.; Luchtenberg, P.; Garcia, C.; Thomassigny, C.; Bouttier, S.; Henriques, J.A.P.; Roesch-Ely, M.; Dumas, F.; Moura, S. Brazilian red propolis: Chemical composition and antibacterial activity determined using bioguided fractionation. Microbiol. Res. 2018, 214, 74–82. [Google Scholar] [CrossRef]
- Silva, F.R.G.; Matias, T.M.S.; Souza, L.I.O.; Matos-Rocha, T.J.; Fonseca, S.A.; Mousinho, K.C.; Santos, A.F. Phytochemical screening and in vitro antibacterial, antifungal, antioxidant and antitumor activities of the red propolis Alagoas. Braz. J. Biol. 2019, 79, 452–459. [Google Scholar] [CrossRef]
- Reis, J.H.d.O.; Barreto, G.d.A.; Cerqueira, J.C.; dos Anjos, J.P.; Andrade, L.N.; Padilha, F.F.; Druzian, J.I.; Machado, B.A.S. Evaluation of the antioxidant profile and cytotoxic activity of red propolis extracts from different regions of northeastern Brazil obtained by conventional and ultrasound-assisted extraction. PLoS ONE 2019, 14, e0219063. [Google Scholar] [CrossRef]
- Bueno-Silva, B.; Kawamoto, D.; Ando-Suguimoto, E.S.; Casarin, R.C.V.; Alencar, S.M.; Rosalen, P.L.; Mayer, M.P.A. Brazilian red propolis effects on peritoneal macrophage activity: Nitric oxide, cell viability, pro-inflammatory cytokines and gene expression. J. Ethnopharmacol. 2017, 207, 100–107. [Google Scholar] [CrossRef]
- Dos Santos, D.A.; Munari, F.M.; Frozza, C.O.d.S.; Moura, S.; Barcellos, T.; Henriques, J.A.P.; Roesch-Ely, M. Brazilian red propolis extracts: Study of chemical composition by ESI-MS/MS (ESI+) and cytotoxic profiles against colon cancer cell lines. Biotechnol. Res. Innov. 2019, 3, 120–130. [Google Scholar] [CrossRef]
- Devequi-Nunes, D.; Machado, B.A.S.; de Abreu Barreto, G.; Rebouças Silva, J.; Da Silva, D.F.; Da Rocha, J.L.C.; Brandão, H.N.; Borges, V.M.; Umsza-Guez, M.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- Regueira-Neto, M.d.S.; Tintino, S.R.; Rolón, M.; Coronal, C.; Vega, M.C.; Balbino, V.d.Q.; Coutinho, H.D.d.M. Antitrypanosomal, antileishmanial and cytotoxic activities of Brazilian red propolis and plant resin of Dalbergia ecastaphyllum (L.) Taub. Food Chem. Toxicol. 2018, 119, 215–221. [Google Scholar] [CrossRef]
- De Mendonça, I.C.G.; Porto, I.C.C.d.M.; Nascimento, T.G.D.; de Souza, N.S.; Oliveira, J.M.d.S.; Arruda, R.E.d.S.; Mousinho, K.C.; dos Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A.; et al. Brazilian red propolis: Phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement. Altern. Med. 2015, 15, 357. [Google Scholar] [CrossRef]
- Skirycz, A.; Kierszniowska, S.; Méret, M.; Willmitzer, L.; Tzotzos, G. Medicinal Bioprospecting of the Amazon Rainforest: A Modern Eldorado? Trends Biotechnol. 2016, 34, 781–790. [Google Scholar] [CrossRef]
- Ishida, V.F.d.C.; Negri, G.; Salatino, A.; Bandeira, M.F.C.L. A new type of Brazilian propolis: Prenylated benzophenones in propolis from Amazon and effects against cariogenic bacteria. Food Chem. 2011, 125, 966–972. [Google Scholar] [CrossRef]
- De Oliveira, M.S.; Cruz, J.N.; Ferreira, O.O.; Pereira, D.S.; Pereira, N.S.; Oliveira, M.E.C.; Venturieri, G.C.; Guilhon, G.M.S.P.; Filho, A.P.d.S.S.; Andrade, E.H.d.A. Chemical Composition of Volatile Compounds in Apis mellifera Propolis from the Northeast Region of Pará State, Brazil. Molecules 2021, 26, 3462. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- World Health Organization Leishmaniasis. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 1 December 2022).
