Adipokines, Vitamin D, and Selected Inflammatory Biomarkers among Parkinson’s Disease Patients with and without Dyskinesia: A Preliminary Examination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Study Design
2.3. Biochemical Analysis
2.4. Statistical Analysis
3. Results
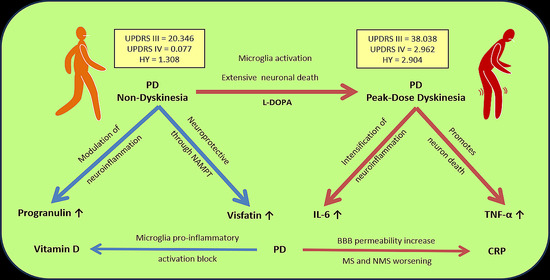
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goh, S.Y.; Chao, Y.X.; Dheen, S.T.; Tan, E.-K.; Tay, S.S.-W. Role of MicroRNAs in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 5649. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the Diagnosis of Parkinson’s Disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Post, B.; van den Heuvel, L.; van Prooije, T.; van Ruissen, X.; van de Warrenburg, B.; Nonnekes, J. Young Onset Parkinson’s Disease: A Modern and Tailored Approach. J. Parkinsons. Dis. 2020, 10, S29–S36. [Google Scholar] [CrossRef]
- Chia, S.J.; Tan, E.-K.; Chao, Y.-X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative Stress in the Aging Substantia Nigra and the Etiology of Parkinson’s Disease. Aging Cell 2019, 18, 1301. [Google Scholar] [CrossRef]
- Marinus, J.; Zhu, K.; Marras, C.; Aarsland, D.; van Hilten, J.J. Risk Factors for Non-Motor Symptoms in Parkinson’s Disease. Lancet Neurol. 2018, 17, 559–568. [Google Scholar] [CrossRef]
- Delamarre, A.; Meissner, W.G. Epidemiology, Environmental Risk Factors and Genetics of Parkinson’s Disease. Presse Med. 2017, 46, 175–181. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Romano, R.; Coelho-Júnior, H.J.; Bucci, C.; Marzetti, E. Mitochondrial Dysfunction, Protein Misfolding and Neuroinflammation in Parkinson’s Disease: Roads to Biomarker Discovery. Biomolecules 2021, 11, 1508. [Google Scholar] [CrossRef] [PubMed]
- Vijiaratnam, N.; Simuni, T.; Bandmann, O.; Morris, H.R.; Foltynie, T. Progress towards Therapies for Disease Modification in Parkinson’s Disease. Lancet Neurol. 2021, 20, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Shiraishi, T.; Umehara, T.; Omoto, S.; Iguchi, Y. Recent Advances in Drug Therapy for Parkinson’s Disease. Intern. Med. 2023, 62, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.C.; de Hemptinne, C.; Thompson, M.C.; Miocinovic, S.; Miller, A.M.; Gilron, R.; Ostrem, J.L.; Chizeck, H.J.; Starr, P.A. Adaptive Deep Brain Stimulation for Parkinson’s Disease Using Motor Cortex Sensing. J. Neural Eng. 2018, 15, 046006. [Google Scholar] [CrossRef]
- Qureshi, A.R.; Jamal, M.K.; Rahman, E.; Paul, D.A.; Oghli, Y.S.; Mulaffer, M.T.; Qureshi, D.; Danish, M.A.; Rana, A.Q. Non-pharmacological Therapies for Pain Management in Parkinson’s Disease: A Systematic Review. Acta Neurol. Scand. 2021, 144, 115–131. [Google Scholar] [CrossRef]
- Modi, M.; Singh, R.; Goyal, M.; Gairolla, J.; Singh, G.; Rishi, V.; Thakur, J.; Sehgal, R.; Garg, V.; Khandelwal, N.; et al. Prevalence of Epilepsy and Its Association with Exposure to Toxocara Canis: A Community Based, Case–Control Study from Rural Northern India. Ann. Indian Acad. Neurol. 2018, 21, 263. [Google Scholar] [CrossRef]
- Kwon, D.K.; Kwatra, M.; Wang, J.; Ko, H.S. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Pathogenesis and Emerging Treatment Strategies. Cells 2022, 11, 3736. [Google Scholar] [CrossRef]
- Pandey, S. Chorea. In Neurological Cinematographic Atlas; Columbia University Press: New York, NY, USA, 1944; Volume 61, pp. 1–4. [Google Scholar]
