The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters
Abstract
:1. Introduction
2. General Organization of the Interfollicular DEJ
2.1. The DEJ Undulating Pattern and the Rete Ridges
2.2. Expected Impact of Rete Ridges in Epidermal Regeneration
2.3. The Epidermis Is a Mechanosentive Tissue
3. Molecular Organization of the DEJ: Several Molecularly Interconnected Networks
3.1. The Archetypal Laminin/Collagen IV Networks
3.2. The Interconnecting Molecules Central to the DEJ Integrity
3.3. The Distinctive and Essential Anchoring Complexes
4. Tense Environment at the DEJ during Skin Aging
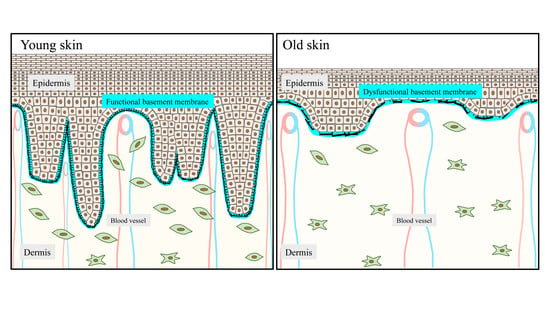
4.1. Flattening of Epidermal Rete Ridges
4.2. Deterioration of the DEJ Molecular Scaffold during Aging
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koster, M.I.; Roop, D.R. Mechanisms regulating epithelial stratification. Annu. Rev. Cell Dev. Biol. 2007, 23, 93–113. [Google Scholar] [CrossRef]
- Breitkreutz, D.; Koxholt, I.; Thiemann, K.; Nischt, R. Skin basement membrane: The foundation of epidermal integrity—BM functions and diverse roles of bridging molecules nidogen and perlecan. Biomed Res. Int. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [Green Version]
- Pozzi, A.; Yurchenco, P.D.; Iozzo, R.V. The nature and biology of basement membranes. Matrix Biol. 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Pareja, J.C. Atypical basement membranes and basement membrane diversity–what is normal anyway? J. Cell Sci. 2020, 133, jcs241794. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Amano, S.; Burgeson, R. Structure and molecular assembly of the dermal-epidermal attachment complex in skin. Connect. Tissue 1998, 30, 213–217. [Google Scholar]
- McMillan, J.R.; Akiyama, M.; Shimizu, H. Epidermal basement membrane zone components: Ultrastructural distribution and molecular interactions. J. Dermatol. Sci. 2003, 31, 169–177. [Google Scholar] [CrossRef]
- Ellison, J.; Garrod, D.R. Anchoring filaments of the amphibian epidermal-dermal junction transverse the basal lamina entirely from plasma membranes of hemidesmosomes to the dermis. J. Cell. Sci. 1984, 72, 163–172. [Google Scholar] [PubMed]
- Rousselle, P.; Lunstrum, G.P.; Keene, D.R.; Burgeson, R.E. Kalinin: An epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J. Cell. Biol. 1991, 114, 567–576. [Google Scholar] [CrossRef]
- Keene, D.R.; Sakai, L.Y.; Lunstrum, G.P.; Morris, N.P.; Burgeson, R.E. Type VII collagen forms an extended network of anchoring fibrils. J. Cell Biol. 1987, 104, 611–621. [Google Scholar] [CrossRef] [Green Version]
- Villone, D.; Fritsch, A.; Koch, M.; Bruckner-Tuderman, L.; Hansen, U.; Bruckner, P. Supramolecular interactions in the dermo-epidermal junction zone: Anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J. Biol. Chem. 2008, 283, 24506–24513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 529–544. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.; Rittié, L. Restoration of the basement membrane after wounding: A hallmark of young human skin altered with aging. J. Cell Commun. Signal. 2018, 12, 401–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekiguchi, R.; Yamada, K.M. Basement membranes in development and disease. Curr. Top. Dev. Biol. 2018, 130, 143–191. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Michopoulou, A.; Montmasson, M.; Garnier, C.; Lambert, E.; Dayan, G.; Rousselle, P. A novel mechanism in wound healing: Laminin 332 drives MMP9/14 activity by recruiting syndecan-1 and CD44. Matrix Biol. 2020, S0945-053X, 30073-1. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 207–223. [Google Scholar] [CrossRef]
- Rousselle, P.; Beck, K. Laminin 332 processing impacts cellular behavior. Cell Adhes. Migr. 2013, 7, 122–134. [Google Scholar] [CrossRef] [Green Version]
- Iozzo, R.V. Basement membrane proteoglycans: From cellar to ceiling. Nat. Rev. Mol. Cell Biol. 2005, 6, 646–656. [Google Scholar] [CrossRef]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. 2017, 27, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Halfter, W.; Oertle, P.; Monnier, C.A.; Camenzind, L.; Reyes-Lua, M.; Hu, H.; Candiello, J.; Labilloy, A.; Balasubramani, M.; Henrich, P.B.; et al. New concepts in basement membrane biology. FEBS J. 2015, 282, 4466–4479. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.T.; Kaur, P. Dermal contributions to human interfollicular epidermal architecture and self-renewal. Int. J. Mol. Sci. 2015, 16, 28098–28107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodley, D.T.; Peterson, H.D.; Herzog, S.R.; Stricklin, G.P.; Burgeson, R.E.; Briggaman, R.A.; Cronce, D.J.; O’Keefe, E.J. Burn wounds resurfaced by cultured epidermal autografts show abnormal reconstitution of anchoring fibrils. JAMA 1988, 259, 2566–2571. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Graham, H.K.; McConnell, J.C.; Sherratt, M.J.; Griffiths, C.E.M.; Watson, R.E.B. Organization of the dermal matrix impacts the biomechanical properties of skin. Br. J. Dermatol. 2017, 177, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Braverman, I.M. The cutaneous microcirculation. J. Investig. Dermatol. Symp. Proc. 2000, 5, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odland, G.F. The morphology of the attachment between the dermis and the epidermis. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1950, 108, 399–413. [Google Scholar] [CrossRef]
- Fawcett, D.W.; William, B. Skin. In Bloom and Fawcett: A Textbook of Histology, 6th ed.; Fawcett, D.W., Ed.; Chapman & Hall: New York, NY, USA, 1994; pp. 525–558. [Google Scholar]
- Newton, V.L.; Bradley, R.S.; Seroul, P.; Cherel, M.; Griffiths, C.E.M.; Rawlings, A.V.; Voegeli, R.; Watson, R.E.B.; Sherratt, M.J. Novel approaches to characterize age-related remodelling of the dermal-epidermal junction in 2D, 3D and in vivo. Skin Res. Technol. 2017, 23, 131–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyauchi, Y.; Shimaoka, Y.; Fujimura, T.; Koike, Y.; Yatabe, M.; Nishikawa, M.; Hayashi, M.; Sugata, K.; Moriwaki, S.; Hatamochi, A. Developmental changes in neonatal and infant skin structures during the first 6 months: In vivo observation. Pediatr. Dermatol. 2016, 33, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Neerken, S.; Lucassen, G.W.; Bisschop, M.A.; Lenderink, E.; Nuijs, T.A. Characterization of age-related effects in human skin: A comparative study that applies confocal laser scanning microscopy and optical coherence tomography. J. Biomed. Opt. 2004, 9, 274–281. [Google Scholar] [CrossRef]
- Lin, K.-H.; Liao, Y.-H.; Wei, M.-L.; Sun, C.-K. Comparative analysis of intrinsic skin aging between Caucasian and Asian subjects by slide-free in vivo harmonic generation microscopy. J. Biophotonics 2020, 13, e201960063. [Google Scholar] [CrossRef] [Green Version]
- Giangreco, A.; Goldie, S.J.; Failla, V.; Saintigny, G.; Watt, F.M. Human skin aging is associated with reduced expression of the stem cell markers beta1 integrin and MCSP. J. Investig. Dermatol. 2010, 130, 604–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercurio, D.G.; Jdid, R.; Morizot, F.; Masson, P.; Maia Campos, P.M.B.G. Morphological, structural and biophysical properties of French and Brazilian photoaged skin. Br. J. Dermatol. 2016, 174, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, E.; Bovi, C.; Tonini, G.; Labeille, B.; Heusèle, C.; Nizard, C.; Schnebert, S.; Aubailly, S.; Barthélémy, J.; Cambazard, F.; et al. Structural skin changes in elderly people investigated by reflectance confocal microscopy. J. Eur. Acad. Dermatol. Venereol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lagarrigue, S.G.; George, J.; Questel, E.; Lauze, C.; Meyer, N.; Lagarde, J.-M.; Simon, M.; Schmitt, A.-M.; Serre, G.; Paul, C. In vivo quantification of epidermis pigmentation and dermis papilla density with reflectance confocal microscopy: Variations with age and skin phototype. Exp. Dermatol. 2012, 21, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hao, T.; Li, C.; Wang, X.; Yu, X.; Liu, L. Automatic evaluation of stratum basale and dermal papillae using ultrahigh resolution optical coherence tomography. Biomed. Signal. Process. Control. 2019, 53, 101527. [Google Scholar] [CrossRef]
- Robic, J.; Perret, B.; Nkengne, A.; Couprie, M.; Talbot, H. Three-dimensional conditional random field for the dermal–epidermal junction segmentation. J. Med. Imaging 2019, 6, 024003. [Google Scholar] [CrossRef]
- Wu, T.; Xiong, X.; Zhang, W.; Zou, H.; Xie, H.; He, S. Morphogenesis of rete ridges in human oral mucosa: A pioneering morphological and immunohistochemical study. Cells Tissues Organs 2013, 197, 239–248. [Google Scholar] [CrossRef]
