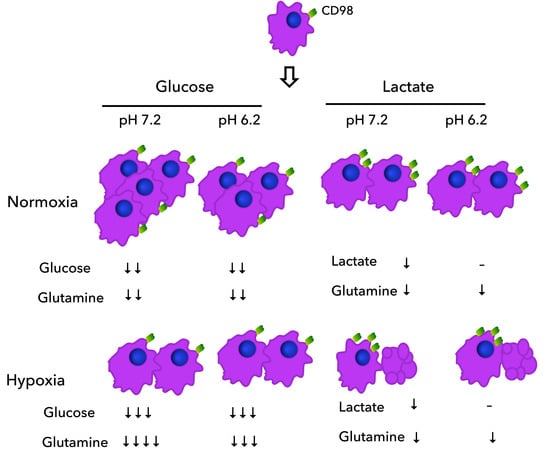
Metabolic Responses of Lung Adenocarcinoma Cells to Survive under Stressful Conditions Associated with Tumor Microenvironment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Cell Culture
2.3. Tested Culture Conditions
2.4. Growth Kinetics of Tumor Cells
2.5. Determination of Metabolites
2.6. Evaluation of Cell Morphology
2.7. CD98 Surface Determination
2.8. Cell Cycle Analysis
2.9. Statistical Analysis
3. Results
3.1. Most Tumor Cells Increased Their Proliferation Rate under Acidosis when Glucose Was Available
3.2. Specific Rates of Glucose Consumption and Lactate Production of Tumor Cells Diminished under Acidosis
3.3. Most Lung Cancer Cell Lines Consumed Lactate under Normoxia or Hypoxia
3.4. Glutamine Consumption Diminished under Acidosis or Hypoxia
3.5. Lung Cancer Cells Increased CD98 Expression When Lactate Was the Predominant Carbon Source
3.6. Acidosis Induced G0/G1 Arrest in A427 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Riemann, A.; Schneider, B.; Ihling, A.; Nowak, M.; Sauvant, C.; Thews, O.; Gekle, M. Acidic environment leads to ROS-induced MAPK signaling in cancer cells. PLoS ONE 2011, 6, e22445. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Lopez-Gonzalez, J.S.; Baez-Viveros, J.L.; Aguilar-Cazares, D.; Prado-Garcia, H. Tumor cell metabolism: An integral view. Cancer Biol. Ther. 2011, 12, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Fais, S.; Venturi, G.; Gatenby, B. Microenvironmental acidosis in carcinogenesis and metastases: New strategies in prevention and therapy. Cancer Metastasis Rev. 2014, 33, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.; Prado-Garcia, H.; Sanchez-Garcia, F.J. Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.M.; Ginsberg, M.H. CD98 at the crossroads of adaptive immunity and cancer. J. Cell Sci. 2012, 125, 1373–1382. [Google Scholar] [CrossRef]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. Membrane transporters for the special amino acid glutamine: Structure/function relationships and relevance to human health. Front. Chem. 2014, 2, 61. [Google Scholar] [CrossRef] [PubMed]
- Prado-Garcia, H.; Campa-Higareda, A.; Romero-Garcia, S. Lactic Acidosis in the Presence of Glucose Diminishes Warburg Effect in Lung Adenocarcinoma Cells. Front. Oncol. 2020, 10, 807. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Prado-Garcia, H.; Valencia-Camargo, A.D.; Alvarez-Pulido, A. Lactic Acidosis Promotes Mitochondrial Biogenesis in Lung Adenocarcinoma Cells, Supporting Proliferation Under Normoxia or Survival Under Hypoxia. Front. Oncol. 2019, 9, 1053. [Google Scholar] [CrossRef]
- Kennedy, K.M.; Scarbrough, P.M.; Ribeiro, A.; Richardson, R.; Yuan, H.; Sonveaux, P.; Landon, C.D.; Chi, J.-T.; Pizzo, S.; Schroeder, T.; et al. Catabolism of exogenous lactate reveals it as a legitimate metabolic substrate in breast cancer. PLoS ONE 2013, 8, e75154. [Google Scholar] [CrossRef]
