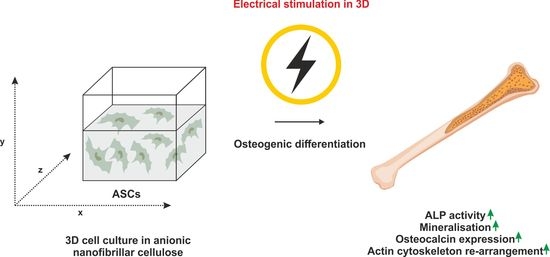
Electrical Stimulation of Adipose-Derived Stem Cells in 3D Nanofibrillar Cellulose Increases Their Osteogenic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. aNFC
2.2. Human ASCs
2.3. Cultivation of ASCs as 2D Monolayer
2.4. Osteogenic and Adipogenic Differentiation in 2D
2.5. ALP Activity in 2D
2.6. Alizarin Red S Staining in 2D
2.7. Oil Red O Staining in 2D
2.8. ES in 2D
2.9. Cultivation of ASCs in 3D aNFC Hydrogels
2.10. ES of ASCs in 3D
2.11. Osteogenic Differentiation, ALP Activity Assay, and Alizarin Red S Staining in 3D
2.12. Viability Assays
2.13. Immunocytochemistry
2.14. F-actin Staining
2.15. Statistical Analysis
3. Results
3.1. ES Increases Osteogenic Differentiation Potential of ASCs under 2D Cell Culture Conditions
3.2. ES Decreases Adipogenic Differentiation of ASCs under 2D Cell Culture Conditions
3.3. Long-Term Cultivation of ASCs in Anionic Nanofibrillar Cellulose Does Not Affect Their Viability under Standard and Osteogenic Differentiation Conditions
3.4. ES of ADSCs Embedded in 3D aNFC Hydrogel Only Moderately Decreases Their Viability under Standard and Osteogenic Conditions
3.5. ASCs Exposed to ES in 3D Show Increased ALP Activity and Higher Levels of Calcium Deposition during Osteogenic Differentiation
3.6. ES under Osteogenic Conditions in 3D Increases Expression of OCN But Does Not Affect OPN Level in ASCs
3.7. ASCs Exposed to ES Display Highly Arranged Actin Cytoskeleton and Formation of Cell-Free Pores within the 3D aNFC Hydrogel
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2011, 4, 61–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballane, G.; Cauley, J.A.; Luckey, M.M.; Fuleihan, G.E.-H. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos. Int. 2017, 28, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, C.; Williams, D.J. Safety of drugs used in the treatment of osteoporosis. Ther. Adv. Drug Saf. 2011, 2, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterling, J.A.; Guelcher, S.A. Biomaterial Scaffolds for Treating Osteoporotic Bone. Curr. Osteoporos. Rep. 2014, 12, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Pape, H.C.; Evans, A.; Kobbe, P. Autologous Bone Graft: Properties and Techniques. J. Orthop. Trauma 2010, 24, S36–S40. [Google Scholar] [CrossRef]
- Giannoudis, P.; Arts, J.J.; Schmidmaier, G.; Larsson, S. What should be the characteristics of the ideal bone graft substitute? Injury 2011, 42, S1–S2. [Google Scholar] [CrossRef]
- Myeroff, C.; Archdeacon, M. Autogenous Bone Graft: Donor Sites and Techniques. J. Bone Jt. Surg. Am. Vol. 2011, 93, 2227–2236. [Google Scholar] [CrossRef]
- O’Keefe, R.J.; Tuan, R.S.; Lane, N.E.; Awad, H.A.; Barry, F.; Bunnell, B.A.; Colnot, C.; Drake, M.T.; Drissi, H.; Dyment, N.A.; et al. American Society for Bone and Mineral Research-Orthopaedic Research Society Joint Task Force Report on Cell-Based Therapies. J. Bone Miner. Res. 2019, 35, 3–17. [Google Scholar] [CrossRef]
- Antebi, B.; Pelled, G.; Gazit, D. Stem Cell Therapy for Osteoporosis. Curr. Osteoporos. Rep. 2014, 12, 41–47. [Google Scholar] [CrossRef]
- Phetfong, J.; Sanvoranart, T.; Nartprayut, K.; Nimsanor, N.; Seenprachawong, K.; Prachayasittikul, V.; Supokawej, A. Osteoporosis: The current status of mesenchymal stem cell-based therapy. Cell. Mol. Biol. Lett. 2016, 21, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Saito, A.; Nagaishi, K.; Iba, K.; Mizuec, Y.; Chikenji, T.; Otani, M.; Nakano, M.; Oyama, K.; Yamashita, T.; Fujimiya, M. Umbilical cord extracts improve osteoporotic abnormalities of bone marrow-derived mesenchymal stem cells and promote their therapeutic effects on ovariectomised rats. Sci. Rep. 2018, 8, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Zhao, B.; Li, C.; Rong, J.-S.; Tao, S.