- Topczewska, J.M.; Ledwon, J.K.; Vaca, E.E.; Gosain, A.K. Mechanical stretching stimulates growth of the basal layer and rete ridges in the epidermis. J. Tissue Eng. Regen. Med. 2019, 13, 2121–2125. [Google Scholar] [CrossRef]
- Boyle, C.; Plotczyk, M.; Villalta, S.F.; Patel, S.; Hettiaratchy, S.; Masouros, S.D.; Masen, M.A.; Higgins, C.A. Morphology and composition play distinct and complementary roles in the tolerance of plantar skin to mechanical load. Sci. Adv. 2019, 5, eaay0244. [Google Scholar] [CrossRef] [Green Version]
- Penrose, L.S.; Ohara, P.T. The development of the epidermal ridges. J. Med. Genet. 1973, 10, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, P.; Guvendiren, M.; Chua, W.; Telerman, S.B.; Liakath-Ali, K.; Burdick, J.A.; Watt, F.M. Mimicking the topography of the epidermal-dermal interface with elastomer substrates. Integr. Biol. 2016, 8, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feito, J.; García-Suárez, O.; García-Piqueras, J.; García-Mesa, Y.; Pérez-Sánchez, A.; Suazo, I.; Cabo, R.; Suárez-Quintanilla, J.; Cobo, J.; Vega, J.A. The development of human digital Meissner’s and Pacinian corpuscles. Ann. Anat. Anat. Anz. 2018, 219, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Okajima, M. Development of dermal ridges in the fetus. J. Med. Genet. 1975, 12, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagna, W.; Carlisle, K. Structural changes in aging human skin. J. Investig. Dermatol. 1979, 73, 47–53. [Google Scholar] [CrossRef] [Green Version]
- McCullough, J.L.; Kelly, K.M. Prevention and treatment of skin aging. Ann. N. Y. Acad. Sci. 2006, 1067, 323–331. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Yonekura, K.; Futagawa, M.; Nakamura, T.; Hirayama, K.; Takahashi, K. Changes in dermal papilla structures due to aging in the facial cheek region. Skin Res. Technol. 2015, 21, 224–231. [Google Scholar] [CrossRef]
- Newton, V.L.; Mcconnell, J.C.; Hibbert, S.A.; Graham, H.K.; Watson, R.E. Skin aging: Molecular pathology, dermal remodelling and the imaging revolution. G. Ital. Dermatol. Venereol. 2015, 150, 665–674. [Google Scholar]
- Fraki, J.E.; Briggaman, R.A.; Lazarus, G.S. Transplantation of psoriatic skin onto nude mice. J. Investig. Dermatol. 1983, 80, 31s–35s. [Google Scholar] [CrossRef]
- Arima, K.; Ohta, S.; Takagi, A.; Shiraishi, H.; Masuoka, M.; Ontsuka, K.; Suto, H.; Suzuki, S.; Yamamoto, K.-I.; Ogawa, M.; et al. Periostin contributes to epidermal hyperplasia in psoriasis common to atopic dermatitis. Allergol. Int. 2015, 64, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Chau, T.; Parsi, K.K.; Ogawa, T.; Kiuru, M.; Konia, T.; Li, C.-S.; Fung, M.A. Psoriasis or not? Review of 51 clinically confirmed cases reveals an expanded histopathologic spectrum of psoriasis. J. Cutan. Pathol. 2017, 44, 1018–1026. [Google Scholar] [CrossRef]
- Lavker, R.M.; Sun, T.T. Heterogeneity in epidermal basal keratinocytes: Morphological and functional correlations. Science 1982, 215, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Sun, T.-T. Epidermal stem cells: Properties, markers, and location. Proc. Natl. Acad. Sci. USA 2000, 97, 13473–13475. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.; Li, A.; Kaur, P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation 2004, 72, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.H.; Harper, S.; Watt, F.M. Stem cell patterning and fate in human epidermis. Cell 1995, 80, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Jensen, U.B.; Lowell, S.; Watt, F.M. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: A new view based on whole-mount labelling and lineage analysis. Development 1999, 126, 2409–2418. [Google Scholar] [PubMed]
- Yamada, T.; Hasegawa, S.; Miyachi, K.; Date, Y.; Inoue, Y.; Yagami, A.; Arima, M.; Iwata, Y.; Yamamoto, N.; Nakata, S.; et al. Laminin-332 regulates differentiation of human interfollicular epidermal stem cells. Mech. Ageing Dev. 2018, 171, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, S.; Taichman, L.B. Organization of stem cells and their progeny in human epidermis. J. Investig. Dermatol. 2005, 124, 367–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolar, J.; Wagner, J.E. Allogeneic blood and bone marrow cells for the treatment of severe epidermolysis bullosa: Repair of the extracellular matrix. Lancet 2013, 382, 1214–1223. [Google Scholar] [CrossRef] [Green Version]
- Chambert, J.; Lihoreau, T.; Joly, S.; Chatelain, B.; Sandoz, P.; Humbert, P.; Jacquet, E.; Rolin, G. Multimodal investigation of a keloid scar by combining mechanical tests in vivo with diverse imaging techniques. J. Mech. Behav. Biomed. Mater. 2019, 99, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Gallant-Behm, C.L.; Olson, M.E.; Hart, D.A. Molecular, histologic, and gross phenotype of skin wound healing in red Duroc pigs reveals an abnormal healing phenotype of hypercontracted, hyperpigmented scarring. Wound Repair Regen. 2004, 12, 305–319. [Google Scholar] [CrossRef]
- Alkhalil, A.; Tejiram, S.; Travis, T.E.; Prindeze, N.J.; Carney, B.C.; Moffatt, L.T.; Johnson, L.S.; Ramella-Roman, J.; Shupp, J.W. A translational animal model for scar compression therapy using an automated pressure delivery system. Eplasty 2015, 15, e29. [Google Scholar] [PubMed]
- Lin, C.; Chiu, P.; Hsueh, Y.; Shieh, S.; Wu, C.; Wong, T.; Chuong, C.; Hughes, M.W. Regeneration of rete ridges in Lanyu pig (Sus scrofa): Insights for human skin wound healing. Exp. Dermatol. 2019, 28, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef] [PubMed]
- Simman, R.; Priebe, C.J.; Simon, M. Reconstruction of aplasia cutis congenita of the trunk in a newborn infant using a cellular allogenic dermal graft and cultured epithelial autografts. Ann. Plast. Surg. 2000, 44, 451–454. [Google Scholar] [CrossRef]
- Bannasch, H.; Stark, G.; Knam, F.; Horch, R.E.; Föhn, M. Decellularized dermis in combination with cultivated keratinocytes in a short- and long-term animal experimental investigation. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 41–49. [Google Scholar] [CrossRef]
- Yim, H.; Cho, Y.S.; Seo, C.H.; Lee, B.C.; Ko, J.H.; Kim, D.; Hur, J.; Chun, W.; Kim, J.H. The use of AlloDerm on major burn patients: AlloDerm prevents post-burn joint contracture. Burns 2010, 36, 322–328. [Google Scholar] [CrossRef]
- Dussoyer, M.; Michopoulou, A.; Rousselle, P. Decellularized scaffolds for skin repair and regeneration. Appl. Sci. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Rue, L.W., 3rd; Cioffi, W.G.; McManus, W.F.; Pruitt, B.A., Jr. Wound closure and outcome in extensively burned patients treated with cultured autologous keratinocytes. J. Trauma 1993, 34, 662–668. [Google Scholar] [CrossRef]
- Carsin, H.; Ainaud, P.; Le Bever, H.; Rives, J.-M.; Lakhel, A.; Stephanazzi, J.; Lambert, F.; Perrot, J. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: A five year single-center experience with 30 patients. Burns 2000, 26, 379–387. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M. Cultured epithelial autograft (CEA) in burn treatment: Three decades later. Burns 2007, 33, 405–413. [Google Scholar] [CrossRef]
- Matsumura, H.; Gondo, M.; Imai, R.; Shibata, D.; Watanabe, K. Chronological histological findings of cultured epidermal autograft over bilayer artificial dermis. Burns 2013, 39, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhao, Y.; Zhang, W.; Xie, W.; He, S. In vitro engineering of a palatal mucosa equivalent with acellular porcine dermal matrix. J. Biomed. Mater. Res. A 2008, 86, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Rizzi, S.C.; Dawson, R.; Lynam, E.; Richards, S.; Leavesley, D.I.; Upton, Z. Development of a three-dimensional human skin equivalent wound model for investigating novel wound healing therapies. Tissue Eng. Part C Methods 2010, 16, 1111–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaudoin Cloutier, C.; Goyer, B.; Perron, C.; Guignard, R.; Larouche, D.; Moulin, V.J.; Germain, L.; Gauvin, R.; Auger, F.A. In vivo evaluation and imaging of a bilayered self-assembled skin substitute using a decellularized dermal matrix grafted on mice. Tissue Eng. Part A 2017, 23, 313–322. [Google Scholar] [CrossRef]
- Pins, G.D.; Toner, M.; Morgan, J.R. Microfabrication of an analog of the basal lamina: Biocompatible membranes with complex topographies. FASEB J. 2000, 14, 593–602. [Google Scholar] [CrossRef]
- Downing, B.R.; Cornwell, K.G.; Toner, M.; Pins, G.D. The influence of microtextured basal lamina analog topography on keratinocyte function and epidermal organization. J. Biomed. Mater. Res. A 2004, 72, 47–56. [Google Scholar] [CrossRef]