- Balint, B.; Mencacci, N.E.; Valente, E.M.; Pisani, A.; Rothwell, J.; Jankovic, J.; Vidailhet, M.; Bhatia, K.P. Dystonia. Nat. Rev. Dis. Prim. 2018, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Leta, V.; Jenner, P.; Chaudhuri, K.R.; Antonini, A. Can Therapeutic Strategies Prevent and Manage Dyskinesia in Parkinson’s Disease? An Update. Expert Opin. Drug Saf. 2019, 18, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- di Biase, L.; Pecoraro, P.M.; Carbone, S.P.; Caminiti, M.L.; Di Lazzaro, V. Levodopa-Induced Dyskinesias in Parkinson’s Disease: An Overview on Pathophysiology, Clinical Manifestations, Therapy Management Strategies and Future Directions. J. Clin. Med. 2023, 12, 4427. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.V.; Lenka, A.; Dhamija, R.K.; Fasano, A. A Video-Atlas of Levodopa-Induced Dyskinesia in Parkinson’s Disease: Terminology Matters. Neurol. Sci. 2023, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of Inflammation: An Organizing Principle in Biology and Medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-W.; Chen, C.-M.; Chang, K.-H. Biomarker of Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 4148. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Quick, J.D.; Silva, C.; Wong, J.H.; Lim, K.L.; Reynolds, R.; Barron, A.M.; Zeng, J.; Lo, C.H. Lysosomal Acidification Dysfunction in Microglia: An Emerging Pathogenic Mechanism of Neuroinflammation and Neurodegeneration. J. Neuroinflamm. 2023, 20, 185. [Google Scholar] [CrossRef]
- Rocha, E.M.; De Miranda, B.; Sanders, L.H. Alpha-Synuclein: Pathology, Mitochondrial Dysfunction and Neuroinflammation in Parkinson’s Disease. Neurobiol. Dis. 2018, 109, 249–257. [Google Scholar] [CrossRef]
- Barczuk, J.; Siwecka, N.; Lusa, W.; Rozpędek-Kamińska, W.; Kucharska, E.; Majsterek, I. Targeting NLRP3-Mediated Neuroinflammation in Alzheimer’s Disease Treatment. Int. J. Mol. Sci. 2022, 23, 8979. [Google Scholar] [CrossRef] [PubMed]
- Contaldi, E.; Magistrelli, L.; Milner, A.V.; Cosentino, M.; Marino, F.; Comi, C. Expression of Transcription Factors in CD4 + T Cells as Potential Biomarkers of Motor Complications in Parkinson’s Disease. J. Parkinsons. Dis. 2021, 11, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Starhof, C.; Winge, K.; Heegaard, N.H.H.; Skogstrand, K.; Friis, S.; Hejl, A. Cerebrospinal Fluid Pro-Inflammatory Cytokines Differentiate Parkinsonian Syndromes. J. Neuroinflamm. 2018, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Pastorello, Y.; Slevin, M. A Meta-Analysis Investigating the Relationship between Inflammation in Autoimmune Disease, Elevated CRP, and the Risk of Dementia. Front. Immunol. 2023, 14, 1087571. [Google Scholar] [CrossRef] [PubMed]
- Na, K.-S.; Jung, H.-Y.; Kim, Y.-K. The Role of Pro-Inflammatory Cytokines in the Neuroinflammation and Neurogenesis of Schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Müller, J.; Schuetze, K.; Rolfes, V.; Bissinger, R.; Rosero, N.; Ahmad, A.; Franklin, B.S.; Zur, B.; Fröhlich, H.; et al. Comprehensive Profiling of Blood Coagulation and Fibrinolysis Marker Reveals Elevated Plasmin-Antiplasmin Complexes in Parkinson’s Disease. Biology 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Cheng, K.K.; Hoo, R.L.; Siu, P.M.; Yau, S. The Novel Perspectives of Adipokines on Brain Health. Int. J. Mol. Sci. 2019, 20, 5638. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Nie, L.; Ali, T.; Liu, Z.; Li, W.; Gao, R.; Zhang, Z.; Liu, J.; Dai, Z.; Xie, Y.; et al. Adiponectin Deficiency Accelerates Brain Aging via Mitochondria-Associated Neuroinflammation. Immun. Ageing 2023, 20, 15. [Google Scholar] [CrossRef]
