Marine Algae Metabolites as Promising Therapeutics for the Prevention and Treatment of HIV/AIDS
Abstract
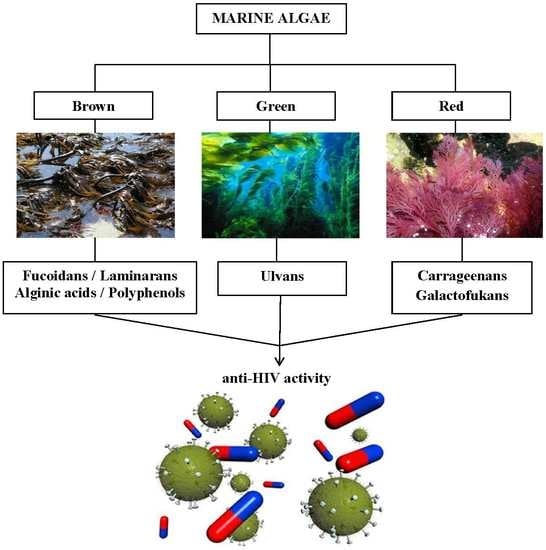
:1. Introduction
2. Infectious Agent
- -
- gag—group specific antigen—encodes the synthesis of viral proteins of the inner layer;
- -
- pol—polymerasae—encodes the enzymes reverse transcriptase (revertase), protease, integrase and ribonuclease;
- -
- env—envelope—encodes the synthesis of the glycoprotein of the outer layer of gp160, which is further split into gp120 and gp41.
3. Anti-HIV Therapy
- -
- Nucleoside. Nucleoside analogs replacing natural pyrimidine and purine nucleosides, disrupting the synthesis of proviral DNA and suppressing viral replication) and non-nucleoside (directly linked to reverse transcriptase near the nucleoside binding site; as a result of complexing with the drugs, this site have an impact and the enzyme binds to a smaller number nucleosides, which significantly slows down the conversion of RNA to DNA);
- -
- Protease inhibitors. Suppression of protease activity leads to the formation of immature viral particles, which is unable to infect new cells;
- -
- Fusion inhibitors. By specifically binding to the gp41 of HIV-1 outside the cell, the drug blocks the penetration of the virus into the target cell and the fusion of the outer membrane of the virus with the cell membrane;
- -
- CCR5 receptor inhibitors. Prevent penetration of HIV to the target cell by acting on the CCR5 co-receptor;
- -
- Virus integrase inhibitors. Block the virus enzyme that is involved in the insertion of proviral DNA into the genome of the target cell. Integrase inhibitors affect one of the stages of the process of inserting proviral DNA—the transfer of a DNA strand. After the transfer of the pre-integration complex (proviral DNA in association with integrase) from the cytoplasm to the cell nucleus, integrase joins the cell DNA, which leads to irreversible binding of the proviral DNA and the DNA of the infected cell.
4. Marine Algae Metabolites
4.1. Lectins
4.2. Sulfated Polysaccharides (SPSs)
4.2.1. Carrageenans
4.2.2. Fucoidans
- -
- SCF—fully sulfated α-L-fucan from the brown alga Saccharina cichorioides—the polysaccharide chain is built mainly from (1→3)-linked α-L-fucopyranose residues;
- -
- FcF—α-L-fucan from the brown alga Fucus evanescens—the main chain consists of alternating (1→3) and (1→4) -linked residues of α-L-fucose;
- -
- GF—SgGF, AoGF, SiGF galactofucans—from Saccharina gurjanovae, Alaria ochotensis, Saccharina japonica algae, respectively.
4.3. Laminarans
4.4. Alginic Acid (Sopolymer of Manuronic and Guluronic Acids)
4.5. Polyphenols
- -
- fugalols and phloretols (phlorotannins with an ether bond);
- -
- fukol (with phenyl bond);
- -
- fucofloretols (with ether and phenyl bond);
- -
- eckols (with dibenzodioxin bond).