- Dutra, R.P.; Bezerra, J.L.; da Silva, M.C.P.; Batista, M.C.A.; Patrício, F.J.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Antileishmanial activity and chemical composition from Brazilian geopropolis produced by stingless bee Melipona fasciculata. Rev. Bras. Farm. 2019, 29, 287–293. [Google Scholar] [CrossRef]
- Dutra, R.P.; Abreu, B.V.d.B.; Cunha, M.S.; Batista, M.C.A.; Torres, L.M.B.; Nascimento, F.R.F.; Ribeiro, M.N.S.; Guerra, R.N.M. Phenolic Acids, Hydrolyzable Tannins, and Antioxidant Activity of Geopropolis from the Stingless Bee Melipona fasciculata Smith. J. Agric. Food Chem. 2014, 62, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.A.N.; Silva, R.H.M.; Queiroz, P.F.d.S.; Fernandes, C.V.; Garcia, J.B.S.; Ramos, R.M.; da Rocha, C.Q.; Lima, S.T.d.J.R.M.; et al. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculata. Int. J. Mol. Sci. 2019, 20, 4512. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.C.P.; Brito, J.M.; Ferreira, A.d.S.; Vale, A.A.M.; dos Santos, A.P.A.; Silva, L.A.; Pereira, P.V.S.; Nascimento, F.R.F.; Nicolete, R.; Guerra, R.N.M. Antileishmanial and Immunomodulatory Effect of Babassu-Loaded PLGA Microparticles: A Useful Drug Target to Leishmania amazonensis Infection. Evidence-Based Complement. Altern. Med. 2018, 2018, 3161045. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Neto, J.G.; Filho, J.G.S.; Bittar, E.M.; Silva, L.M.; de Sousa, F.F.; Domingos, H.V.; Costa-Lotufo, L.V.; Reis, A.S.; dos Santos, A.O. Structural, thermal, electronic, vibrational, magnetic, and cytotoxic properties of chloro(glycinato-N,O)(1,10-phenanthroline-N,N′)-copper(II) trihydrate coordination complex. J. Inorg. Biochem. 2022, 226, 111658. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian, 09 A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Olson, A.J. Using AutoDock for Ligand-Receptor Docking. Curr. Protoc. Bioinform. 2008, 24, 8.14.11–18.14.40. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Calado, G.P.; Fróes, Y.N.; de Araújo, S.A.; França, L.M.; Paes, A.M.d.A.; de Morais, S.V.; da Rocha, C.Q.; Vasconcelos, C.C. Plant Metabolites as SARS-CoV-2 Inhibitors Candidates: In Silico and In Vitro Studies. Pharmaceuticals 2022, 15, 1045. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Stierand, K.; Maaß, P.C.; Rarey, M. Molecular complexes at a glance: Automated generation of two-dimensional complex diagrams. Bioinformatics 2006, 22, 1710–1716. [Google Scholar] [CrossRef] [PubMed]
- Osorio, E.; Arango, G.J.; Jiménez, N.; Alzate, F.; Ruiz, G.; Gutiérrez, D.; Paco, M.A.; Giménez, A.; Robledo, S. Antiprotozoal and cytotoxic activities in vitro of Colombian Annonaceae. J. Ethnopharmacol. 2007, 111, 630–635. [Google Scholar] [CrossRef]