-Q.; Tao, T.-Z. Decreased proliferation ability and differentiation potential of mesenchymal stem cells of osteoporosis rat. Asian Pac. J. Trop. Med. 2014, 7, 358–363. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Xu, X.; Tong, Z.; Lin, J.; Yu, Q.; Lin, Y.; Kuang, W. Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: A possible mechanism of age related osteoporosis. Bone Res. 2015, 3, 15003. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.-X.; Sui, B.-D.; Liu, N.; Hu, C.-H.; He, T.; Zhang, X.-Y.; Zhao, P.; Chen, J.; Xuan, K.; Jin, Y. Adipose mesenchymal stem cells from osteoporotic donors preserve functionality and modulate systemic inflammatory microenvironment in osteoporotic cytotherapy. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Greiner, J.F.W.; Gottschalk, M.; Fokin, N.; Büker, B.; Kaltschmidt, B.P.; Dreyer, A.; Vordemvenne, T.; Kaltschmidt, C.; Hütten, A.; Kaltschmidt, B. Natural and synthetic nanopores directing osteogenic differentiation of human stem cells. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 319–328. [Google Scholar] [CrossRef]
- Vordemvenne, T.; Wähnert, D.; Koettnitz, J.; Merten, M.; Fokin, N.; Becker, A.; Büker, B.; Vogel, A.; Kronenberg, D.; Stange, R.; et al. Bone Regeneration: A Novel Osteoinductive Function of Spongostan by the Interplay between Its Nano- and Microtopography. Cells 2020, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Olivares-Navarrete, R.; Lee, E.M.; Smith, K.; Hyzy, S.L.; Doroudi, M.; Williams, J.K.; Gall, K.; Boyan, B.; Schwartz, Z. Substrate Stiffness Controls Osteoblastic and Chondrocytic Differentiation of Mesenchymal Stem Cells without Exogenous Stimuli. PLoS ONE 2017, 12, e0170312. [Google Scholar] [CrossRef]
- Yourek, G.; McCormick, S.M.; Mao, J.J.; Reilly, G.C. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regen. Med. 2010, 5, 713–724. [Google Scholar] [CrossRef] [Green Version]
- Tsimbouri, P.M.; Childs, P.G.; Pemberton, G.D.; Yang, J.; Jayawarna, V.; Orapiriyakul, W.; Burgess, K.; González-García, C.; Blackburn, G.; Thomas, D.; et al. Stimulation of 3D osteogenesis by mesenchymal stem cells using a nanovibrational bioreactor. Nat. Biomed. Eng. 2017, 1, 758–770. [Google Scholar] [CrossRef] [Green Version]
- Orapiriyakul, W.; Tsimbouri, M.P.; Childs, P.; Campsie, P.; Wells, J.; Fernandez-Yague, M.A.; Burgess, K.; Tanner, K.E.; Tassieri, M.; Meek, D.; et al. Nanovibrational Stimulation of Mesenchymal Stem Cells Induces Therapeutic Reactive Oxygen Species and Inflammation for Three-Dimensional Bone Tissue Engineering. ACS Nano 2020, 14, 10027–10044. [Google Scholar] [CrossRef]
- Eischen-Loges, M.; Oliveira, K.M.C.; Bhavsar, M.; Barker, J.H.; Leppik, L. Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ 2018, 6, e4959. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppik, L.; Oliveira, K.M.C.; Bhavsar, M.; Barker, J.H. Electrical stimulation in bone tissue engineering treatments. Eur. J. Trauma Emerg. Surg. 2020, 46, 231–244. [Google Scholar] [CrossRef] [Green Version]
- Sundelacruz, S.; Levin, M.; Kaplan, D.L. Membrane Potential Controls Adipogenic and Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2008, 3, e3737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundelacruz, S.; Moody, A.T.; Levin, M.; Kaplan, D.L. Membrane Potential Depolarization Alters Calcium Flux and Phosphate Signaling During Osteogenic Differentiation of Human Mesenchymal Stem Cells. Bioelectricity 2019, 1, 56–66. [Google Scholar] [CrossRef]
- Jing, D.; Li, F.; Jiang, M.; Cai, J.; Wu, Y.; Xie, K.; Wu, X.; Tang, C.; Liu, J.; Guo, W.; et al. Pulsed Electromagnetic Fields Improve Bone Microstructure and Strength in Ovariectomized Rats through a Wnt/Lrp5/β-Catenin Signaling-Associated Mechanism. PLoS ONE 2013, 8, e79377. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Liu, Y.; Lipsky, S.; Cho†, M. Physical manipulation of calcium oscillations facilitates osteodifferentiation of human mesenchymal stem cells. FASEB J. 2007, 21, 1472–1480. [Google Scholar] [CrossRef] [Green Version]