- Kinfe, T.; Buchfelder, M.; Chaudhry, S.; Chakravarthy, K.; Deer, T.; Russo, M.; Georgius, P.; Hurlemann, R.; Rasheed, M.; Muhammad, S.; et al. Leptin and Associated Mediators of Immunometabolic Signaling: Novel Molecular Outcome Measures for Neurostimulation to Treat Chronic Pain. Int. J. Mol. Sci. 2019, 20, 4737. [Google Scholar] [CrossRef]
- Barichella, M.; Garrì, F.; Caronni, S.; Bolliri, C.; Zocchi, L.; Macchione, M.C.; Ferri, V.; Calandrella, D.; Pezzoli, G. Vitamin D Status and Parkinson’s Disease. Brain Sci. 2022, 12, 790. [Google Scholar] [CrossRef] [PubMed]
- Pignolo, A.; Mastrilli, S.; Davì, C.; Arnao, V.; Aridon, P.; dos Santos Mendes, F.A.; Gagliardo, C.; D’Amelio, M. Vitamin D and Parkinson’s Disease. Nutrients 2022, 14, 1220. [Google Scholar] [CrossRef] [PubMed]
- Koduah, P.; Paul, F.; Dörr, J.-M. Vitamin D in the Prevention, Prediction and Treatment of Neurodegenerative and Neuroinflammatory Diseases. EPMA J. 2017, 8, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a Major Cytokine in the Central Nervous System. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, M. Tumor Necrosis Factor Alpha: A Major Cytokine of Brain Neuroinflammation. In Cytokines; IntechOpen: London, UK, 2020. [Google Scholar]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression and Mortality. Neurology 1967, 17, 427. [Google Scholar] [CrossRef] [PubMed]
- Regnault, A.; Boroojerdi, B.; Meunier, J.; Bani, M.; Morel, T.; Cano, S. Does the MDS-UPDRS Provide the Precision to Assess Progression in Early Parkinson’s Disease? Learnings from the Parkinson’s Progression Marker Initiative Cohort. J. Neurol. 2019, 266, 1927–1936. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef] [PubMed]
- Spooren, A.; Kolmus, K.; Laureys, G.; Clinckers, R.; De Keyser, J.; Haegeman, G.; Gerlo, S. Interleukin-6, a Mental Cytokine. Brain Res. Rev. 2011, 67, 157–183. [Google Scholar] [CrossRef]
- Karpenko, M.N.; Vasilishina, A.A.; Gromova, E.A.; Muruzheva, Z.M.; Bernadotte, A. Interleukin-1β, Interleukin-1 Receptor Antagonist, Interleukin-6, Interleukin-10, and Tumor Necrosis Factor-α Levels in CSF and Serum in Relation to the Clinical Diversity of Parkinson’s Disease. Cell. Immunol. 2018, 327, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and Chronic Inflammation. Arthritis Res. Ther. 2006, 8 (Suppl. S2), S3. [Google Scholar] [CrossRef]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 Cytokine: An Overview of the Immune Regulation, Immune Dysregulation, and Therapeutic Approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Y.; Zhang, S.P.; Cao, C.; Loh, Y.P.; Cheng, Y. Aberrations in Peripheral Inflammatory Cytokine Levels in Parkinson Disease: A Systematic Review and Meta-Analysis. JAMA Neurol. 2016, 73, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Bartl, M.; Dakna, M.; Galasko, D.; Hutten, S.J.; Foroud, T.; Quan, M.; Marek, K.; Siderowf, A.; Franz, J.; Trenkwalder, C.; et al. Biomarkers of Neurodegeneration and Glial Activation Validated in Alzheimer’s Disease Assessed in Longitudinal Cerebrospinal Fluid Samples of Parkinson’s Disease. PLoS ONE 2021, 16, e0257372. [Google Scholar] [CrossRef]