- Makwali, J.A.; Wanjala, F.M.E.; Ingonga, J.; Anjili, C.O. In vitro Studies on the Antileishmanial Activity of Herbicides and Plant Extracts Against Leishmania major Parasites. Res. J. Med. Plant 2015, 9, 90–104. [Google Scholar] [CrossRef]
- Cristina Marcucci, M.; Salatino, A.; Farias Azevedo de Magalhães Oliveira, L.; Passarelli Gonçalves, C. Accessible Methodologies for Quantification of Flavonoids and Total Phenols in Propolis. Rev. Virtual Quím. 2021, 13, 61–73. [Google Scholar] [CrossRef]
- Frozza, C.O.d.S.; Garcia, C.S.C.; Gambato, G.; de Souza, M.D.O.; Salvador, M.; Moura, S.; Padilha, F.F.; Seixas, F.K.; Collares, T.; Borsuk, S.; et al. Chemical characterization, antioxidant and cytotoxic activities of Brazilian red propolis. Food Chem. Toxicol. 2013, 52, 137–142. [Google Scholar] [CrossRef]
- Araujo, J.M.E.; Mendonça-Melo, L.S.; Araujo, E.D.; Fernandes, R.P.M.; Scher, R. Phenolic Composition and Leishmanicidal Activity of Red Propolis and Dalbergia ecastaphyllum (L.) Taub (Fabaceae) Extracts from Sergipe, Brazil. Braz. Arch. Biol. Technol. 2018, 61, 1–10. [Google Scholar] [CrossRef]
- Azevedo, L.F.; Silva, P.d.F.; Brandão, M.P.; da Rocha, L.G.; Aragão, C.F.S.; da Silva, S.A.S.; Porto, I.C.C.M.; Basílio-Júnior, I.D.; Fonseca, E.J.d.S.; de Moura, M.A.B.F.; et al. Polymeric nanoparticle systems loaded with red propolis extract: A comparative study of the encapsulating systems, PCL-Pluronic versus Eudragit®E100-Pluronic. J. Apic. Res. 2018, 57, 255–270. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; Da Silva, P.F.; Azevedo, L.F.; Da Rocha, L.G.; de Moraes Porto, I.C.C.; Lima E Moura, T.F.A.; Basílio-Júnior, I.D.; Grillo, L.A.M.; Dornelas, C.B.; da Silva Fonseca, E.J.; et al. Polymeric Nanoparticles of Brazilian Red Propolis Extract: Preparation, Characterization, Antioxidant and Leishmanicidal Activity. Nanosc. Res. Lett. 2016, 11, 301. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Lotti, C.; Campone, L.; Cuesta-Rubio, O.; Fernandez, M.C.; Rastrelli, L. Cuban and Brazilian Red Propolis: Botanical Origin and Comparative Analysis by High-Performance Liquid Chromatography–Photodiode Array Detection/Electrospray Ionization Tandem Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 6484–6491. [Google Scholar] [CrossRef]
- Ccana-Ccapatinta, G.V.; Mejía, J.A.A.; Tanimoto, M.H.; Groppo, M.; de Carvalho, J.C.A.S.; Bastos, J.K. Dalbergia ecastaphyllum (L.) Taub. and Symphonia globulifera L.f.: The Botanical Sources of Isoflavonoids and Benzophenones in Brazilian Red Propolis. Molecules 2020, 25, 2060. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.P.; da Silva, T.M.G.; Camara, C.A.; Santana, A.L.B.D.; Moreira, M.S.A.; Silva, T.M.S. Isolation and structure elucidation of flavonoids from Amburana cearensis resin and identification of human DNA topoisomerase II-α inhibitors. Phytochem. Lett. 2017, 22, 61–70. [Google Scholar] [CrossRef]