- Mobini, S.; Talts, Ü.-L.; Xue, R.; Cassidy, N.J.; Cartmell, S.H. Electrical Stimulation Changes Human Mesenchymal Stem Cells Orientation and Cytoskeleton Organization. J. Biomater. Tissue Eng. 2017, 7, 829–833. [Google Scholar] [CrossRef] [Green Version]
- Leppik, L.; Han, Z.; Mobini, S.; Parameswaran, V.T.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.C.; Bhavsar, M.B.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lee, G.S.; Chun, H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 2016, 6, 39302. [Google Scholar] [CrossRef]
- Ben-David, U.; Mayshar, Y.; Benvenisty, N. Large-Scale Analysis Reveals Acquisition of Lineage-Specific Chromosomal Aberrations in Human Adult Stem Cells. Cell Stem Cell 2011, 9, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bara, J.J.; Richards, R.G.; Alini, M.; Stoddart, M.J. Concise Review: Bone Marrow-Derived Mesenchymal Stem Cells Change Phenotype Following In Vitro Culture: Implications for Basic Research and the Clinic. Stem Cells 2014, 32, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Xu, N.; Xu, D.; Dong, M.; Shi, X.; Wang, Y.; Hao, Z.; Zhu, S.; Zhao, D.; Jin, H.; et al. Comparison of senescence-related changes between three- and two-dimensional cultured adipose-derived mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Edreira, E.R.U.; Hayrapetyan, A.; Wolke, J.G.C.; Croes, H.J.E.; Klymov, A.; Jansen, J.A.; Beucken, J.V.D. Effect of calcium phosphate ceramic substrate geometry on mesenchymal stromal cell organization and osteogenic differentiation. Biofabrication 2016, 8, 025006. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Nicholson, L.; Al Harbi, N.; Mancuso, E.; Jones, E.; Dickinson, A.M.; Wang, X.N.; Dalgarno, K. Osteogenic potential of heterogeneous and CD271-enriched mesenchymal stromal cells cultured on apatite-wollastonite 3D scaffolds. BMC Biomed. Eng. 2019, 1, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Guarino, V.; Scaglione, S.; Sandri, M.; Alvarez-Perez, M.A.; Tampieri, A.; Quarto, R.; Ambrosio, L. MgCHA particles dispersion in porous PCL scaffolds: In vitromineralization andin vivobone formation. J. Tissue Eng. Regen. Med. 2014, 8, 291–303. [Google Scholar] [CrossRef]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.; Vissers, C.B.; Leach, J.K. Increased Survival and Function of Mesenchymal Stem Cell Spheroids Entrapped in Instructive Alginate Hydrogels. Stem Cells Transl. Med. 2016, 5, 773–781. [Google Scholar] [CrossRef]
- Lund, A.; Stegemann, J.; Plopper, G. Mesenchymal Stem Cells Sense Three Dimensional Type I Collagen through Discoidin Domain Receptor. Open Stem Cell J. 2009, 1, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Ohno, J.; Sato, A.; Kido, H.; Fukushima, T. Mesenchymal stem cell spheroids exhibit enhanced in-vitro and in-vivo osteoregenerative potential. BMC Biotechnol. 2014, 14, 105. [Google Scholar] [CrossRef] [Green Version]
- Favi, P.M.; Benson, R.S.; Neilsen, N.R.; Hammonds, R.L.; Bates, C.C.; Stephens, C.P.; Dhar, M.S. Cell proliferation, viability, and in vitro differentiation of equine mesenchymal stem cells seeded on bacterial cellulose hydrogel scaffolds. Mater. Sci. Eng. C 2013, 33, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Grad, S.; Stoddart, M.J.; Farè, S.; Altomare, L.; Azzimonti, B.; Alini, M.; Rimondini, L. Bioreactor mechanically guided 3D mesenchymal stem cell chondrogenesis using a biocompatible novel thermo-reversible methylcellulose-based hydrogel. Sci. Rep. 2017, 7, srep45018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azoidis, I.; Metcalfe, J.; Reynolds, J.; Keeton, S.; Hakki, S.S.; Sheard, J.; Widera, D. Three-dimensional cell culture of human mesenchymal stem cells in nanofibrillar cellulose hydrogels. MRS Commun. 