- Lammers, G.; Roth, G.; Heck, M.; Zengerle, R.; Tjabringa, G.S.; Versteeg, E.M.; Hafmans, T.; Wismans, R.; Reinhardt, D.P.; Verwiel, E.T.P.; et al. Construction of a microstructured collagen membrane mimicking the papillary dermis architecture and guiding keratinocyte morphology and gene expression. Macromol. Biosci. 2012, 12, 675–691. [Google Scholar] [CrossRef]
- Bush, K.A.; Pins, G.D. Development of microfabricated dermal epidermal regenerative matrices to evaluate the role of cellular microenvironments on epidermal morphogenesis. Tissue Eng. Part A 2012, 18, 2343–2353. [Google Scholar] [CrossRef] [Green Version]
- Clement, A.L.; Moutinho, T.J., Jr.; Pins, G.D. Micropatterned dermal-epidermal regeneration matrices create functional niches that enhance epidermal morphogenesis. Acta Biomater. 2013, 9, 9474–9484. [Google Scholar] [CrossRef] [Green Version]
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.-M.; Park, Y.J.; Hong, S.-D.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B.-M. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461. [Google Scholar] [CrossRef]
- Fu, X.; Xu, M.; Liu, J.; Qi, Y.; Li, S.; Wang, H. Regulation of migratory activity of human keratinocytes by topography of multiscale collagen-containing nanofibrous matrices. Biomaterials 2014, 35, 1496–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobasseri, S.A.; Zijl, S.; Salameti, V.; Walko, G.; Stannard, A.; Garcia-Manyes, S.; Watt, F.M. Patterning of human epidermal stem cells on undulating elastomer substrates reflects differences in cell stiffness. Acta Biomater. 2019, 87, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Helling, A.L.; Viswanathan, P.; Cheliotis, K.; Mobasseri, S.A.; Yang, Y.; El Haj, A.J.; Watt, F.M. Dynamic culture substrates that mimic the topography of the epidermal-dermal junction. Tissue Eng. Part A 2019, 25, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackstone, B.N.; Malara, M.M.; Baumann, M.E.; McFarland, K.L.; Supp, D.M.; Powell, H.M. Fractional CO2 laser micropatterning of cell-seeded electrospun collagen scaffolds enables rete ridge formation in 3D engineered skin. Acta Biomater. 2020, 102, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Malara, M.M.; Blackstone, B.N.; Baumann, M.E.; Bailey, J.K.; Supp, D.M.; Powell, H.M. Cultured epithelial autograft combined with micropatterned dermal template forms rete ridges in vivo. Tissue Eng. Part A 2020, 26, 1138–1146. [Google Scholar] [CrossRef]
- Morrissey, M.A.; Sherwood, D.R. An active role for basement membrane assembly and modification in tissue sculpting. J. Cell Sci. 2015, 128, 1661–1668. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Lewis, W.; Page-McCaw, A. Basement membrane mechanics shape development: Lessons from the fly. Matrix Biol. 2019, 75–76, 72–81. [Google Scholar] [CrossRef]
- Oakford, M.E.; Dixon, S.V.; August, S.; Pickard, C.; Ardern-Jones, M.; Lackie, P.; Friedmann, P.S.; Healy, E. Migration of immunocytes across the basement membrane in skin: The role of basement membrane pores. J. Investig. Dermatol. 2011, 131, 1950–1953. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.T. Mechanical properties of basement membrane in health and disease. Matrix Biol. 2017, 57–58, 366–373. [Google Scholar] [CrossRef]
- Candiello, J.; Balasubramani, M.; Schreiber, E.M.; Cole, G.J.; Mayer, U.; Halfter, W.; Lin, H. Biomechanical properties of native basement membranes. FEBS J. 2007, 274, 2897–2908. [Google Scholar] [CrossRef]
- Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphya, C.J. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. J. Struct. Biol. 2009, 167, 19–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crest, J.; Diz-Muñoz, A.; Chen, D.-Y.; Fletcher, D.A.; Bilder, D. Organ sculpting by patterned extracellular matrix stiffness. Elife 2017, 6, e24958. [Google Scholar] [CrossRef] [PubMed]
- Chlasta, J.; Milani, P.; Runel, G.; Duteyrat, J.-L.; Arias, L.; Lamiré, L.-A.; Boudaoud, A.; Grammont, M. Variations in basement membrane mechanics are linked to epithelial morphogenesis. Development 2017, 144, 4350–4362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.; Chaudhuri, O. Beyond proteases: Basement membrane mechanics and cancer invasion. J. Cell Biol. 2019, 218, 2456–2469. [Google Scholar] [CrossRef] [Green Version]
- Sproule, T.J.; Roopenian, D.C.; Sundberg, J.P. A direct method to determine the strength of the dermal-epidermal junction in a mouse model for epidermolysis bullosa. Exp. Dermatol. 2012, 21, 453–455. [Google Scholar] [CrossRef]
- Jung, J.P.; Lin, W.-H.; Riddle, M.J.; Tolar, J.; Ogle, B.M. A 3D in vitro model of the dermoepidermal junction amenable to mechanical testing. J. Biomed. Mater. Res. Part A 2018, 106, 3231–3238. [Google Scholar] [CrossRef]
- LaRose, A.; Dakiw-Piaceski, A.; Barbier, M.A.; Larouche, D.; Gauvin, R.; Caruso, M.; Pope, E.; Germain, L. Peel test to assess the adhesion strength of the dermal-epidermal junction in tissue-engineered skin. Tissue Eng. Part C Methods 2020, 26, 180–189. [Google Scholar] [CrossRef]
- Ciarletta, P.; Amar, M.B. Papillary network in the dermal-epidemal junction of skin: A biochemical model. Mech. Res. Commun. 2012, 42, 68–76. [Google Scholar] [CrossRef]
- Lejeune, E.; Javili, A.; Linder, C. Understanding geometric instabilities in thin films via a multi-layer model. Soft Matter 2016, 12, 806–816. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Skin aging as a mechanical phenomenon: The main weak links. Nutr. Healthy Aging 2018, 4, 291–307. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Bershadsky, A.D.; Balaban, N.Q.; Geiger, B. Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 2003, 19, 677–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.H.-C.; Thampatty, B.P. An Introductory Review of Cell Mechanobiology. Biomech. Model. Mechanobiol. 2006, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [Green Version]
- Mammoto, A.; Connor, K.M.; Mammoto, T.; Yung, C.W.; Huh, D.; Aderman, C.M.; Mostoslavsky, G.; Smith, A.T.; Ingber, D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 2009, 457, 1103–1108. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.S.; Mrksich, M.; Huang, S.; Whitesides, G.M.; Ingber, D.E. Geometric control of cell life and death. Science 1997, 276, 1425–1428. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.H.; Collier, J.H.; Sfeir, C.S.; Healy, K.E. Engineering gene expression and protein synthesis by modulation of nuclear shape. Proc. Natl. Acad. Sci. USA 2002, 99, 1972–1977. [Google Scholar] [CrossRef] [Green Version]
- Curtis, A.; Wilkinson, C. Topographical control of cells. Biomaterials 1997, 18, 1573–1583. [Google Scholar] [CrossRef]
- Evans, N.D.; Oreffo, R.O.; Healy, E.; Thurner, P.J.; Man, Y.H. Epithelial mechanobiology, skin wound healing, and the stem cell niche. J. Mech. Behav. Biomed. Mater. 2013, 28, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Olenius, M.; Dalsgaard, C.-J.; Wickman, M. Mitotic activity in expanded human skin. Plast. Reconstr. Surg. 1993, 91, 213–216. [Google Scholar] [CrossRef]
- Takei, T.; Han, O.; Ikeda, M.; Male, P.; Mills, I.; Sumpio, B.E. Cyclic strain stimulates isoform-specific PKC activation and translocation in cultured human keratinocytes. J. Cell. Biochem. 1997, 67, 327–337. [Google Scholar] [CrossRef]
- Kippenberger, S.; Bernd, A.; Guschel, M.; Müller, J.; Kaufmann, R.; Loitsch, S.; Bereiter-Hahn, J. Signaling of mechanical stretch in human keratinocytes via MAP kinases. J. Investig. Dermatol. 2000, 114, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Komine, M.; Fujimoto, M.; Okochi, H.; Tamaki, K. Mechanical stretching in vitro regulates signal transduction pathways and cellular proliferation in human epidermal keratinocytes. J. Investig. Dermatol. 2004, 122, 783–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watt, F.M.; Jordan, P.W.; O’Neill, C.H. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc. Natl. Acad. Sci. USA 1988, 85, 5576–5580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connelly, J.T.; Gautrot, J.E.; Trappmann, B.; Tan, D.W.; Donati, G.; Huck, W.T.; Watt, F.M. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol. 2010, 12, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Zijl, S.; Vasilevich, A.S.; Viswanathan, P.; Helling, A.L.; Beijer, N.; Walko, G.; Chiappini, C.; De Boer, J.; Watt, F.M. Micro-scaled topographies direct differentiation of human epidermal stem cells. Acta Biomater. 2019, 84, 133–145. [Google Scholar] [CrossRef]