- Falcão, S.I.; Lopes, M.; Vilas-Boas, M. A First Approach to the Chemical Composition and Antioxidant Potential of Guinea-Bissau Propolis. Nat. Prod. Commun. 2019, 14, 1934578X1984413. [Google Scholar] [CrossRef]
- Omar, R.M.K.; Igoli, J.; Gray, A.I.; Ebiloma, G.U.; Clements, C.; Fearnley, J.; Edrada-Ebel, R.A.; Zhang, T.; De Koning, H.P.; Watson, D.G. Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma Brucei. Phytochem. Anal. 2016, 27, 107–115. [Google Scholar] [CrossRef]
- Carvalho, A.; Santos, L.; Freitas, J.; Chaves, M. Isoflavonoides Da Tribo Dalbergieae: Uma Contribuição Quimiossistemática Para a Subfamília Papilionoideae. Quim. Nova 2020, 43, 1294–1311. [Google Scholar] [CrossRef]
- Santos, R.L.; Pereira, D.S.; Júnior, S.R.X.; Venturieri, G.C. Levantamento fitogeográfico de Dalbergia L.f. (Leguminosae-papilionoideae) com potencial produtivo para própolis vermelha no Estado do Pará. Rev. Verde Agroecol. Desenvolv. Sustent. 2017, 12, 590. [Google Scholar] [CrossRef]
- Araujo, C.A.C.; Alegrio, L.V.; Leon, L.L. Antileishmanial activity of compounds extracted and characterized from Centrolobium sclerophyllum. Phytochemistry 1998, 49, 751–754. [Google Scholar] [CrossRef]
- Salem, M.M.; Werbovetz, K.A. Isoflavonoids and Other Compounds from Psorothamnus arborescens with Antiprotozoal Activities. J. Nat. Prod. 2006, 69, 43–49. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and Antileishmanial Activities of Flavonoids and Their Analogues: In Vitro, In Vivo, Structure-Activity Relationship, and Quantitative Structure-Activity Relationship Studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, D.B.; Lemos, J.A.; Miranda, S.E.M.; Di Filippo, L.D.; Duarte, J.L.; Ferreira, L.A.M.; Barros, A.L.B.; Oliveira, A.E.M.F.M. Current Applications of Plant-Based Drug Delivery Nano Systems for Leishmaniasis Treatment. Pharmaceutics 2022, 14, 2339. [Google Scholar] [CrossRef]
- Chen, M.; Zhai, L.; Christensen, S.B.; Theander, T.G.; Kharazmi, A. Inhibition of Fumarate Reductase in Leishmania major and L. donovani by Chalcones. Antimicrob. Agents Chemother. 2001, 45, 2023–2029. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, J.; Li, H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-κB and NLRP3 inflammasome pathways. Biomed. Pharmacother. 2018, 106, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wong, H.-K.; Feng, Y.-B.; Zhang, Z.-J. Liquiritigenin exhibits antitumour action in pituitary adenoma cells via Ras/ERKs and ROS-dependent mitochondrial signalling pathways. J. Pharm. Pharmacol. 2014, 66, 408–417. [Google Scholar] [CrossRef]