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- News Bulletin. Global HIV Statistics for 2017. Available online: www.unaids.org/ru/resources/fact-sheet (accessed on 23 November 2018).
- Golubeva, M.V.; Jackson, A.; Vergunova, I.V.; Gvozdik, A.L. Comparative characteristics of HIV infection in certain territories of Russia and Ireland. Med. Vest. North Cauc. 2015, 10, 43–50. [Google Scholar]
- Pokrovsky, V.V.; Ladnaya, N.N.; Pokrovskaya, A.V. VICH/AIDS reduces the number of Russians and their life expectancy. Demogr. Rev. 2017, 4, 65–82. (In Russian). Available online: https://demreview.hse.ru/issue/view/616/DemRev_4_1_2017 (accessed on 30 April 2019).
- HIV Infection and AIDS. National Leadership. Brief edition [Electronic resource] V.V. Pokrovsky, M., Ed.; GEOTAR-Media. 2014, p. 528. Available online: http://wwwrosmedlib.ru/book/ICBN9785970428917.html (accessed on 14 May 2014).
- Vo, T.S.; Kim, S.K. Potential anti-HIV agents from marine resources: An overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef]
- Huskens, D.; Schols, D. Algal lectins as potential HIV microbicide candidates. Mar. Drugs 2012, 10, 1476–1497. [Google Scholar] [CrossRef]
- Praseptiangga, D. Algal lectins and their potential uses. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2015, 10, 89–98. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K. Lectins from red algae and their biomedical potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar] [CrossRef]
- Alexandre, K.B.; Gray, E.S.; Hazel, M.; McMahon, J.B.; Ereck, C.; O’Keefe, B.R.; Rachel, C.; Morris, L. The lectins Griffithsin, Cyanovirin-N and Scytovirin inhibit HIV-1 binding to the DC-SIGN receptor and transfer to CD4+ cells. Virology 2012, 20, 175–186. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Ramessar, K.; O’Keefe, B.R. Antiviral lectins: Selective inhibitors of viral entry. Antivir. Res. 2017, 142, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, K.B.; Gray, E.S.; Pantophlet, R.; Moore, P.L.; McMahon, J.B.; Chakauya, E.; O’Keefe, B.R.; Chikwamba, R.; Morris, L. Binding of the mannose-specific lectin, griffithsin, to HIV-1 gp120 exposes the CD4-binding site. J. Virol. 2011, 85, 9039–9050. [Google Scholar] [CrossRef]
- Patel, P.; Borkowf, C.B.; Brooks, J.T.; Lasry, A.; Lansky, A.; Mermin, J. Estimating per-act HIV transmission risk: A systematic review. AIDS 2014, 28, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.S.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W., Jr.; McMahon, J.B.; et al. Isolation and characterization of griffithsin, a novel HIV inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Hoorelbeke, B.; Kagiampakis, I.; Demeler, B.; Balzarini, J.; Liwang, P.J. The griffithsin dimer is required for high-potency inhibition of HIV-1: Evidence for the manipulation of the structure of gp120 as part of the griffithsin dimer mechanism. Antimicrob. Agents Chemother. 2013, 57, 3976–3989. [Google Scholar] [CrossRef]
- Barton, C.; Kouokam, J.C.; Hurst, H.; Palmer, K.E.; O’Keefe, B.R. Pharmacokinetics of the antiviral lectin griffithsin administered by different routes indicates multiple potential uses. Viruses 2016, 8, 331. [Google Scholar] [CrossRef]
- Girard, L.; Birse, K.; Holm, J.B.; Gajer, P.; Humphrys, M.S.; Garber, D.; Guenthner, P.; Noel-Romas, L.; Abou, M.; McCorrister, S.; et al. Impact of griffitsin anti-HIV microbicide and placebo gels on the rectal mucosal proteome and microbiome in non-human primates. Sci. Rep. 2018, 8, 8059. [Google Scholar] [CrossRef] [PubMed]
- Mazalovska, M.; Kouokam, J.C. Lectins as Promising Therapeutics for the Prevention and Treatment of HIV and Other Potential Coinfections. Biomed. Res. Int. 2018, 3750646. [Google Scholar] [CrossRef] [PubMed]