- Simon, D.K.; Simuni, T.; Elm, J.; Clark-Matott, J.; Graebner, A.K.; Baker, L.; Dunlop, S.R.; Emborg, M.; Kamp, C.; Morgan, J.C.; et al. Peripheral Biomarkers of Parkinson’s Disease Progression and Pioglitazone Effects. J. Parkinsons. Dis. 2015, 5, 731–736. [Google Scholar] [CrossRef]
- Xiromerisiou, G.; Marogianni, C.; Lampropoulos, I.C.; Dardiotis, E.; Speletas, M.; Ntavaroukas, P.; Androutsopoulou, A.; Kalala, F.; Grigoriadis, N.; Papoutsopoulou, S. Peripheral Inflammatory Markers TNF-α and CCL2 Revisited: Association with Parkinson’s Disease Severity. Int. J. Mol. Sci. 2022, 24, 264. [Google Scholar] [CrossRef]
- Gupta, V.; Garg, R.K.; Khattri, S. Levels of IL-8 and TNF-α Decrease in Parkinson’s Disease. Neurol. Res. 2016, 38, 98–102. [Google Scholar] [CrossRef]
- Frankola, A.K.; Greig, H.N.; Luo, W.; Tweedie, D. Targeting TNF-Alpha to Elucidate and Ameliorate Neuroinflammation in Neurodegenerative Diseases. CNS Neurol. Disord. Drug Targets 2011, 10, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Bottigliengo, D.; Foco, L.; Seibler, P.; Klein, C.; König, I.R.; Del Greco, F.M. A Mendelian Randomization Study Investigating the Causal Role of Inflammation on Parkinson’s Disease. Brain 2022, 145, 3444–3453. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hong, W.; Yang, Z.; Ding, J.; Ren, Y. Efficacy of Pramipexole Combined with Levodopa for Parkinson’s Disease Treatment and Their Effects on QOL and Serum TNF-α Levels. J. Int. Med. Res. 2020, 48, 22449. [Google Scholar] [CrossRef]
- Qiu, X.; Xiao, Y.; Wu, J.; Gan, L.; Huang, Y.; Wang, J. C-Reactive Protein and Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2019, 10, 384. [Google Scholar] [CrossRef]
- Hall, S.; Janelidze, S.; Surova, Y.; Widner, H.; Zetterberg, H.; Hansson, O. Cerebrospinal Fluid Concentrations of Inflammatory Markers in Parkinson’s Disease and Atypical Parkinsonian Disorders. Sci. Rep. 2018, 8, 13276. [Google Scholar] [CrossRef]
- Mayo, S.; Benito-León, J.; Peña-Bautista, C.; Baquero, M.; Cháfer-Pericás, C. Recent Evidence in Epigenomics and Proteomics Biomarkers for Early and Minimally Invasive Diagnosis of Alzheimer’s and Parkinson’s Diseases. Curr. Neuropharmacol. 2021, 19, 1273–1303. [Google Scholar] [CrossRef]
- Shen, J.; Amari, N.; Zack, R.; Skrinak, R.T.; Unger, T.L.; Posavi, M.; Tropea, T.F.; Xie, S.X.; Van Deerlin, V.M.; Dewey, R.B.; et al. Plasma MIA, CRP, and Albumin Predict Cognitive Decline in Parkinson’s Disease. Ann. Neurol. 2022, 92, 255–269. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Cao, Z.; Liu, Q.; Cheng, Y. Cerebrospinal Fluid Inflammatory Cytokine Aberrations in Alzheimer’s Disease, Parkinson’s Disease and Amyotrophic Lateral Sclerosis: A Systematic Review and Meta-Analysis. Front. Immunol. 2018, 9, 2122. [Google Scholar] [CrossRef]
- Erten, M. Visfatin as a Promising Marker of Cardiometabolic Risk. Acta Cardiol. Sin. 2021, 37, 464–472. [Google Scholar] [CrossRef]