- Biggs, L.C.; Kim, C.S.; Miroshnikova, Y.A.; Wickström, S.A. Mechanical forces in the skin: Roles in tissue architecture, stability, and function. J. Investig. Dermatol. 2020, 140, 284–290. [Google Scholar] [CrossRef]
- Butcher, D.T.; Alliston, T.; Weaver, V.M. A tense situation: Forcing tumour progression. Nat. Rev. Cancer 2009, 9, 108–122. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, M.A.; Desai, R.; Solski, P.A.; Der, C.J.; Keely, P.J. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J. Cell Biol. 2003, 163, 583–595. [Google Scholar] [CrossRef]
- Samuel, M.S.; Lopez, J.I.; McGhee, E.J.; Croft, D.R.; Strachan, D.; Timpson, P.; Munro, J.; Schröder, E.; Zhou, J.; Brunton, V.G.; et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer Cell 2011, 19, 776–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokuyama, E.; Nagai, Y.; Takahashi, K.; Kimata, Y.; Naruse, K. Mechanical stretch on human skin equivalents increases the epidermal thickness and develops the basement membrane. PLoS ONE 2015, 10, e0141989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Stuart, M.A.C.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Castellan, M.; Battilana, G.; Zanconato, F.; Azzolin, L.; Giulitti, S.; Cordenonsi, M.; Piccolo, S. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat. Commun. 2017, 8, 15206. [Google Scholar] [CrossRef] [PubMed]
- Trepat, X.; Wasserman, M.R.; Angelini, T.E.; Millet, E.; Weitz, D.A.; Butler, J.P.; Fredberg, J.J. Physical forces during collective cell migration. Nat. Phys. 2009, 5, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, G.; Luo, X.; Qiu, J.; Tang, C. Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burns 2012, 38, 414–420. [Google Scholar] [CrossRef]
- Wickert, L.E.; Pomerenke, S.; Mitchell, I.; Masters, K.S.; Kreeger, P.K. Hierarchy of cellular decisions in collective behavior: Implications for wound healing. Sci. Rep. 2016, 6, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Gautham Hari Narayana, S.N.; Kasiviswanathan, U.; Agarwal, T.; Senthilguru, K.; Mukhopadhyay, D.; Pal, K.; Giri, S.; Maitib, T.K.; Banerjee, I. Substrate stiffness does affect the fate of human keratinocytes. RSC Adv. 2016, 6, 3539–3551. [Google Scholar] [CrossRef]
- Kenny, F.N.; Drymoussi, Z.; Delaine-Smith, R.; Kao, A.P.; Laly, A.C.; Knight, M.; Philpott, M.P.; Connelly, J.T. Tissue stiffening promotes keratinocyte proliferation through activation of epidermal growth factor signaling. J. Cell Sci. 2018, 131, jcs215780. [Google Scholar] [CrossRef] [Green Version]
- Ya, C.; Carrancá, M.; Sigaudo-Roussel, D.; Faure, P.; Fromy, B.; Debret, R. Substrate softness promotes terminal differentiation of human keratinocytes without altering their ability to proliferate back into a rigid environment. Arch. Dermatol. Res. 2019, 311, 741–751. [Google Scholar] [CrossRef]
- Zarkoob, H.; Bodduluri, S.; Ponnaluri, S.V.; Selby, J.C.; Sander, E.A. Substrate stiffness affects human keratinocyte colony formation. Cell. Mol. Bioeng. 2015, 8, 32–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yurchenco, P.D.; Schittny, J.C. Molecular architecture of basement membranes. FASEB J. 1990, 4, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Behrens, D.T.; Villone, D.; Koch, M.; Brunner, G.; Sorokin, L.; Robenek, H.; Bruckner-Tuderman, L.; Bruckner, P.; Hansen, U. The epidermal basement membrane is a composite of separate laminin- or collagen IV-containing networks connected by aggregated perlecan, but not by nidogens. J. Biol. Chem. 2012, 287, 18700–18709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Lewis, W.; LaFever, K.S.; Page-McCaw, A. A scar-like lesion is apparent in basement membrane after wound repair in vivo. Matrix Biol. 2018, 74, 101–120. [Google Scholar] [CrossRef]
- Nystroem, A.; Bruckner-Tuderman, L. Matrix molecules and skin biology. Semin. Cell Dev. Biol. 2019, 89, 136–146. [Google Scholar] [CrossRef]
- McKee, K.K.; Harrison, D.; Capizzi, S.; Yurchenco, P.D. Role of laminin terminal globular domains in basement membrane assembly. J. Biol. Chem. 2007, 282, 21437–21447. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Fässler, R.; Hohenester, E. Laminin: The crux of basement membrane assembly. J. Cell Biol. 2004, 164, 959–963. [Google Scholar] [CrossRef] [Green Version]
- Aumailley, M.; Smyth, N. The role of laminins in basement membrane function. J. Anat. 1998, 193, 1–21. [Google Scholar] [CrossRef]
- Hohenester, E. Structural biology of laminins. Essays Biochem. 2019, 63, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Yap, L.; Tay, H.G.; Nguyen, M.T.; Tjin, M.S.; Tryggvason, K. Laminins in cellular differentiation. Trends Cell Biol. 2019, 29, 987–1000. [Google Scholar] [CrossRef] [Green Version]
- Yurchenco, P.D.; Cheng, Y.S.; Colognato, H. Laminin forms an independent network in basement membranes. J. Cell. Biol. 1992, 117, 1119–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Edgar, D.; Fässler, R.; Wadsworth, W.; Yurchenco, P.D. The role of laminin in embryonic cell polarization and tissue organization. Dev. Cell 2003, 4, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.S.; Champliaud, M.F.; Burgeson, R.E.; Marinkovich, M.P.; Yurchenco, P.D. Self-assembly of laminin isoforms. J. Biol. Chem. 1997, 272, 31525–31532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohenester, E.; Yurchenco, P.D. Laminins in basement membrane assembly. Cell Adhes. Migr. 2013, 7, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Aumailley, M.; Rousselle, P. Laminins of the dermo-epidermal junction. Matrix Biol. 1999, 18, 19–28. [Google Scholar] [CrossRef]
- McMillan, J.R.; Akiyama, M.; Nakamura, H.; Shimizu, H. Colocalization of multiple laminin isoforms predominantly beneath hemidesmosomes in the upper lamina densa of the epidermal basement membrane. J. Histochem. Cytochem. 2006, 54, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegner, J.; Loser, K.; Apsite, G.; Nischt, R.; Eckes, B.; Krieg, T.; Werner, S.; Sorokin, L.M. Laminin α5 in the keratinocyte basement membrane is required for epidermal-dermal intercommunication. Matrix Biol. 2016, 56, 24–41. [Google Scholar] [CrossRef]
- Sampaolo, S.; Napolitano, F.; Tirozzi, A.; Reccia, M.G.; Lombardi, L.; Farina, O.; Barra, A.; Cirillo, F.; Melone, M.A.B.; Gianfrancesco, F.; et al. Identification of the first dominant mutation of LAMA5 gene causing a complex multisystem syndrome due to dysfunction of the extracellular matrix. J. Med. Genet. 2017, 54, 710–720. [Google Scholar] [CrossRef]
- Julia, T.; Tzu, J.; Chen, Y.; Zhang, Y.; Nguyen, N.T.; Gao, J.; Bradley, M.; Keene, D.R.; Oro, A.E.; Miner, J.H.; et al. Laminin-10 is crucial for hair morphogenesis. EMBO J. 2003, 22, 2400–2410. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; DeRouen, M.C.; Chen, C.-H.; Nguyen, M.; Nguyen, N.T.; Ido, H.; Harada, K.; Sekiguchi, K.; Morgan, B.A.; Miner, J.H.; et al. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008, 22, 2111–2124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.K.; Lam, R.; McKee, K.K.; Aleksandrova, M.; Dowling, J.; Alexander, S.I.; Mallawaarachchi, A.; Cottle, D.L.; Short, K.M.; Pais, L.; et al. A mutation affecting laminin alpha 5 polymerisation gives rise to a syndromic developmental disorder. Development 2020, 147, dev189183. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Pouliot, N.; Redvers, R.; Kaur, P. Extensive tissue-regenerative capacity of neonatal human keratinocyte stem cells and their progeny. J. Clin. Investig. 2004, 113, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Fifield, S.; Schlüter, H.; Li, A.; Aitken, T.; Gangatirkar, P.; Blashki, D.; Koelmeyer, R.L.; Pouliot, N.; Palatsides, M.; Ellis, S.; et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J. Clin. Investig. 2009, 119, 2795–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, L.; Kaur, P. The aging epidermal skin niche. In Advances in Stem Cells and Their Niches; Nilsson, S., Ed.; Elsevier: London, UK, 2020; Volume 4, pp. 65–98. [Google Scholar]
- Khoshnoodi, J.; Pedchenko, V.; Hudson, B.G. Mammalian collagen IV. Microsc. Res. Tech. 2008, 71, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.C.; Timpl, R. The collagen superfamily. Int. Arch. Allergy Immunol. 1995, 107, 484–490. [Google Scholar] [CrossRef]
- Oguchi, M.; Kobayasi, T.; Asboe-Hansen, G. Secretion of type IV collagen by keratinocytes of human adult. J. Investig. Dermatol. 1985, 85, 79–81. [Google Scholar] [CrossRef] [Green Version]