- Kouokam, J.C.; Huskens, D.; Schols, D.; Johannemann, A.; Riedell, S.K.; Walter, W.; Walker, J.M.; Matoba1, N.; O’Keefe, B.R.; Palmer, K.E. Investigation of Griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS ONE 2011, 6, e22635. [Google Scholar] [CrossRef] [PubMed]
- Kouokam, J.C.; Lasnik, A.B.; Palmer, K.E. Studies in a murine model confirm the safety of Griffithsin and advocate its further development as a microbicide targeting HIV-1 and other enveloped viruses. Viruses 2016, 8, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Stenfanidor, M.; Mesquitai, M.M.; Fakioglu, E.; Segarra, T.; Rohan, L.; Halford, W.; Palmer, K.E.; Herold, B.C. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 2013, 87, 6257–6269. [Google Scholar] [CrossRef]
- Moulaei, T.; Alexandre, K.B.; Shenoy, S.R.; Meyerson, J.R.; Krumpe, L.R.; Constantine, B.; Wilson, J.; Buckheit, R.W., Jr.; McMahon, J.B.; Subramaniam, S.; et al. Griffithsin tandemers: Flexible and potent lectin inhibitors of the human immunodeficiency virus. Retrovirology 2015, 12, 6. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Vojdani, F.; Buffa, V.; Shattock, R.J.; Montefiori, D.C.; Bakke, J.; Mirsalis, J.; d’Andrea, A.-L.; Hume, S.D.; Bratcher, B.; et al. Scaleablemanufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Nalt. Acad. Sci. USA 2009, 106, 6099–6104. [Google Scholar] [CrossRef]
- Fontenelle, T.P.C.; Lima, G.C.; Mesquita, J.X.; Lopes, J.L.S.; de Brito, T.V.; Junior, F.C.V.; Sales, A.B.; Aragao, K.S.; Souza, M.H.L.; Barbosa, A.L.R.; et al. Lectin obtained from the red seaweed Bryothamnion triquetrum: Secondary structure and and anti-inflammatory activity in mice. Int J. Biol Macromol. 2018, 112, 1122–1130. [Google Scholar] [CrossRef]
- Alban, S.; Franz, G. Partial synthetic glucan sulfates as potential new antithrombotics: A review. Biomacromolecules 2001, 2, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Kharlamenko, V.I.; Zvyagintseva, T.N. Optimization of the extraction process of fucoidan from the brown alga Fucus evanescens. Chem. Plant Mater. 2012, 1, 143–147. [Google Scholar]
- Ahmadi, A.; Moghadamtousi, S.Z.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. Biomed. Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef]
- Sheng, G.J.; Oh, J.I.; Chang, S.K.; Hsieh-Wilson, L.C. Tunable heparin sulfate mimetics for modulating chemokine activity. J. Am. Chem. Soc. 2013, 135, 10898–10901. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Yoshida, O.; Tochikura, T.S.; Yoshida, T.; Mimura, T.; Kido, Y.; Motoki, Y.; Kaneko, Y.; Uryu, T.; Yamamoto, N. Sulfation of polysaccharides generates potent and selective inhibitors of human immunodeficiency virus infection and replication in vitro. Jpn. J. Cancer Res. 1987, 78, 1164–1168. [Google Scholar]
- Chattopadhyay, N.; Ghosh, T.; Sinha, S.; Chattopadhyay, K.; Karmakar, P.; Ray, B. Polysaccharides from Turbinaria conoides:structural features and antioxidant capacity. Food Chem. 2010, 118, 823–829. [Google Scholar] [CrossRef]
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural features and antiviral activity of sulfated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007, 18, 153–162. [Google Scholar] [CrossRef]
- Mandal, P.; Pujol, C.A.; Carlucci, M.J.; Chattopadhyay, K.; Damonte, E.B.; Ray, B. Sulfated xylomannan of Scinaia hetai: Isolation, structural features and antiviral activity. Phytochemistry 2008, 69, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Yermak, I.M.; Byankina, A.O.; Sokolova, E.V. Structural features and biological activity of carrageenans—sulphated polysaccharides of red algae of the Far Eastern seas of Russia. Bull Far East Branch Russ. Acad. Sci. 2014, 1, 80–92. (In Russian). Available online: https://elibrary.ru/download/elibrary_22936902_54741494.pdf (accessed on 29 January 2015).