- Al Abdulsalam, E.A.; Al Harithy, R.N. Visfatin and Global Histone H3K9me Levels in Colon Cancer. Ann. Med. 2021, 53, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Opatrilova, R.; Caprnda, M.; Kubatka, P.; Valentova, V.; Uramova, S.; Nosal, V.; Gaspar, L.; Zachar, L.; Mozos, I.; Petrovic, D.; et al. Adipokines in Neurovascular Diseases. Biomed. Pharmacother. 2018, 98, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Shimon, D.; Youssef, S.; Yankovitz, G.; Tessler, A.; Chernobylsky, T.; Gaoni-Yogev, A.; Perelroizen, R.; Budick-Harmelin, N.; Steinman, L.; et al. NAD + Metabolism Drives Astrocyte Proinflammatory Reprogramming in Central Nervous System Autoimmunity. Proc. Natl. Acad. Sci. USA 2022, 119, e2211310119. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.D.; Guo, S.Q.; Hu, Z.W.; Li, W.L. NAMPT Protects against 6-Hydroxydopamine-Induced Neurotoxicity in PC12 Cells through Modulating SIRT1 Activity. Mol. Med. Rep. 2016, 13, 4058–4064. [Google Scholar] [CrossRef]
- Santiago, J.A.; Littlefield, A.M.; Potashkin, J.A. Integrative Transcriptomic Meta-Analysis of Parkinson’s Disease and Depression Identifies NAMPT as a Potential Blood Biomarker for de Novo Parkinson’s Disease. Sci. Rep. 2016, 6, 34579. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Pino, J.; Scotece, M.; Conde, J.; Lago, F.; Gonzalez-Gay, M.A.; Mera, A.; Gómez, R.; Mobasheri, A.; Gualillo, O. Progranulin as a Biomarker and Potential Therapeutic Agent. Drug Discov. Today 2017, 22, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, H.; Song, A.; Huang, J.; Zhang, Y.; Jin, S.; Li, S.; Zhang, L.; Yang, C.; Yang, P. Progranulin Inhibits LPS-Induced Macrophage M1 Polarization via NF-KB and MAPK Pathways. BMC Immunol. 2020, 21, 32. [Google Scholar] [CrossRef]
- Yao, Y.N.; Wang, M.D.; Tang, X.C.; Wu, B.; Sun, H.M. Reduced Plasma Progranulin Levels Are Associated with the Severity of Parkinson’s Disease. Neurosci. Lett. 2020, 725, 134873. [Google Scholar] [CrossRef]
- Deneubourg, C.; Ramm, M.; Smith, L.J.; Baron, O.; Singh, K.; Byrne, S.C.; Duchen, M.R.; Gautel, M.; Eskelinen, E.L.; Fanto, M.; et al. The Spectrum of Neurodevelopmental, Neuromuscular and Neurodegenerative Disorders Due to Defective Autophagy. Autophagy 2022, 18, 496–517. [Google Scholar] [CrossRef]
- Rodrigues, P.S.; Kale, P.P. Mini Review—The Role of Glucocerebrosidase and Progranulin as Possible Targets in the Treatment of Parkinson’s Disease. Rev. Neurol. 2021, 177, 1082–1089. [Google Scholar] [CrossRef]
- Mendsaikhan, A.; Tooyama, I.; Walker, D.G. Microglial Progranulin: Involvement in Alzheimer’s Disease and Neurodegenerative Diseases. Cells 2019, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Mateo, I.; González-Aramburu, I.; Pozueta, A.; Vázquez-Higuera, J.L.; Rodríguez-Rodríguez, E.; Sánchez-Juan, P.; Calero, M.; Dobato, J.L.; Infante, J.; Berciano, J.; et al. Reduced Serum Progranulin Level Might Be Associated with Parkinson’s Disease Risk. Eur. J. Neurol. 2013, 20, 1571–1573. [Google Scholar] [CrossRef]
- Rhinn, H.; Tatton, N.; McCaughey, S.; Kurnellas, M.; Rosenthal, A. Progranulin as a Therapeutic Target in Neurodegenerative Diseases. Trends Pharmacol. Sci. 2022, 43, 641–652. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, F.; Tang, J.; Jia, L.; Feng, Y.; Xu, P.; Faramand, A. Association between Vitamin D Supplementation and Mortality: Systematic Review and Meta-Analysis. BMJ 2019, 366, l4673. [Google Scholar] [CrossRef]