- Olsen, D.R.; Uitto, J. Differential expression of type IV procollagen and laminin genes by fetal vs adult skin fibroblasts in culture: Determination of subunit mRNA steady-state levels. J. Investig. Dermatol. 1989, 93, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Abreu-Velez, A.M.; Howard, M.S. Collagen IV in normal and in disease process. N. Am. J. Med. Sci. 2012, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Khoshnoodi, J.; Cartailler, J.-P.; Alvares, K.; Veis, A.; Hudson, B.G. Molecular recognition in the assembly of collagens: Terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J. Biol. Chem. 2006, 281, 38117–38121. [Google Scholar] [CrossRef] [Green Version]
- Timpl, R.; Risteli, J.; Bächinger, H.P. Identification of a new basement membrane collagen by the aid of a large fragment resistant to bacterial collagenase. FEBS Lett. 1979, 101, 265–268. [Google Scholar] [CrossRef] [Green Version]
- Risteli, J.; Timpl, R.; Bachinger, H.P.; Engel, J.; Furthmayr, H. 7-S Collagen: Characterization of an unusual basement membrane structure. JBIC J. Biol. Inorg. Chem. 1980, 108, 239–250. [Google Scholar] [CrossRef]
- Hudson, B.G.; Tryggvason, K.; Sundaramoorthy, M.; Neilson, E.G. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N. Engl. J. Med. 2003, 348, 2543–2556. [Google Scholar] [CrossRef]
- Pastor-Pareja, J.C.; Xu, T. Shaping cells and organs in drosophila by opposing roles of fat body-secreted collagen IV and perlecan. Dev. Cell 2011, 21, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pöschl, E.; Mayer, U.; Stetefeld, J.; Baumgartner, R.; Holak, T.A.; Huber, R.; Timpl, R. Site-directed mutagenesis and structural interpretation of the nidogen binding site of the laminin gamma1 chain. EMBO J. 1996, 15, 5154–5159. [Google Scholar] [CrossRef]
- Fox, J.W.; Mayer, U.; Nischt, R.; Aumailley, M.; Reinhardt, D.; Wiedemann, H.; Mann, K.; Timpl, R.; Krieg, T.; Engel, J.; et al. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991, 10, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Bader, B.L.; Smyth, N.; Nedbal, S.; Miosge, N.; Baranowsky, A.; Mokkapati, S.; Murshed, M.; Nischt, R. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol. Cell. Biol. 2005, 25, 6846–6856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokkapati, S.; Baranowsky, A.; Mirancea, N.; Smyth, N.; Breitkreutz, D.; Nischt, R. Basement membranes in skin are differently affected by lack of nidogen 1 and 2. J. Investig. Dermatol. 2008, 128, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, D.; Mann, K.; Nischt, R.; Fox, J.W.; Chu, M.L.; Krieg, T.; Timpl, R. Mapping of nidogen binding sites for collagen type IV, heparan sulfate proteoglycan, and zinc. J. Biol. Chem. 1993, 268, 10881–10887. [Google Scholar]
- Yurchenco, P.D. Integrating Activities of laminins that drive basement membrane assembly and function. Transp. Syst. 2015, 76, 1–30. [Google Scholar] [CrossRef]
- Gubbiotti, M.A.; Neill, T.; Iozzo, R.V. A current view of perlecan in physiology and pathology: A mosaic of functions. Matrix Biol. 2017, 57–58, 285–298. [Google Scholar] [CrossRef] [Green Version]
- Sher, I.; Zisman-Rozen, S.; Eliahu, L.; Whitelock, J.M.; Maas-Szabowski, N.; Yamada, Y.; Breitkreutz, D.; Fusenig, N.E.; Arikawa-Hirasawa, E.; Iozzo, R.V.; et al. Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J. Biol. Chem. 2006, 281, 5178–5187. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, M.; Michopoulou, A.; André-Frei, V.; Boulesteix, S.; Guicher, C.; Dayan, G.; Whitelock, J.; Damour, O.; Rousselle, P. Perlecan expression influences the keratin 15-positive cell population fate in the epidermis of aging skin. Aging 2016, 8, 751–768. [Google Scholar] [CrossRef] [Green Version]
- Candiello, J.; Cole, G.J.; Halfter, W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010, 29, 402–410. [Google Scholar] [CrossRef]
- Balasubramani, M.; Schreiber, E.M.; Candiello, J.; Balasubramani, G.; Kurtz, J.; Halfter, W. Molecular interactions in the retinal basement membrane system: A proteomic approach. Matrix Biol. 2010, 29, 471–483. [Google Scholar] [CrossRef]
- Hohenester, E.; Maurer, P.; Hohenadl, C.; Timpl, R.; Jansonius, J.N.; Engel, J. Structure of a novel extracellular Ca(2+)-binding module in BM-40. Nat. Struct. Biol. 1996, 3, 67–73. [Google Scholar] [CrossRef]
- Hohenester, E.; Sasaki, T.; Giudici, C.; Farndale, R.W.; Bächinger, H.P. Structural basis of sequence-specific collagen recognition by SPARC. Proc. Natl. Acad. Sci. USA 2008, 105, 18273–18277. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, A.D. The role of SPARC in extracellular matrix assembly. J. Cell Commun. Signal. 2009, 3, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.J.; Sage, E.H. A prototypic matricellular protein in the tumor microenvironment-where there’s SPARC, there’s fire. J. Cell. Biochem. 2008, 104, 721–732. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Dontula, R.; El-Rayes, B.F.; Lakka, S.S. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis 2014, 35, 967–973. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Lane, T.F.; Pallero, M.A.; Sage, E.H. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand. J. Cell. Biochem. 1995, 57, 341–350. [Google Scholar] [CrossRef]
- Chlenski, A.; Guerrero, L.J.; Salwen, H.R.; Yang, Q.; Tian, Y.; La Madrid, A.M.; Mirzoeva, S.; Bouyer, P.G.; Xu, D.; Walker, M.; et al. Secreted protein acidic and rich in cysteine is a matrix scavenger chaperone. PLoS ONE 2011, 6, e23880. [Google Scholar] [CrossRef] [Green Version]
- Harris, B.S.; Zhang, Y.; Card, L.; Rivera, L.B.; Brekken, R.A.; Bradshaw, A.D. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H841–H847. [Google Scholar] [CrossRef]
- Sage, H.; Vernon, R.B.; Funk, S.E.; Everitt, E.A.; Angello, J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J. Cell Biol. 1989, 109, 341–356. [Google Scholar] [CrossRef]
- Martinek, N.; Shahab, J.; Saathoff, M.; Ringuette, M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in drosophila embryos. J. Cell Sci. 2008, 121, 1671–1680. [Google Scholar] [CrossRef] [Green Version]
- Isabella, A.J.; Horne-Badovinac, S. Dynamic regulation of basement membrane protein levels promotes egg chamber elongation in Drosophila. Dev. Biol. 2015, 406, 212–221. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, M.A.; Jayadev, R.; Miley, G.R.; Blebea, C.A.; Chi, Q.; Ihara, S.; Sherwood, D.R. SPARC promotes cell invasion in vivo by decreasing type IV collagen levels in the basement membrane. PLoS Genet. 2016, 12, e1005905. [Google Scholar] [CrossRef] [Green Version]
- Chioran, A.; Duncan, S.; Catalano, A.; Brown, T.J.; Ringuette, M.J. Collagen IV trafficking: The inside-out and beyond story. Dev. Biol. 2017, 431, 124–133. [Google Scholar] [CrossRef]
- Raghunath, M.; Tschödrich-Rotter, M.; Sasaki, T.; Meuli, M.; Chu, M.-L.; Timpl, R. Confocal laser scanning analysis of the association of fibulin-2 with fibrillin-1 and fibronectin define different stages of skin regeneration. J. Investig. Dermatol. 1999, 112, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Kusubata, M.; Hirota, A.; Ebihara, T.; Kuwaba, K.; Matsubara, Y.; Sasaki, T.; Kusakabe, M.; Tsukada, T.; Irie, S.; Koyama, Y.-I. Spatiotemporal changes of fibronectin, tenascin-C, fibulin-1, and fibulin-2 in the skin during the development of chronic contact dermatitis. J. Investig. Dermatol. 1999, 113, 906–912. [Google Scholar] [CrossRef] [Green Version]
- Timpl, R.; Sasaki, T.; Kostka, G.; Chu, M.-L. Fibulins: A versatile family of extracellular matrix proteins. Nat. Rev. Mol. Cell Biol. 2003, 4, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Longmate, W.M.; Monichan, R.; Chu, M.-L.; Tsuda, T.; Mahoney, M.G.; DiPersio, C.M. Reduced fibulin-2 contributes to loss of basement membrane integrity and skin blistering in mice lacking integrin α3β1 in the epidermis. J. Investig. Dermatol. 2014, 134, 1609–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heljasvaara, R.; Aikio, M.; Ruotsalainen, H.; Pihlajaniemi, T. Collagen XVIII in tissue homeostasis and dysregulation—Lessons learned from model organisms and human patients. Matrix Biol. 2017, 57–58, 55–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halfter, W.; Dong, S.; Schurer, B.; Cole, G.J. Collagen XVIII is a basement membrane heparan sulfate proteoglycan. J. Biol. Chem. 1998, 273, 25404–25412. [Google Scholar] [CrossRef] [Green Version]
- Saarela, J.; Rehn, M.; Oikarinen, A.; Autio-Harmainen, H.; Pihlajaniemi, T. The short and long forms of type XVIII collagen show clear tissue specificities in their expression and location in basement membrane zones in humans. Am. J. Pathol. 1998, 153, 611–626. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.P.; Warman, M.L.; Seldin, M.F.; Cheng, S.-D.; Knoll, J.H.; Timmons, S.; Olsen, B.R. Cloning of cDNA and genomic DNA encoding human type XVIII collagen and localization of the alpha1(XVIII) collagen gene to mouse chromosome 10 and human chromosome 21. Genomics 1994, 19, 494–499. [Google Scholar] [CrossRef]