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Muller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Muto, S.; Niimura, K.; Oohara, M.; Oguchi, Y.; Matsunaga, K.; Hirose, K.; Kakuchi, J.; Sugita, N.; Furusho, T. Polysaccharides from Marine Algae and Antiviral Drugs Containing the Same As Active Ingredients. EP295 956, 21 December 1988. Japanese Patent Application 87/152/086 18 June 1987 (Chem. Abstr. 111:54116w). [Google Scholar]
- Bhattachryya, S.; Xue, L.; Devcota, S.; Chang, E.; Morris, S.; Tobacman, J.K. Carrageenan-Induced colonic Inflammation Is reduced in Bcl10 null mice and Increased in IL-10-deficient mice. Mediators Inflamm. 2013, 397642. [Google Scholar] [CrossRef] [PubMed]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmac. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Baba, M.; Snock, R.; Pauwels, R. and DeClercq, E. Sulfated polysaccharide are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef]
- Wang, S.C.; Bligh, S.W.A.; Shi, S.S.; Wang, Z.T.; Hu, Z.B.; Crowder, J.; Branford-White, C.; Vella, C. Structural features and anti-HIV-1 activity of novel polysaccharides from red algae Grateloupia longifolia and Grateloupia filicina. Int. J. Biol. Macromol. 2007, 41, 369–375. [Google Scholar] [CrossRef]
- Skoler-Karpoff, S.; Ramjee, G.; Ahmed, K.; Altini, L.; Plagianos, M.G.; Friedland, B.; Govender, S.; De Kock, A.; Cassim, N.; Palanee, T.; et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1977–1987. [Google Scholar] [CrossRef]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyagintseva, T.N.; Maluarenko, O.S.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from brown alga Fucus evanescens: Structure and biological activity. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Prokofjeva, M.M.; Imbs, T.I.; Shevchenko, N.M.; Spirin, P.V.; Horn, S.; Fehse, B.; Zvyagintseva, T.N.; Prassolov, V.S. Fucoidans as potential inhibitors of HIV-1. Mar. Drugs 2013, 11, 3000–3014. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, I.; Itoh, W.; Kimura, S.; Mori, S.; Shimada, K. Further characterization of sulfated homopolysaccharide as anti-HIV agent. Experientia 1989, 45, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, S.; Menon, T.; Hanna, L.E. In vitro anti HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Meiyu, G.; Fuchuan, L.; Xianliang, X.; Jing, L.; Zuowei, Y.; Huashi, G. The potential molecular targets of marine sulfated polymannuroguluronate interfering with HIV-1 entry: Interaction between SPMG and HIV-1 rgp120 and CD4 molecule. Antivir. Res. 2003, 59, 127–135. [Google Scholar] [CrossRef]
- Haroun-Bouhedja, F.; Ellouali, M.; Sinquin, C.; Boisson-Vidal, C. Relationship between sulfate groups and biological activities of fucans. Thromb. Res. 2000, 100, 453–459. [Google Scholar] [CrossRef]
- Thuy, T.T.T.; Ly, B.M.; Van, T.T.; Quang, N.V.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Moulard, M.; Lortar-Jakob, H.; Mondor, I.; Roca, G.; Wyatt, R.; Sodroski, J.; Zhao, L.; Olson, W.; Kwong, P.D.; Sattentau, Q.J. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 2000, 74, 1948–1980. [Google Scholar] [CrossRef]
- Queiroz, K.C.S.; Medeiros, V.P.; Queiroz, L.S.; Abreu, L.R.D.; Rocha, H.A.O.; Ferreira, C.V.; Juca, M.B.; Aoyama, H.; Leite, E.L. Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 2008, 62, 303–307. [Google Scholar] [CrossRef]