- Wang, D.; Lu, J.; Liu, Y.; Meng, Q.; Xie, J.; Wang, Z.; Teng, L. Liquiritigenin Induces Tumor Cell Death through Mitogen-Activated Protein Kinase- (MPAKs-) Mediated Pathway in Hepatocellular Carcinoma Cells. BioMed. Res. Int. 2014, 2014, 965316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Dai, Y.; Ma, Y.; Yan, Y.; Hua, M.; Gao, Q.; Geng, X.; Zhou, Q. Protective Effects of Liquiritigenin against Cisplatin-Induced Nephrotoxicity via NRF2/SIRT3-Mediated Improvement of Mitochondrial Function. Molecules 2022, 27, 3823. [Google Scholar] [CrossRef]
- Geng, W.; Zhou, G.; Zhao, B.; Xiao, Q.; Li, C.; Fan, S.; Dong, P.; Zheng, J. Liquiritigenin suppresses the activation of hepatic stellate cells via targeting miR-181b/PTEN axis. Phytomedicine 2020, 66, 153108. [Google Scholar] [CrossRef]







| Sample | Total Phenolics (mg GAE/g) | Flavonoids (mg QE/g) |
|---|---|---|
| EHPV-1 | 309.27 ± 18.73 a | 34.38 ± 0.44 a |
| EHPV-2 | 336.91 ± 8.77 b | 25.77 ± 0.94 b |
| EHPV-3 | 192.03 ± 7.06 c | 16.70 ± 0.88 c |
| EHPV-4 | 278.98 ± 10.00 d | 25.23 ± 0.57 b,d |
| FrHX | 77.33 ± 3.25 e | nd |
| FrCL | 328.44 ± 7.51 a,b | 25.46 ± 0.40 b,d |
| FrEA | 182.92 ± 2.77 c | 21.25 ± 0.08 d |
| Sample | IC50 (µg/mL) |
|---|---|
| EHPV-1 | 23.37 ± 1.4 a,e |
| EHPV-2 | 31.38 ± 3.6 a,b |
| EHPV-3 | 36.10 ± 3.7 b,c,d |
| EHPV-4 | 34.31 ± 0.3 a,b,c,d |
| FrHX | 45.99 ± 5.2 d |
| FrCL | 16.11 ± 0.9 e |
| FrEA | 112.0 ± 7.8 f |
| Amphotericin B | 0.013 ± 0.1 |
| Peak | RT (Min) | [M + H]+ (m/z) | Product Ions MS/MS (m/z) | Tentative Identification | Relative Area (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EHPV-1 | FrCL | |||||||||||
| 1 | 23.1 | 301 | 284 | 269 | 255 | 241 | 213 | 137 | 123 | 7,8,3′-trihydroxy-4′-methoxyisoflavone | 6.30 | 7.31 |
| 2 | 30.1 | 257 | 239 | 229 | 211 | 147 | 137 | liquiritigenin a | 5.68 | 6.09 | ||
| 3 | 31.1 | 287 | 269 | 259 | 177 | 163 | 153 | 137 | 123 | vesticarpan | 1.95 | 1.84 |
| 4 | 36.4 | 317 | 299 | 289 | 207 | 179 | 163 | 135 | 107 | violanone | 1.13 | 1.31 |
| 5 | 37.1 | 287 | 255 | 241 | 193 | 177 | 153 | 147 | 123 | 3,8-dihydroxy-9-methoxy-pterocarpan | 0.73 | 0.80 |
| 6 | 38.4 | 285 | 270 | 253 | 225 | 137 | 123 | calycosin a | 21.87 | 23.78 | ||
| 7 | 42.5 | 287 | 269 | 259 | 177 | 161 | 153 | 139 | 137 | 3,4-dihydroxy-9-methoxy-pterocarpan | 4.54 | 4.97 |
| 8 | 49.6 | 303 | 285 | 193 | 181 | 167 | 149 | 123 | 107 | mucronulatol | 1.49 | 1.73 |
| 9 | 50.6 | 301 | 269 | 241 | 191 | 167 | 147 | 123 | 107 | 3-hydroxy-8,9-dimethoxy-pterocarpan | 0.65 | 0.72 |
| 10 | 58.9 | 273 | 163 | 151 | 137 | 123 | vestitol | 10.95 | 11.43 | |||
| 11 | 59.9 | 257 | 239 | 229 | 211 | 163 | 147 | 137 | isoliquiritigenin a | 0.54 | 0.60 | |
| 12 | 62.4 | 269 | 177 | 161 | 147 | 137 | 123 | formononetin a | 12.72 | 14.42 | ||
| 13 | 63.7 | 271 | 243 | 229 | 177 | 161 | 147 | 137 | medicarpin | 3.30 | 1.83 | |