- Marneros, A.G.; Keene, U.R.; Hansen, U.; Fukai, N.; Moulton, K.; Goletz, P.L.; Moiseyev, G.; Pawlyk, B.S.; Halfter, W.; Dong, S.; et al. Collagen XVIII/endostatin is essential for vision and retinal pigment epithelial function. EMBO J. 2004, 23, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Elamaa, H.; Sormunen, R.; Rehn, M.; Soininen, R.; Pihlajaniemi, T. Endostatin overexpression specifically in the lens and skin leads to cataract and ultrastructural alterations in basement membranes. Am. J. Pathol. 2006, 166, 221–229. [Google Scholar] [CrossRef] [Green Version]
- Miosge, N.; Simniok, T.; Sprysch, P.; Herken, R. The collagen type XVIII endostatin domain is co-localized with perlecan in basement membranes in vivo. J. Histochem. Cytochem. 2003, 51, 285–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marneros, A.G.; Olsen, B.R. Physiological role of collagen XVIII and endostatin. FASEB J. 2005, 19, 716–728. [Google Scholar] [CrossRef] [PubMed]
- Fukai, N.; Eklund, L.; Marneros, A.G.; Oh, S.P.; Keene, U.R.; Tamarkin, L.; Niemelä, M.; Ilves, M.; Li, E.; Pihlajaniemi, T.; et al. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002, 21, 1535–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, I.; Jobeili, L.; Cadau, S.; Berthélémy, N.; Pierrot, A.; Tedeschi, C.; Bardey, V.; Fargier, G.; Rival, D.; Jeanmaire, C.; et al. Collagen XVIII: A key interfacial component of the skin architecture. J. Cosmet. Sci. 2017, 68, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Carter, W.G.; Ryan, M.C.; Gahr, P.J. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell 1991, 65, 599–610. [Google Scholar] [CrossRef]
- Ghohestani, R.F.; Li, K.; Rousselle, P.; Uitto, J. Molecular organization of the cutaneous basement membrane zone. Clin. Dermatol. 2001, 19, 551–562. [Google Scholar] [CrossRef]
- Litjens, S.H.; De Pereda, J.M.; Sonnenberg, A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006, 16, 376–383. [Google Scholar] [CrossRef]
- Sterk, L.M.T.; Geuijen, C.A.W.; Oomen, L.C.J.M.; Calafat, J.; Janssen, H.; Sonnenberg, A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J. Cell Biol. 2000, 149, 969–982. [Google Scholar] [CrossRef] [Green Version]
- Nahidiazar, L.; Kreft, M.; van den Broek, B.; Secades, P.; Manders, E.M.M.; Sonnenberg, A.; Jalink, K. The molecular architecture of hemidesmosomes, as revealed with super-resolution microscopy. J. Cell Sci. 2015, 128, 3714–3719. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zuidema, A.; Molder, L.T.; Nahidiazar, L.; Hoekman, L.; Schmidt, T.; Coppola, S.; Sonnenberg, A. Hemidesmosomes modulate force generation via focal adhesions. J. Cell Biol. 2020, 219, e201904137. [Google Scholar] [CrossRef]
- Champliaud, M.F.; Lunstrum, G.P.; Rousselle, P.; Nishiyama, T.; Keene, D.R.; Burgeson, R.E. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial-stromal attachment. J. Cell Biol. 1996, 132, 1189–1198. [Google Scholar] [CrossRef]
- Carulli, S.; Beck, K.; Dayan, G.; Boulesteix, S.; Lortat-Jacob, H.; Rousselle, P. Cell surface proteoglycans syndecan-1 and -4 bind overlapping but distinct sites in laminin α3 LG45 protein domain. J. Biol. Chem. 2012, 287, 12204–12216. [Google Scholar] [CrossRef] [Green Version]
- Senyürek, I.; Kempf, W.; Klein, G.; Maurer, A.; Kalbacher, H.; Schäfer, L.; Wanke, I.; Christ, C.; Stevanovic, S.; Schaller, M.; et al. Processing of laminin α chains generates peptides involved in wound healing and host defense. J. Innate Immun. 2014, 6, 467–484. [Google Scholar] [CrossRef]
- Sakai, L.Y.; Keene, D.R.; Morris, N.P.; Burgeson, R.E. Type VII collagen is a major structural component of anchoring fibrils. J. Cell Biol. 1986, 103, 1577–1586. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Natsuga, K.; Shinkuma, S.; Shimizu, H. Epidermal aspects of type VII collagen: Implications for dystrophic epidermolysis bullosa and epidermolysis bullosa acquisita. J. Dermatol. 2018, 45, 515–521. [Google Scholar] [CrossRef]
- Burgeson, R.E. Type VII collagen, anchoring fibrils and epidermolysis bullosa. J. Investig. Dermatol. 1993, 101, 252–255. [Google Scholar] [CrossRef] [Green Version]
- Rousselle, P.; Keene, D.R.; Ruggiero, F.; Champliaud, M.F.; van der Rest, M.; Burgeson, R.E. Laminin 5 binds the NC-1 domain of type VII collagen. J. Cell Biol. 1997, 138, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Marinkovich, M.P.; Veis, A.; Cai, X.; Rao, C.N.; O’Toole, E.A.; Woodley, D.T. Interactions of the amino-terminal noncollagenous (NC1) domain of type VII collagen with extracellular matrix components. J. Biol. Chem. 1997, 272, 14516–14522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Jones, J.C.; O’Toole, E.A.; Li, Y.-Y.; Woodley, D.T.; Marinkovich, M.P. NC1 domain of type VII collagen binds to the beta 3 chain of laminin 5 via a unique subdomain within the fibronectin-like repeats. J. Investig. Dermatol. 1999, 112, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, Y.; Kariya, Y.; Yasuda, C.; Miyazaki, K. Regulation of cell adhesion and type VII collagen binding by the beta3 chain short arm of laminin-5: Effect of its proteolytic cleavage. J. Biochem. 2005, 138, 539–552. [Google Scholar] [CrossRef]
- Van den Bergh, F.; Eliason, S.; Giudice, G.J. Type XVII collagen (BP180) can function as a cell−matrix adhesion molecule via binding to laminin 332. Matrix Biol. 2011, 30, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Nishie, W.; Kiritsi, D.; Nyström, A.; Hofmann, S.C.; Bruckner-Tuderman, L. Dynamic interactions of epidermal collagen XVII with the extracellular matrix: Laminin 332 as a major binding partner. Am. J. Pathol. 2011, 179, 829–837. [Google Scholar] [CrossRef]
- Franzke, C.V.; Tasanen, K.; Schumann, H.; Bruckner-Tuderman, L. Collagenous transmembrane proteins: Collagen XVII as a prototype. Matrix Biol. 2003, 22, 299–309. [Google Scholar] [CrossRef]
- Jones, V.A.; Patel, P.M.; Gibson, F.T.; Cordova, A.; Amber, K.T. The role of collagen XVII in cancer: Squamous cell carcinoma and beyond. Front. Oncol. 2020, 10, 352. [Google Scholar] [CrossRef]
- Natsuga, K.; Watanabe, M.; Nishie, W.; Shimizu, H. Life before and beyond blistering: The role of collagen XVII in epidermal physiology. Exp. Dermatol. 2019, 28, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Fine, J.-D.; Bruckner-Tuderman, L.; Eady, R.A.J.; Bauer, E.A.; Bauer, J.W.; Has, C.; Heagerty, A.; Hintner, H.; Hovnanian, A.; Jonkman, M.F.; et al. Inherited epidermolysis bullosa: Updated recommendations on diagnosis and classification. J. Am. Acad. Dermatol. 2014, 70, 1103–1126. [Google Scholar] [CrossRef]
- McGrath, J.A. Recently identified forms of epidermolysis bullosa. Ann. Dermatol. 2015, 27, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Rousselle, P.; Michopoulou, A. Laminin 332 in junctional epidermolysis and as an autoantigen in mucous membrane pemphigoid. In Blistering Diseases; Murrell, D.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 91–102. [Google Scholar]
- Kiritsi, D.; Nyström, A. Recent advances in understanding and managing epidermolysis bullosa. F1000Research 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Bruckner-Tuderman, L. Skin fragility: Perspectives on evidence-based therapies. Acta Derm. Venereol. 2020, 100, adv00053-101. [Google Scholar] [CrossRef] [Green Version]
- Prodinger, C.; Reichelt, J.; Bauer, J.W.; Laimer, M. Epidermolysis bullosa: Advances in research and treatment. Exp. Dermatol. 2019, 28, 1176–1189. [Google Scholar] [CrossRef] [Green Version]
- Goletz, S.; Zillikens, D.; Schmidt, E. Structural proteins of the dermal-epidermal junction targeted by autoantibodies in pemphigoid diseases. Exp. Dermatol. 2017, 26, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Bernard, P.; Antonicelli, F.; Bedane, C.; Joly, P.; Le Roux-Villet, C.; Duvert-Lehembre, S.; Rousselle, P.; Prost-Squarcioni, C. Prevalence and clinical significance of anti-Laminin 332 autoantibodies detected by a novel ELISA in mucous membrane pemphigoid. JAMA Dermatol. 2013, 20, 1–8. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. Molecular mechanisms of skin aging: State of the art. Ann. N.Y. Acad. Sci. 2007, 1119, 40–50. [Google Scholar] [CrossRef]
- Lavker, R.M.; Zheng, P.S.; Dong, G. Aged skin: A study by light, transmission electron, and scanning electron microscopy. J. Investig. Dermatol. 1987, 88, 44s–51s. [Google Scholar] [CrossRef]
- Kurban, R.S.; Bhawan, J. Histologic changes in skin associated with aging. J. Dermatol. Surg. Oncol. 1990, 16, 908–914. [Google Scholar] [CrossRef]