- Burke, P.J. Mitochondria, Bioenergetics and Apoptosis in Cancer. Trends Cancer 2017, 3, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.Y.; Kim, S.G.; Vander Heiden, M.G.; Mahoney, S.J.; Vu, H.; Yoon, S.-O.; Cantley, L.C.; Blenis, J. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol. Cell 2010, 38, 487–499. [Google Scholar] [CrossRef]
- van den Heuvel, A.P.; Jing, J.; Wooster, R.F.; Bachman, K.E. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol. Ther. 2012, 13, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Birsoy, K.; Possemato, R.; Lorbeer, F.K.; Bayraktar, E.C.; Thiru, P.; Yucel, B.; Wang, T.; Chen, W.W.; Clish, C.B.; Sabatini, D.M. Metabolic determinants of cancer cell sensitivity to glucose limitation and biguanides. Nature 2014, 508, 108–112. [Google Scholar] [CrossRef]
- Rohren, E.M.; Turkington, T.G.; Coleman, R.E. Clinical applications of PET in oncology. Radiology 2004, 231, 305–332. [Google Scholar] [CrossRef]
- Weber, W. Clinical PET/MR. Recent. Results Cancer Res. 2020, 216, 747–764. [Google Scholar]
- Pavlides, S.; Whitaker-Menezes, D.; Castello-Cros, R.; Flomenberg, N.; Witkiewicz, A.K.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; et al. The reverse Warburg effect: Aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 2009, 8, 3984–4001. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, M.P.; Martinez-Outschoorn, U.E.; Sotgia, F. Oncogenes induce the cancer-associated fibroblast phenotype: Metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle 2013, 12, 2723–2732. [Google Scholar] [CrossRef]
- Wu, H.; Ding, Z.; Hu, D.; Sun, F.; Dai, C.; Xie, J.; Hu, X. Central role of lactic acidosis in cancer cell resistance to glucose deprivation-induced cell death. J. Pathol. 2012, 227, 189–199. [Google Scholar] [CrossRef]
- Xie, J.; Wu, H.; Dai, C.; Pan, Q.; Ding, Z.; Hu, D.; Ji, B.; Luo, Y.; Hu, X. Beyond Warburg effect–dual metabolic nature of cancer cells. Sci. Rep. 2014, 4, 4927. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.M.; Meadows, G.G. Differential effects of specific amino acid restriction on glucose metabolism, reduction/oxidation status and mitochondrial damage in DU145 and PC3 prostate cancer cells. Oncol. Lett. 2011, 2, 349–355. [Google Scholar] [CrossRef]
- Kolesnik, D.L.; Pyaskovskaya, O.N.; Solyanik, G.I. Impact of lactic acidosis on the survival of Lewis lung carcinoma cells. Exp. Oncol. 2017, 39, 112–116. [Google Scholar] [CrossRef]
- Poettler, M.; Unseld, M.; Braemswig, K.; Haitel, A.; Zielinski, C.C.; Prager, G.W. CD98hc (SLC3A2) drives integrin-dependent renal cancer cell behavior. Mol. Cancer 2013, 12, 169. [Google Scholar] [CrossRef]