- Chang, S.-W.; Lee, H.-C. Vitamin D and Health—The Missing Vitamin in Humans. Pediatr. Neonatol. 2019, 60, 237–244. [Google Scholar] [CrossRef]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D Supplementation for Improvement of Chronic Low-Grade Inflammation in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Wang, K.; Chen, L.; Jiang, P. Neuroprotective Effects of Vitamin D and 17ß-Estradiol against Ovariectomy-Induced Neuroinflammation and Depressive-like State: Role of the AMPK/NF-ΚB Pathway. Int. Immunopharmacol. 2020, 86, 106734. [Google Scholar] [CrossRef]
- Huang, Y.-N.; Ho, Y.-J.; Lai, C.-C.; Chiu, C.-T.; Wang, J.-Y. 1,25-Dihydroxyvitamin D3 Attenuates Endotoxin-Induced Production of Inflammatory Mediators by Inhibiting MAPK Activation in Primary Cortical Neuron-Glia Cultures. J. Neuroinflamm. 2015, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shah, S.A.; Zaman, N.; Uddin, M.N.; Khan, W.; Ali, A.; Riaz, M.; Kamil, A. Vitamin D Exerts Neuroprotection via SIRT1/Nrf-2/ NF-KB Signaling Pathways against D-Galactose-Induced Memory Impairment in Adult Mice. Neurochem. Int. 2021, 142, 104893. [Google Scholar] [CrossRef] [PubMed]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Chlebna-Sokół, D.; Konstantynowicz, J.; Abramowicz, P.; Kulik-Rechberger, B.; Niedziela, M.; Obuchowicz, A.; Ziora, K.; Karalus-Gach, J.; Golec, J.; Michałus, I.; et al. Evidence of a Significant Vitamin D Deficiency among 9–13-Year-Old Polish Children: Results of a Multicentre Study. Eur. J. Nutr. 2019, 58, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Czech-Kowalska, J.; Latka-Grot, J.; Bulsiewicz, D.; Jaworski, M.; Pludowski, P.; Wygledowska, G.; Chazan, B.; Pawlus, B.; Zochowska, A.; Borszewska-Kornacka, M.K.; et al. Impact of Vitamin D Supplementation during Lactation on Vitamin D Status and Body Composition of Mother-Infant Pairs: A MAVID Randomized Controlled Trial. PLoS ONE 2014, 9, e107708. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Grant, W.B.; Bhattoa, H.P.; Bayer, M.; Povoroznyuk, V.; Rudenka, E.; Ramanau, H.; Varbiro, S.; Rudenka, A.; Karczmarewicz, E.; et al. Vitamin D Status in Central Europe. Int. J. Endocrinol. 2014, 2014, 589587. [Google Scholar] [CrossRef] [PubMed]
- Titova, N.; Martinez-Martin, P.; Katunina, E.; Chaudhuri, K.R. Advanced Parkinson’s or “Complex Phase” Parkinson’s Disease? Re-Evaluation Is Needed. J. Neural Transm. 2017, 124, 1529–1537. [Google Scholar] [CrossRef] [PubMed]



| Parameter | Parkinson’s Disease | Control | p-Value | Power of a Test | ||
|---|---|---|---|---|---|---|
| Non-Dyskinesia | With Dyskinesia | |||||
| n (Female/Male) | 26 (13/13) | 26 (12/14) | 26 (13/13) | - | - | |
| Age [years] | Mean | 67.462 | 68.423 | 66.308 | 0.0933 | 0.9998 |
| SEM | 1.137 | 1.154 | 1.109 | |||
| Median | 68.500 | 68.500 | 66.000 | |||
| IQR | 7.750 | 6.000 | 4.750 | |||
| Body Mass [kg] | Mean | 74.654 | 73.346 | 70.308 | 0.2785 | 1.0000 |
| SEM | 2.250 | 2.974 | 1.897 | |||
| Median | 74.500 | 73.000 | 86.000 | |||
| IQR | 14.750 | 13.000 | 16.000 | |||
| Height [cm] | Mean | 168.692 | 166.808 | 167.231 | 0.5200 | 0.4212 |
| SEM | 1.542 | 1.299 | 0.618 | |||
| Median | 168.000 | 167.500 | 167.000 | |||
| IQR | 11.000 | 7.500 | 3.500 | |||
| BMI [kg/m2] | Mean | 26.158 | 26.221 | 25.121 | 0.5025 | 0.4376 |
| SEM | 0.611 | 0.941 | 0.633 | |||
| Median | 25.619 | 25.457 | 24.562 | |||
| IQR | 4.163 | 3.709 | 5.346 | |||
| SBP [mmHg] | Mean | 125.038 | 125.962 | 123.423 | 0.7033 | 1.0000 |