- Branchet, M.; Boisnic, S.; Frances, C.; Robert, A. Skin thickness changes in normal aging skin. Gerontology 1990, 36, 28–35. [Google Scholar] [CrossRef]
- Huzaira, M.; Rius, F.; Rajadhyaksha, M.; Anderson, R.R.; González, S. Topographic variations in normal skin, as viewed by in vivo reflectance confocal microscopy. J. Investig. Dermatol. 2001, 116, 846–852. [Google Scholar] [CrossRef] [Green Version]
- Sauermann, K.; Clemann, S.; Jaspers, S.; Gambichler, T.; Altmeyer, P.; Hoffmann, K.; Ennen, J. Age related changes of human skin investigated with histometric measurements by confocal laser scanning microscopy in vivo. Skin Res. Technol. 2002, 8, 52–56. [Google Scholar] [CrossRef]
- Timár, F.; Soós, G.; Szende, B.; Horváth, A. Interdigitation index–a parameter for differentiating between young and older skin specimens. Skin Res. Technol. 2000, 6, 17–20. [Google Scholar] [CrossRef]
- Liao, Y.H.; Kuo, W.C.; Chou, S.Y.; Tsai, C.S.; Lin, G.L.; Tsai, M.R.; Shih, Y.T.; Lee, G.G.; Sun, C.K. Quantitative analysis of intrinsic skin aging in dermal papillae by in vivo harmonic generation microscopy. Biomed. Opt. Express 2014, 5, 3266–3279. [Google Scholar] [CrossRef] [Green Version]
- Haytoglu, N.S.; Gurel, M.S.; Erdemir, A.; Falay, T.; Dolgun, A.; Haytoglu, T.G. Assessment of skin photoaging with reflectance confocal microscopy. Skin Res. Technol. 2014, 20, 363–372. [Google Scholar] [CrossRef]
- Lavker, R.M. Structural alterations in exposed and unexposed aged skin. J. Investig. Dermatol. 1979, 73, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farage, M.A.; Miller, K.W.; Berardesca, E.; Maibach, H.I. Clinical implications of aging skin: Cutaneous disorders in the elderly. Am. J. Clin. Dermatol. 2009, 10, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Zoubouliss, C.C.; Makrantonaki, E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011, 29, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A. Age-associated changes in the skin. J. Am. Geriatr. Soc. 1982, 30, 139–143. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [Green Version]
- Marcos-Garcés, V.; Molina Aguilar, P.; Bea Serrano, C.; García Bustos, V.; Benavent Seguí, J.; Ferrández Izquierdo, A.; Ruiz-Saurí, A. Age-related dermal collagen changes during development, maturation and ageing–a morphometric and comparative study. J. Anat. 2014, 225, 98–108. [Google Scholar] [CrossRef]
- Mine, S.; Fortunel, N.O.; Pageon, H.; Asselineau, D. Aging alters functionally human dermal papillary fibroblasts but not reticular fibroblasts: A new view of skin morphogenesis and aging. PLoS ONE 2008, 3, e4066. [Google Scholar] [CrossRef] [Green Version]
- Pageon, H.; Zucchi, H.; Asselineau, D. Distinct and complementary roles of papillary and reticular fibroblasts in skin morphogenesis and homeostasis. Eur. J. Dermatol. EJD 2012, 22, 324–332. [Google Scholar] [CrossRef]
- Janson, D.G.; Saintigny, G.; van Adrichem, A.; Mahé, C.; El Ghalbzouri, A. Different gene expression patterns in human papillary and reticular fibroblasts. J. Investig. Dermatol. 2012, 132, 2565–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haydont, V.; Bernard, B.A.; Fortunel, N.O. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Graham, H.K.; Hodson, N.W.; Hoyland, J.A.; Millward-Sadler, S.J.; Garrod, D.; Scothern, A.; Griffiths, C.E.; Watson, R.E.; Cox, T.R.; Erler, J.T.; et al. Tissue section AFM: In situ ultrastructural imaging of native biomolecules. Matrix Biol. 2010, 29, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Wurm, E.; Longo, C.; Curchin, C.; Soyer, H.; Prow, T.W.; Pellacani, G. In vivo assessment of chronological ageing and photoageing in forearm skin using reflectance confocal microscopy. Br. J. Dermatol. 2012, 167, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Longo, C.; Casari, A.; Beretti, F.; Cesinaro, A.M.; Pellacani, G. Skin aging: In vivo microscopic assessment of epidermal and dermal changes by means of confocal microscopy. J. Am. Acad. Dermatol. 2013, 68, e73–e82. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.A.L.M.; Suppa, M.; Marneffe, A.; Miyamoto, M.; Jemec, G.B.E.; Del Marmol, V. High-definition optical coherence tomography intrinsic skin ageing assessment in women: A pilot study. Arch. Dermatol. Res. 2015, 307, 705–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Runel, G.; Cario, M.; Lopez-Ramirez, N.; Malbouyres, M.; Ruggiero, F.; Bernard, L.; Puisieux, A.; Caramel, J.; Chlasta, J.; Masse, I. Stiffness measurement is a biomarker of skin ageing in vivo. Exp. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, P.; Oh, J.; Wlaschek, M.; Wenk, J.; Briviba, K.; Hommel, C.; Herrmann, G.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts. Photochem. Photobiol. 1996, 64, 877–885. [Google Scholar] [CrossRef]
- Sherratt, M.J.; Bayley, C.P.; Reilly, S.M.; Gibbs, N.K.; Griffiths, C.E.M.; Watson, R.E.B. Low-dose ultraviolet radiation selectively degrades chromophore-rich extracellular matrix components. J. Pathol. 2010, 222, 32–40. [Google Scholar] [CrossRef]
- Hibbert, S.A.; Watson, R.E.B.; Griffiths, C.E.M.; Gibbs, N.K.; Sherratt, M.J. Selective proteolysis by matrix metalloproteinases of photo-oxidised dermal extracellular matrix proteins. Cell. Signal. 2019, 54, 191–199. [Google Scholar] [CrossRef]
- Vázquez, F.; Palacios, S.; Alemañ, N.; Guerrero, F. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas 1996, 25, 209–215. [Google Scholar] [CrossRef]
- Le Varlet, B.; Chaudagne, C.; Saunois, A.; Barré, P.; Sauvage, C.; Berthouloux, B.; Meybeck, A.; Dumas, M.; Bonté, F. Age-related functional and structural changes in human dermo-epidermal junction components. J. Investig. Dermatol. Symp. Proc. 1998, 3, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Langton, A.K.; Halai, P.; Griffiths, C.E.; Sherratt, M.J.; Watson, R.E. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech. Ageing Dev. 2016, 156, 14–16. [Google Scholar] [CrossRef]
- Feru, J.; Delobbe, E.; Ramont, L.; Brassart, B.; Terryn, C.; Dupont-Deshorgue, A.; Garbar, C.; Monboisse, J.-C.; Maquart, F.-X.; Brassart-Pasco, S. Aging decreases collagen IV expression in vivo in the dermo-epidermal junction and in vitro in dermal fibroblasts: Possible involvement of TGF-β1. Eur. J. Dermatol. EJD 2016, 26, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, Y.K.; Jung, J.Y.; Shin, J.E.; Chung, J. Changes in glycosaminoglycans and related proteoglycans in intrinsically aged human skin in vivo. Exp. Dermatol. 2011, 20, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Matsumura, H.; Kato, T.; Ichinose, S.; Takada, A.; Namiki, T.; Asakawa, K.; Morinaga, H.; Mohri, Y.; De Arcangelis, A.; et al. Stem cell competition orchestrates skin homeostasis and ageing. Nature 2019, 568, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Natsuga, K.; Nishie, W.; Kobayashi, Y.; Donati, G.; Suzuki, S.; Fujimura, Y.; Tsukiyama, T.; Ujiie, H.; Shinkuma, S.; et al. Type XVII collagen coordinates proliferation in the interfollicular epidermis. eLife 2017, 6, e26635. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Obara, K.; Tohgi, N.; Yamazaki, A.; Aki, R.; Hamada, Y.; Arakawa, N.; Singh, S.R.; Hoffman, R.M.; Amoh, Y. Expression of anti-aging type-XVII collagen (COL17A1/BP180) in hair follicle-associated pluripotent (HAP) stem cells during differentiation. Tissue Cell 2019, 59, 33–38. [Google Scholar] [CrossRef]
- Matsumura, H.; Mohri, Y.; Binh, N.T.; Morinaga, H.; Fukuda, M.; Ito, M.; Kurata, S.; Hoeijmakers, J.; Nishimura, E.K. Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 2016, 351, 559–613. [Google Scholar] [CrossRef]
- Sbardella, D.; Fasciglione, G.F.; Gioia, M.; Ciaccio, C.; Tundo, G.R.; Marini, S.; Coletta, M. Human matrix metalloproteinases: An ubiquitarian class of enzymes involved in several pathological processes. Mol. Asp. Med. 2012, 33, 119–208. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Q.; Mauviel, A.; Ryynänen, J.; Sollberg, S.; Uitto, J. Type VII collagen gene expression by human skin fibroblasts and keratinocytes in culture: Influence of donor age and cytokine responses. J. Investig. Dermatol. 1994, 102, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Craven, N.M.; Watson, R.E.; Jones, C.J.; Shuttleworth, C.A.; Kielty, C.M.; Griffiths, C.E. Clinical features of photodamaged human skin are associated with a reduction in collagen VII. Br. J. Dermatol. 1997, 137, 344–350. [Google Scholar] [CrossRef]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, K.; Capell, B.C. The senescence-associated secretory phenotype: Critical effector in skin cancer and aging. J. Investig. Dermatol. 2016, 136, 2133–2139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.S.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Amano, S. Possible involvement of basement membrane damage in skin photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Q.; Nash, J.F. Pathology of Aging Skin. In Textbook of Aging Skin; Farage, M., Miller, K., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–23. [Google Scholar]