- Mishra, A.P.; Salehi, B.; Sharifi-Rad, M.; Pezzani, R.; Kobarfard, F.; Sharifi-Rad, J.; Nigam, M. Programmed Cell Death, from a Cancer Perspective: An Overview. Mol. Diagn. Ther. 2018, 22, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Wojtkowiak, J.W.; Rothberg, J.M.; Kumar, V.; Schramm, K.J.; Haller, E.; Proemsey, J.B.; Lloyd, M.C.; Sloane, B.F.; Gillies, R.J. Chronic autophagy is a cellular adaptation to tumor acidic pH microenvironments. Cancer Res. 2012, 72, 3938–3947. [Google Scholar] [CrossRef] [PubMed]





| Glucose pH 7.2 | Glucose pH 6.2 | ||||||
|---|---|---|---|---|---|---|---|
| Tumor Cell Line | %O2 | Growth Phase Length (h) | Doubling Time (h) | μ (×10−2 h−1) | Growth Phase Length (h) | Doubling Time (h) | μ (×10−2 h−1) |
| MRC-5 | N | 24–96 | 46.11 | 1.5 | 24–72 | 105.2 | 0.66 |
| H | 24–72 | 86.28 | 0.8 | 48–72 | 437.4 | 0.16 | |
| A427 | N | 0–72 | 30.46 | 2.28 | 0–48 | 19.44 | 3.57 |
| H | 0–48 | 57.13 | 1.21 | 0–32 | 34.02 | 2.04 | |
| A549 | N | 0–45 | 18.75 | 3.7 | 6–69 | 29.71 | 2.33 |
| H | 0–45 | 23.32 | 2.97 | 22–69 | 31.24 | 2.22 | |
| Calu-1 | N | 22–46 | 29.42 | 2.36 | 22–54 | 103.3 | 0.67 |
| H | 22–46 | 54.56 | 1.27 | 22–54 | 33.22 | 2.09 | |
| SKMES-1 | N | 0–48 | 23.34 | 2.97 | 0–48 | 26.41 | 2.62 |
| H | 0–72 | 50.1 | 1.38 | 0–48 | 26.18 | 2.65 | |
| MCF-7 | N | 0–48 | 31.11 | 2.23 | 0–48 | 40.69 | 1.7 |
| H | 0–48 | 36.18 | 1.91 | 0–48 | 29.6 | 2.3 | |
| Lactate pH 7.2 | Lactate pH 6.2 | ||||||
|---|---|---|---|---|---|---|---|
| Tumor Cell Line | %O2 | Growth Phase Length (h) | Doubling Time (h) | μ (×10−2 h−1) | Growth Phase Length (h) | Doubling Time (h) | μ (×10−2 h−1) |
| MRC-5 | N | 24–96 | 36.4 | 1.9 | 24–72 | 61.78 | 1.12 |
| H | - | - | - | 24–96 | 102.9 | 0.67 | |
| A427 | N | 0–32 | 32.44 | 2.14 | 0–48 | 31.95 | 2.17 |
| H | - | - | - | - | - | - | |
| A549 | N | 6–69 | 35.87 | 1.9 | 22–69 | 22.86 | 3.03 |
| H | - | - | - | 6–45 | 87.11 | 0.8 | |
| Calu-1 | N | - | - | - | - | - | - |
| H | - | - | - | - | - | - | |
| SKMES-1 | N | 0–48 | 23.38 | 2.96 | 0–72 | 77.55 | 0.89 |
| H | 0–48 | 36.95 | 1.88 | 0–48 | 39.66 | 1.75 | |
| MCF-7 | N | 0–48 | 31.84 | 2.18 | 0–72 | 55.65 | 1.25 |
| H | - | - | - | 0–48 | 128.2 | 0.54 | |
| Lactate pH 7.2 | Lactate pH 6.2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tumor Cell Line | %O2 | a qS Lactate (μmol/106 cells × h) | b qP Lactate (μmol/106 cells × h) | c qS Glutamine (μmol/106 cells × h) | d qP Glutamate (μmol/106 cells × h) | qS Lactate (μmol/106 cells × h) | qP Lactate (μmol/106 cells × h) | qS Glutamine (μmol/106 cells × h) | qP Glutamate (μmol/106 cells × h) |
| MRC-5 | N | 0.67 | - | 0.08 | 0 | - | 0.19 | 0.14 | 0.07 |
| H | - | - | - | - | 1.25 | - | 0.01 | 0 | |
| A427 | N | 0.28 | - | 0.08 | 0.02 | - | 0.003 | 0.05 | 0.01 |