| SEM | 1.133 | 2.481 | 1.702 | |||
| Median | 122.500 | 125.000 | 123.500 | |||
| IQR | 10.000 | 10.000 | 14.750 | |||
| DBP [mmHg] | Mean | 76.923 | 75.385 | 73.962 | 0.8623 | 1.0000 |
| SEM | 3.972 | 1.817 | 1.088 | |||
| Median | 72.500 | 72.500 | 72.500 | |||
| IQR | 10.000 | 10.000 | 8.750 | |||
| UPDRS I | Mean | 0.885 | 2.346 | - | <0.0001 | 0.9909 |
| SEM | 0.150 | 0.248 | - | |||
| Median | 1.000 | 2.000 | - | |||
| IQR | 1.000 | 1.000 | - | |||
| Min | 0.000 | 0.000 | - | |||
| Max | 2.000 | 5.000 | - | |||
| UPDRS II | Mean | 6.154 | 12.462 | - | <0.0001 | 1.0000 |
| SEM | 0.760 | 0.728 | - | |||
| Median | 6.000 | 12.000 | - | |||
| IQR | 3.000 | 4.000 | - | |||
| Min | 1.000 | 5.000 | - | |||
| Max | 15.000 | 20.000 | - | |||
| UPDRS III | Mean | 20.346 | 38.038 | - | <0.0001 | 1.0000 |
| SEM | 1.331 | 1.442 | - | |||
| Median | 19.000 | 37.000 | - | |||
| IQR | 11.000 | 7.000 | - | |||
| Min | 10.000 | 26.000 | - | |||
| Max | 33.000 | 54.000 | - | |||
| UPDRS IV | Mean | 0.077 | 2.962 | - | <0.0001 | 1.0000 |
| SEM | 0.053 | 0.218 | - | |||
| Median | 0.000 | 3.000 | - | |||
| IQR | 0.000 | 2.000 | - | |||
| Min | 0.000 | 1.000 | - | |||
| Max | 1.000 | 6.000 | - | |||
| Hoehn–Yahr Scale | Mean | 1.308 | 2.904 | - | <0.0001 | 0.9956 |
| SEM | 0.049 | 0.079 | - | |||
| Median | 1.500 | 3.000 | - | |||
| IQR | 0.500 | 0.500 | - | |||
| Min | 1.000 | 2.500 | - | |||
| Max | 1.500 | 4.000 | - | |||
| Schwab and England Activities of Daily Living scale | Mean | 87.308 | 62.308 | - | <0.0001 | 1.0000 |
| SEM | 1.046 | 1.393 | - | |||
| Median | 90.000 | 60.000 | - | |||
| IQR | 10.000 | 10.000 | - | |||
| Min | 80.000 | 50.000 | - | |||
| Max | 100.000 | 80.000 | - | |||
| Years since Diagnosis [years] | Mean | 1.615 | 8.346 | - | <0.0001 | 1.0000 |
| SEM | 0.097 | 0.271 | - | |||
| Median | 2.000 | 8.000 | - | |||
| IQR | 1.000 | 3.000 | - | |||
| Min | 1.000 | 6.000 | - | |||
| Max | 2.000 | 10.000 | - | |||
| Place of Residence [n] | City over 100,000 | 14 | 17 | 12 | - | - |
| City up to 100,000 | 5 | 3 | 4 | |||
| City up to 50,000 | 2 | 4 | 8 | |||
| Village | 5 | 2 | 2 | |||
| Education [n] | Higher | 1 | 5 | 13 | - | - |
| Secondary | 14 | 13 | 9 | |||
| Vacational | 10 | 7 | 4 | |||
| Primary | 1 | 1 | 0 | |||
| Drugs, Dose | PD Non-Dyskinesia [n] | PD With Dyskinesia [n] |
|---|---|---|
| no treatment | 1 | 0 |
| madopar HBS (LEDD = 125) | 19 | 12 |
| madopar 250 + HBS (LEDD = 375) | 0 | 7 |
| madopar HBS + madopar 125 (LEDD = 250) | 0 | 4 |
| madopar HBS + madopar 125 + madopar 62.5 (LEDD = 313) | 1 | 1 |
| madopar 62.5 + HBS (LEDD = 188) | 4 | 1 |
| madopar 125 + madopar 125 (LEDD = 250) | 1 | 0 |
| madopar × 4 (LEDD = 500) | 0 | 1 |
| Parameter | Parkinson’s Disease | Control | p-Value | Power of a Test | ||
|---|---|---|---|---|---|---|
| Non-Dyskinesia | With Dyskinesia | |||||
| IL-6 [pg/mL] | Mean | 2.823 | 4.468 | 2.180 | 0.0206 | 0.9999 |
| SEM | 0.447 | 0.753 | 0.188 | |||
| Median | 2.685 | 3.153 | 2.258 | |||
| IQR | 3.054 | 1.977 | 1.613 | |||
| TNF-α [pg/mL] | Mean | 15.879 | 19.154 | 10.149 | <0.0001 | 1.0000 |
| SEM | 1.151 | 1.498 | 0.272 | |||
| Median | 16.606 | 18.698 | 10.107 | |||
| IQR | 9.605 | 6.341 | 1.319 | |||
| CRP [mg/mL] | Mean | 2.948 | 3.057 | 2.340 | 0.9468 | 0.6410 |
| SEM | 0.540 | 0.523 | 0.164 | |||