- Trinchero, J.; Ponce, N.M.A.; Cordoba, O.L.; Flores, M.L.; Pampuro, S.; Stortz, D.; Salomon, H.; Turk, G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother. Res. 2009, 23, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Prokofjeva, M.M.; Orlova, N.N.; Gornostaeva, A.S.; Shulgin, A.A.; Nikitenko, N.A.; Senchenko, V.N.; Lebedev, T.D.; Spirin, P.V.; Riecken, K.; Fhese, B.; et al. Universal modular system for in vitro screening of potential inhibitors of HIV-1 replication. J. Mol. Biol. 2014, 48, 297–300. [Google Scholar] [CrossRef]
- Stonik, V.A. Marine natural products: A way to new drugs. Acta Naturae 2009, 1, 15–25. [Google Scholar]
- O’Doherty, J.V.; Dillon, S.; Figat, S.; Callan, J.J.; Sweeney, T. The effects of lactose inclusion and seaweed extract derived from Laminaria spp. on performance, digestibility of diet components and microbial populations in newly weaned pigs. Anim. Feed Sci. Technol. 2010, 157, 173–180. [Google Scholar] [CrossRef]
- Xianliang, X.; Meiyu, G.; Huashi, G.; Zelin, L. Study on the mechanism of inhibitory action of 911 on replication of HIV-1 in vitro. Chin. J. Mar. Drugs 2000, 19, 15–22. [Google Scholar]
- Teas, J.; Irhimeh, M.R. Dietary algae and HIV AIDS: Proof of concept clinical date. J. Appl. Phycol. 2012, 24, 575–582. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, UNAIDS & UNICEF. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access: Progress Report 2011. 2011. Available online: http://www.who.int/iris/handle/10665/44787 (accessed on 30 April 2019).
- Kubanek, J.; Jensen, P.R.; Keifer, P.A.; Sullards, M.C.; Collins, D.O.; Fenical, W. Seaweed resistance to microbialattak a targeted chemical defense against marine fungi. Proc. Natl. Acad. Sci. USA 2003, 100, 6916–6921. [Google Scholar] [CrossRef]
- Lane, C.E.; Mayes, C.; Druehl, L.D.; Saunders, G.W. A vulti-gene molecular investigation of the kelp (Laminariales, Pheophyceae) supports substantial taxonomic reorganization. J. Phycol. 2006, 42, 493–512. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2007, 31, 195–212. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2007, 20, 705–711. [Google Scholar] [CrossRef]
- Shibata, T.; Kawaguchi, S.; Hama, Y.; Inagaki, M.; Yamaguchi, K.; Nakamura, T. Local and chemical distribution of phlorotannins in brown algae. J. Appl. Phycol. 2004, 16, 291–296. [Google Scholar] [CrossRef]
- Singh, I.P.; Bharate, S.B. Phloroglucinol compounds of natural origin. Nat. Prod. Rep. 2006, 23, 558–591. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Lee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Ahn, M.J.; Yoon, K.D.; Min, S.Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.G.; Huh, H.; Kim, J. Inhibition of HIV-1 reverse transcriptase and proptease by phlorotannins from the brown alga Ecklonia cava. Biol. Pharm. Bull. 2004, 2, 544–547. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Ambrosova, J.V.; Orsavova, J.; Micek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef]
- Bogolitsyn, K.G.; Druzhinina, A.S.; Ovchinnikov, D.V.; Kaplitsyn, P.A.; Shulgina, E.V.; Parshina, A.E. Brown Alga Polyphenols. Chem. Plant Raw Mater. 2018, 3, 5–21. [Google Scholar] [CrossRef]
- Tkach, A.V.; Obluchinskaya, E.D. Sterols and polyphenols of fucoids from the Murmansk coast of the Barents Sea. Bull. Murm. State Tech. Univ. 2017, 20, 326–335. (In Russian). Available online: https://readera.ru/14294997 (accessed on 30 April 2019). [CrossRef]