| 14 | 77.7 | 285 | 270 | 253 | 181 | 163 | 137 | 123 | biochanin A a | 0.31 | 0.44 | |
| 15 | 83.9 | 523 | 399 | 387 | 373 | retusapurpurin B | 0.57 | 0.63 | ||||
| CYP51 | TR | ||
|---|---|---|---|
| Compound | ΔGbind (kcal/mol) | Compound | ΔGbind (kcal/mol) |
| liquiritigenin | −9.3 | liquiritigenin | −8.9 |
| calycosin | −9.3 | 7,8,3′-trihydroxy-4′-methoxyisoflavone | −8.2 |
| 7,8,3′-trihydroxy-4′-methoxyisoflavone | −9.1 | biochanin A | −8.5 |
| formononetin | −8.9 | retusapurpurin B | −8.5 |
| 3,8-dihydroxy-9-methoxy-pterocarpan | −8.9 | calycosin | −8.4 |
| biochanin A | −8.8 | vestitol | −8.4 |
| medicarpin | −8.7 | formononetin | −8.3 |
| 3,4-dihydroxy-9-methoxy-pterocarpan | −8.6 | isoliquiritigenin | −8.1 |
| vestitol | −8.4 | 3,4-dihydroxy-9-methoxy-pterocarpan | −8.1 |
| violanone | −8.2 | mucronulatol | −8.0 |
| vesticarpan | −8.2 | violanone | −7.7 |
| isoliquiritigenin | −8.1 | 3-hydroxy-8,9-dimethoxy-pterocarpan | −7.2 |
| mucronulatol | −7.9 | medicarpin | −7.1 |
| 3-hydroxy-8,9-dimethoxy-pterocarpan | −7.5 | 3,4-dihydroxy-9-methoxy-pterocarpan | −7.1 |
| retusapurpurin B | −6.2 | vesticarpan | −7.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutra, R.P.; de Sousa, M.M., Jr.; Mignoni, M.S.P.M.; de Oliveira, K.G.M.; Pereira, E.B.; Figueredo, A.S.; da Costa, A.A.C.; Dias, T.G.; Vasconcelos, C.C.; Silva, L.A.; et al. Brazilian Amazon Red Propolis: Leishmanicidal Activity and Chemical Composition of a New Variety of Red Propolis. Metabolites 2023, 13, 1027. https://doi.org/10.3390/metabo13091027
Dutra RP, de Sousa MM Jr., Mignoni MSPM, de Oliveira KGM, Pereira EB, Figueredo AS, da Costa AAC, Dias TG, Vasconcelos CC, Silva LA, et al. Brazilian Amazon Red Propolis: Leishmanicidal Activity and Chemical Composition of a New Variety of Red Propolis. Metabolites. 2023; 13(9):1027. https://doi.org/10.3390/metabo13091027
Chicago/Turabian StyleDutra, Richard Pereira, Marcos Marinho de Sousa, Jr., Maria Simone Pereira Maciel Mignoni, Karla Gabriela Mota de Oliveira, Euzineti Borges Pereira, Aline Santana Figueredo, Arthur André Castro da Costa, Tatielle Gomes Dias, Cleydlenne Costa Vasconcelos, Lucilene Amorim Silva, and et al. 2023. "Brazilian Amazon Red Propolis: Leishmanicidal Activity and Chemical Composition of a New Variety of Red Propolis" Metabolites 13, no. 9: 1027. https://doi.org/10.3390/metabo13091027
APA StyleDutra, R. P., de Sousa, M. M., Jr., Mignoni, M. S. P. M., de Oliveira, K. G. M., Pereira, E. B., Figueredo, A. S., da Costa, A. A. C., Dias, T. G., Vasconcelos, C. C., Silva, L. A., Reis, A. S., & Lopes, A. J. O. (2023). Brazilian Amazon Red Propolis: Leishmanicidal Activity and Chemical Composition of a New Variety of Red Propolis. Metabolites, 13(9), 1027. https://doi.org/10.3390/metabo13091027