| Median | 2.300 | 1.855 | 2.214 | |||
| IQR | 2.620 | 1.850 | 1.201 | |||
| Visfatin [ng/mL] | Mean | 0.818 | 0.477 | 0.585 | 0.0009 | 0.1751 |
| SEM | 0.080 | 0.067 | 0.043 | |||
| Median | 0.742 | 0.402 | 0.556 | |||
| IQR | 0.449 | 0.310 | 0.330 | |||
| Progranulin [ng/mL] | Mean | 32.641 | 38.141 | 38.840 | 0.0215 | 1.0000 |
| SEM | 2.630 | 2.360 | 2.260 | |||
| Median | 31.406 | 38.417 | 38.421 | |||
| IQR | 9.410 | 11.480 | 13.686 | |||
| 25(OH)-vitamin D [ng/mL] | Mean | 23.792 | 22.973 | 27.917 | 0.1966 | 1.0000 |
| SEM | 0.918 | 1.117 | 1.808 | |||
| Median | 24.050 | 23.500 | 27.920 | |||
| IQR | 6.725 | 7.725 | 14.108 | |||
| PT [s] (normal range: 11–13.5 s) | Mean | 12.373 | 11.804 | - | 0.3780 | 0.4898 |
| SEM | 0.610 | 0.155 | - | |||
| Median | 11.950 | 11.600 | - | |||
| IQR | 0.550 | 0.675 | - | |||
| INR (normal range: 0.8–1.1) | Mean | 1.095 | 1.037 | - | 0.2870 | 0.0546 |
| SEM | 0.058 | 0.015 | - | |||
| Median | 1.050 | 1.020 | - | |||
| IQR | 0.045 | 0.075 | - | |||
| APTT [s] (normal range: 27–41 s) | Mean | 30.458 | 28.746 | - | 0.1810 | 0.9976 |
| SEM | 0.927 | 0.466 | - | |||
| Median | 29.450 | 28.000 | - | |||
| IQR | 4.600 | 2.650 | - | |||
| Fibrinogen [g/L] (normal range: 2–4 g/L) | Mean | 3.373 | 3.412 | - | 0.8020 | 0.0570 |
| SEM | 0.093 | 0.121 | - | |||
| Median | 3.300 | 3.300 | - | |||
| IQR | 0.675 | 0.975 | - | |||
| Parameter | p-Value | ||
|---|---|---|---|
| Non-Dyskinesia vs. With Dyskinesia | Non-Dyskinesia vs. Control | With Dyskinesia vs. Control | |
| IL-6 | >0.05 | >0.05 | 0.012 |
| TNF-α | >0.05 | 0.003 | <0.001 |
| Visfatin | 0.002 | >0.05 | >0.05 |
| Progranulin | >0.05 | 0.032 | >0.05 |
| Parameter | OR [95% CI] | p-Value |
|---|---|---|
| Sex | 2.114; [1.197; 22.589] | 0.6905 |
| IL-6 | 3.229; [2.368; 4.925] | 0.3111 |
| TNF-α | 2.968; [2.611; 3.431] | 0.1877 |
| CRP | 2.994; [2.257; 4.38] | 0.5444 |
| Visfatin | 1.036; [1.003; 1.511] | 0.0076 |
| Progranulin | 2.826; [2.644; 3.035] | 0.2600 |
| 25(OH)-vitamin D | 2.782; [2.472; 3.181] | 0.7132 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milanowski, J.; Nuszkiewicz, J.; Lisewska, B.; Lisewski, P.; Szewczyk-Golec, K. Adipokines, Vitamin D, and Selected Inflammatory Biomarkers among Parkinson’s Disease Patients with and without Dyskinesia: A Preliminary Examination. Metabolites 2024, 14, 106. https://doi.org/10.3390/metabo14020106
Milanowski J, Nuszkiewicz J, Lisewska B, Lisewski P, Szewczyk-Golec K. Adipokines, Vitamin D, and Selected Inflammatory Biomarkers among Parkinson’s Disease Patients with and without Dyskinesia: A Preliminary Examination. Metabolites. 2024; 14(2):106. https://doi.org/10.3390/metabo14020106
Chicago/Turabian StyleMilanowski, Jan, Jarosław Nuszkiewicz, Beata Lisewska, Paweł Lisewski, and Karolina Szewczyk-Golec. 2024. "Adipokines, Vitamin D, and Selected Inflammatory Biomarkers among Parkinson’s Disease Patients with and without Dyskinesia: A Preliminary Examination" Metabolites 14, no. 2: 106. https://doi.org/10.3390/metabo14020106
APA StyleMilanowski, J., Nuszkiewicz, J., Lisewska, B., Lisewski, P., & Szewczyk-Golec, K. (2024). Adipokines, Vitamin D, and Selected Inflammatory Biomarkers among Parkinson’s Disease Patients with and without Dyskinesia: A Preliminary Examination. Metabolites, 14(2), 106. https://doi.org/10.3390/metabo14020106