- Moran-Santibanez, K.; Pena-Hemandez, M.A.; Cruz-Suarez, L.E.; Rieque, M.D.; Skouta, R.; Vasquez, A.H.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. Virucidal and synergistic activity of polyphenol-rich extracts of seaweeds against measles virus. Viruses 2018, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.J.; Yoon, K.D.; Kim, J.H.; Shin, C.G.; Kim, J. Inhibitory activity on HIV-1 reverse trancriptase and integrase of a carmalol derivative from brown alga Ishige okamurae. Phytother. Res. 2006, 20, 711–713. [Google Scholar] [CrossRef]
- Artan, M.; Li, Y.; Karadeniz, F. Anti HIV-1 activity of phloroglucinol derivate, 6,6’-biecol, from Ecklonia cava. Bioorg Med. Chem. 2008, 16, 7921–7926. [Google Scholar] [CrossRef]
- Kim, M.M.; Kim, S.K.; Lee, S.H. Hiv-1 Inhibiting Pharmaceutical Composition Containing an Ecklonia Cava-Derived Phloroglucinol Polymer Compound US13/964,687 2013-08-12. Available online: https://patents.google.com/patent/US20130338218A1/en (accessed on 30 April 2019).
- Karadeniz, F.; Kang, K.H.; Park, J.W.; Park, S.J.; Kim, S.K. Anti-HIV-1 activity of phlorotannin derivative 8.4′′′-dieckol from Korean brown alga Ecklonia cava. Biosci. Biotechnol. Biochem. 2014, 78, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, Q.; Yang, G.; Zhu, D.; Shao, Y.; Zhang, J.; Cui, Y.; Wang, R.; Zhang, L. The anti-HIV-1 activity of polyphenols from Phyllanhus urinaria and the pharmacokinetics and tissue distribution of its marker compound, gallic acid. J. Tradit. Chin. Med. Sci. 2017, 4, 158–166. [Google Scholar]
- Ow, Y.Y.; Stupans, I. Gallic acid and gallic acid derivates: Effect on drug metabolizing enzymes. Curr. Drug Metab. 2003, 4, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, S.; Kusaykin, M.; Trincone, A.; Zvyagintseva, T. Are multifunctional marine polysaccharides a myth or reality? Front. Chem. 2015, 3, 39. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besednova, N.N.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Makarenkova, I.D.; Smolina, T.P.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Zaporozhets, T.S. Marine Algae Metabolites as Promising Therapeutics for the Prevention and Treatment of HIV/AIDS. Metabolites 2019, 9, 87. https://doi.org/10.3390/metabo9050087
Besednova NN, Zvyagintseva TN, Kuznetsova TA, Makarenkova ID, Smolina TP, Fedyanina LN, Kryzhanovsky SP, Zaporozhets TS. Marine Algae Metabolites as Promising Therapeutics for the Prevention and Treatment of HIV/AIDS. Metabolites. 2019; 9(5):87. https://doi.org/10.3390/metabo9050087
Chicago/Turabian StyleBesednova, Natalya N., Tatyana N. Zvyagintseva, Tatyana A. Kuznetsova, Ilona D. Makarenkova, Tatyana P. Smolina, Ludmila N. Fedyanina, Sergey P. Kryzhanovsky, and Tatyana S. Zaporozhets. 2019. "Marine Algae Metabolites as Promising Therapeutics for the Prevention and Treatment of HIV/AIDS" Metabolites 9, no. 5: 87. https://doi.org/10.3390/metabo9050087
APA StyleBesednova, N. N., Zvyagintseva, T. N., Kuznetsova, T. A., Makarenkova, I. D., Smolina, T. P., Fedyanina, L. N., Kryzhanovsky, S. P., & Zaporozhets, T. S. (2019). Marine Algae Metabolites as Promising Therapeutics for the Prevention and Treatment of HIV/AIDS. Metabolites, 9(5), 87. https://doi.org/10.3390/metabo9050087