| H | - | - | - | - | - | - | - | - | |
| A549 | N | - | 0.13 | 0.06 | 0.03 | - | 0.32 | 0.05 | 0.02 |
| H | - | - | - | - | - | 0.24 | 0.03 | 0.02 | |
| Calu-1 | N | - | - | - | - | - | - | - | - |
| H | - | - | - | - | - | - | - | - | |
| SKMES-1 | N | 0.13 | - | 0.05 | 0.06 | 0.13 | - | 0.04 | 0.04 |
| H | 0.01 | - | 0.03 | 0.05 | 0.25 | - | 0 | 0.03 | |
| MCF-7 | N | 0.31 | - | 0.075 | 0.001 | 0.21 | - | 0.036 | 0.006 |
| H | - | - | - | - | - | 0.095 | 0.014 | 0.017 | |
| Tumor Cell | Glucose pH 7.2 | Glucose pH 6.2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Line | %O2 | a qS Glucose | b qP Lactate | c qS Glutamine | d qP Glutamate | qS Glucose | qP Lactate | qS Glutamine | qP Glutamate |
| (μmol/106 cells × h) | (μmol/106 cells × h) | (μmol/106 cells × h) | (μmol/106 cells × h) | (μmol/106 cells × h) | (μmol/106 cells × h) | (μmol/106 cells × h) | (μmol/106 cells × h) | ||
| MRC-5 | N | 0.91 | 1.25 | 0.07 | 0 | 1.49 | 1.94 | 0.03 | 0.001 |
| H | 1.28 | 1.96 | 0.02 | 0.08 | 1.11 | 1.7 | 0 | 0 | |
| A427 | N | 0.43 | 1.19 | 0.05 | 0.01 | 0.43 | 0.72 | 0.08 | 0.001 |
| H | 0.87 | 1.91 | 0.07 | 0.02 | 0.65 | 1.32 | 0.06 | 0.014 | |
| A549 | N | 0.58 | 0.89 | 0.1 | 0.02 | 0.2 | 0.42 | 0.06 | 0.01 |
| H | 0.52 | 1.04 | 0.01 | 0.02 | 0.09 | 0.64 | 0.06 | 0.015 | |
| Calu-1 | N | 0.84 | 2.68 | 0.08 | 0.005 | 0.32 | 1.33 | 0.01 | 0.007 |
| H | 1.06 | 1.75 | 0.12 | 0 | 0.49 | 1.55 | 0 | 0.004 | |
| SKMES-1 | N | 0.38 | 0.77 | 0.036 | 0.06 | 0.3 | 0.47 | 0.03 | 0.054 |
| H | 0.28 | 0.56 | 0.014 | 0.02 | 0.44 | 0.93 | 0.04 | 0.036 | |
| MCF-7 | N | 0.3 | 0.5 | 0.052 | 0 | 0.14 | 0.41 | 0.094 | 0 |
| H | 0.46 | 0.91 | 0.051 | 0 | 0.34 | 0.78 | 0.062 | 0.003 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carlos-Reyes, A.; Romero-Garcia, S.; Prado-Garcia, H. Metabolic Responses of Lung Adenocarcinoma Cells to Survive under Stressful Conditions Associated with Tumor Microenvironment. Metabolites 2024, 14, 103. https://doi.org/10.3390/metabo14020103
Carlos-Reyes A, Romero-Garcia S, Prado-Garcia H. Metabolic Responses of Lung Adenocarcinoma Cells to Survive under Stressful Conditions Associated with Tumor Microenvironment. Metabolites. 2024; 14(2):103. https://doi.org/10.3390/metabo14020103
Chicago/Turabian StyleCarlos-Reyes, Angeles, Susana Romero-Garcia, and Heriberto Prado-Garcia. 2024. "Metabolic Responses of Lung Adenocarcinoma Cells to Survive under Stressful Conditions Associated with Tumor Microenvironment" Metabolites 14, no. 2: 103. https://doi.org/10.3390/metabo14020103
APA StyleCarlos-Reyes, A., Romero-Garcia, S., & Prado-Garcia, H. (2024). Metabolic Responses of Lung Adenocarcinoma Cells to Survive under Stressful Conditions Associated with Tumor Microenvironment. Metabolites, 14(2), 103. https://doi.org/10.3390/metabo14020103