- Kurdykowski, S.; Mine, S.; Bardey, V.; Danoux, L.; Jeanmaire, C.; Pauly, G.; Brabencova, E.; Wegrowski, Y.; Maquart, F.-X. Ultraviolet-B irradiation induces epidermal up-regulation of heparanase expression and activity. J. Photochem. Photobiol. B Biol. 2012, 106, 107–112. [Google Scholar] [CrossRef]
- Phillip, J.M.; Aifuwa, I.; Walston, J.; Wirtz, D. The mechanobiology of aging. Annu. Rev. Biomed. Eng. 2015, 17, 113–141. [Google Scholar] [CrossRef] [Green Version]
- Théry, M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell Sci. 2010, 123, 4201–4213. [Google Scholar] [CrossRef] [Green Version]
- Bellas, E.; Chen, C.S. Forms, forces, and stem cell fate. Curr. Opin. Cell Biol. 2014, 31, 92–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, M.; Fannin, J.; Rice, K.M.; Wang, B.; Blough, E.R. Effect of aging on cellular mechanotransduction. Ageing Res. Rev. 2011, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Starodubtseva, M.N. Mechanical properties of cells and ageing. Ageing Res. Rev. 2011, 10, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Lulevich, V.; Yang, H.Y.; Isseroff, R.R.; Liu, G.Y. Single cell mechanics of keratinocyte cells. Ultramicroscopy 2010, 110, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Kobiela, T.; Stepulak, M.; Lekka, M.; Malejczyk, M.; Lelen-Kaminska, K.; Arct, J.; Majewski, S. The influence of surfactants and hydrolyzed proteins on keratinocytes viability and elasticity. Skin Res. Technol. 2013, 19, e200–e208. [Google Scholar] [CrossRef] [PubMed]
- Berdyyeva, T.K.; Woodworth, C.D.; Sokolov, I. Human epithelial cells increase their rigidity with ageing in vitro: Direct measurements. Phys. Med. Biol. 2005, 50, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokolov, I.; Iyer, S.; Woodworth, C.D. Recovery of elasticity of aged human epithelial cells in vitro. Nanomedicine 2006, 2, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Pageon, H.; Técher, M.P.; Asselineau, D. Reconstructed skin modified by glycation of the dermal equivalent as a model for skin aging and its potential use to evaluate anti-glycation molecules. Exp. Gerontol. 2008, 43, 584–588. [Google Scholar] [CrossRef]
- Bailey, A.J. Molecular mechanisms of ageing in connective tissues. Mech. Ageing Dev. 2001, 122, 735–755. [Google Scholar] [CrossRef]
- Verzijl, N.; DeGroot, J.; Thorpe, S.R.; Bank, R.A.; Shaw, J.N.; Lyons, T.J.; Bijlsma, J.W.; Lafeber, F.P.; Baynes, J.W.; TeKoppele, J.M. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000, 275, 39027–39031. [Google Scholar] [CrossRef] [Green Version]
- Charonis, A.S.; Tsilbary, E.C. Structural and functional changes of laminin and type IV collagen after nonenzymatic glycation. Diabetes 1992, 41, 49–51. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mott, J.D.; Khalifah, R.G.; Nagase, H.; Shield, C.F., 3rd; Hudson, J.K.; Hudson, B.G. Nonenzymatic glycation of type IV collagen and matrix metalloproteinase susceptibility. Kidney Int. 1997, 52, 1302–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sage, J.; De Quéral, D.; Leblanc-Noblesse, E.; Kurfürst, R.; Schnebert, S.; Perrier, E.; Nizard, C.; Lalmanach, G.; Lecaille, F. Differential expression of cathepsins K, S and V between young and aged Caucasian women skin epidermis. Matrix Biol. 2014, 33, 41–46. [Google Scholar] [CrossRef]
- Pageon, H.; Asselineau, D. An in vitro approach to the chronological aging of skin by glycation of the collagen: The biological effect of glycation on the reconstructed skin model. Ann. N.Y. Acad. Sci. 2005, 1043, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, E.; Kobayashi, T.; Fujimoto, N.; Akiyama, M.; Tajima, S.; Nagai, R. AGE-modified collagens I and III induce keratinocyte terminal differentiation through AGE receptor CD36: Epidermal-dermal interaction in acquired perforating dermatosis. J. Investig. Dermatol. 2010, 130, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Fleming, T.; Humpert, P.M.; Nawroth, P.; Bierhaus, A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process: A mini-review. Gerontologia 2011, 57, 435–443. [Google Scholar] [CrossRef]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef]
- Lohwasser, C.; Neureiter, D.; Weigle, B.; Kirchner, T.; Schuppan, D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J. Investig. Dermatol. 2006, 126, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Zhu, P.; Ren, M.; Yang, C.; Hu, Y.X.; Ran, J.M.; Yan, L. Involvement of RAGE, MAPK and NF-κB pathways in AGEs-induced MMP-9 activation in HaCaT keratinocytes. Exp. Dermatol. 2012, 21, 123–129. [Google Scholar] [CrossRef]
- Kaya, G.; Saurat, J.-H. Dermatoporosis: A chronic cutaneous insufficiency/fragility syndrome. Dermatology 2007, 215, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Sgonc, R.; Gruber, J. Age-related aspects of cutaneous wound healing: A mini-review. Gerontology 2013, 59, 159–164. [Google Scholar] [CrossRef] [PubMed]



| Age of Donors | Cohort | Anatomic Location | Mean Length (µm) | Technology Used | Reference |
|---|---|---|---|---|---|
| 3 months | 7 female, 8 male | Upper thigh | 50 (estimated) | in vivo Confocal Laser Scanning Microscopy | [29] |
| Ventral forearm | 35 (estimated) | ||||
| Buttock | 48 (estimated) | ||||
| 18–30 years | 6 female, 4 male Fitzpatrick skin phototype I-III | Forearm | 37.6 ± 6.9 | Immunostaining on paraffin section and imaging measurements | [28] |
| Buttock | 61.7 ± 13.2 | ||||
| 19–24 years | 8 female, 7 male Caucasian | Face–Temple | 30 ± 8 | in vivo Confocal Laser Scanning Microscopy | [30] |
| Forearm | 41 ± 8 | ||||
| 19–29 years | 5 female, 7 male Caucasian | Volar arm | 97.41 ± 25.93 | in vivo Harmonic Generation Microscopy | [31] |
| 21–33 years | 10 (mix) Caucasian | Abdomen | 60 (estimated) | Immunostaining on frozen sections and imaging measurements | [32] |
| 30–59 years | 5 female, 8 male Caucasian | Volar arm | 69.59 ± 23.96 | in vivo Harmonic Generation Microscopy | [31] |
| 40–65 years | 41 female French Fitzpatrick skin phototype II–IV | Forearm | 27.01 ± 12.73 | in vivo Reflectance Confocal Microscopy | [33] |
| Face | 13.66 ± 12.73 | ||||
| 40–65 years | 41 female Brazilian Fitzpatrick skin phototype II–IV | Forearm | 28.16 ± 8.23 | ||
| Face | 9.21 ± 12.41 | ||||
| 51–59 years | 7 (mix) Caucasian | Abdomen | 35 (estimated) | Immunostaining on frozen sections and imaging measurements | [32] |
| 54–57 years | 5 female, 10 male Caucasian | Face–Temple | 20 ± 6 | in vivo Confocal Laser Scanning Microscopy | [30] |
| Forearm | 25 ± 8 | ||||
| >60 years | 6 (mix) Caucasian | Abdomen | 15 (estimated) | Immunostaining on frozen sections and manual measurements | [32] |
| 60–79 years | 2 female, 4 male Caucasian | Volar arm | 58.97 ± 16.70 | in vivo Harmonic Generation Microscopy | [31] |
| >65 years | 6 female, 4 male Fitzpatrick skin phototype I-III | Forearm | 26.2 ± 4.5 | Immunostaining on paraffin sections and manual measurements | [28] |
| Buttock | 46.0 ± 15.3 | ||||
| 74–81 years | 209 participants Fitzpatrick skin phototype I–IV | Face | 5.98 ± 7.11 | in vivo Reflectance Confocal Microscopy | [34] |
| Forearm | 10.55 ± 8.01 | ||||
| Volar arm | 12.94 ± 5.42 | ||||
| 105 females Fitzpatrick skin phototype I–IV | Face | 6.41 ± 7.08 | |||
| Forearm | 10.00 ± 8.49 | ||||
| Volar arm | 12.37 ± 5.02 | ||||
| 104 males Fitzpatrick skin phototype I–IV | Face | 5.57 ± 7.13 | |||
| Forearm | 11.06 ± 7.54 | ||||
| Volar arm | 13.50 ± 5.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roig-Rosello, E.; Rousselle, P. The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters. Biomolecules 2020, 10, 1607. https://doi.org/10.3390/biom10121607
Roig-Rosello E, Rousselle P. The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters. Biomolecules. 2020; 10(12):1607. https://doi.org/10.3390/biom10121607
Chicago/Turabian StyleRoig-Rosello, Eva, and Patricia Rousselle. 2020. "The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters" Biomolecules 10, no. 12: 1607. https://doi.org/10.3390/biom10121607
APA StyleRoig-Rosello, E., & Rousselle, P. (2020). The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters. Biomolecules, 10(12), 1607. https://doi.org/10.3390/biom10121607