2017, 7, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Sheard, J.J.; Bicer, M.; Meng, Y.; Frigo, A.; Aguilar, R.M.; Vallance, T.M.; Iandolo, D.; Widera, D. Optically Transparent Anionic Nanofibrillar Cellulose Is Cytocompatible with Human Adipose Tissue-Derived Stem Cells and Allows Simple Imaging in 3D. Stem Cells Int. 2019, 2019, 3106929. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Zeuner, M.-T.; Didenko, N.N.; Humphries, D.; Stergiadis, S.; Morash, T.M.; Patel, K.; Grimm, W.-D.; Widera, D. Isolation and Characterization of Neural Crest-Derived Stem Cells From Adult Ovine Palatal Tissue. Front. Cell Dev. Biol. 2018, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.; Widera, D.; Qunneis, F.; Müller, J.; Zander, C.; Greiner, J.; Strauss, C.; Lüningschrör, P.; Heimann, P.; Schwarze, H.; et al. Isolation of Novel Multipotent Neural Crest-Derived Stem Cells from Adult Human Inferior Turbinate. Stem Cells Dev. 2012, 21, 742–756. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Paudyal, A.; Dewan, S.; Ikie, C.; Whalley, B.J.; De Tombe, P.P.; Boateng, S.Y. Nuclear accumulation of myocyte muscle LIM protein is regulated by heme oxygenase 1 and correlates with cardiac function in the transition to failure. J. Physiol. 2016, 594, 3287–3305. [Google Scholar] [CrossRef]
- Schürmann, M.; Wolff, A.; Widera, D.; Hauser, S.; Heimann, P.; Hütten, A.; Kaltschmidt, C.; Kaltschmidt, B. Interaction of adult human neural crest-derived stem cells with a nanoporous titanium surface is sufficient to induce their osteogenic differentiation. Stem Cell Res. 2014, 13, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Titushkin, I.; Cho, M. Modulation of Cellular Mechanics during Osteogenic Differentiation of Human Mesenchymal Stem Cells. Biophys. J. 2007, 93, 3693–3702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.U.; Qu, R.; Fan, T.; Ouyang, J.; Dai, J. A glance on the role of actin in osteogenic and adipogenic differentiation of mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Neoh, K.G.; Kang, E.-T. Electrical stimulation of adipose-derived mesenchymal stem cells and endothelial cells co-cultured in a conductive scaffold for potential orthopaedic applications. J. Tissue Eng. Regen. Med. 2018, 12, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.G.R.; Zerler, B.; Moran, E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl. Acad. Sci. USA 2000, 97, 8352–8357. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Bule, M.L.; Martinez-Botas, J.; Trillo, M.A.; Paino, C.L.; Ubeda, A. Antiadipogenic effects of subthermal electric stimulation at 448 kHz on differentiating human mesenchymal stem cells. Mol. Med. Rep. 2016, 13, 3895–3903. [Google Scholar] [CrossRef] [Green Version]
- Jing, H.; Liao, L.; An, Y.; Su, X.; Liu, S.; Shuai, Y.; Zhang, X.; Jin, Y. Suppression of EZH2 Prevents the Shift of Osteoporotic MSC Fate to Adipocyte and Enhances Bone Formation During Osteoporosis. Mol. Ther. 2016, 24, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.-S.; Zhou, H.-B.; Zhang, H.; Chen, B.; Liu, Z.-P.; Yuan, Y.; Zhou, X.-Z.; Xu, Y. The GDF11-FTO-PPARγ axis controls the shift of osteoporotic MSC fate to adipocyte and inhibits bone formation during osteoporosis. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2018, 1864, 3644–3654. [Google Scholar] [CrossRef]
- Koivuniemi, R.; Hakkarainen, T.; Kiiskinen, J.; Kosonen, M.; Vuola, J.; Valtonen, J.; Luukko, K.; Kavola, H.; Yliperttula, M. Clinical Study of Nanofibrillar Cellulose Hydrogel Dressing for Skin Graft Donor Site Treatment. Adv. Wound Care 2020, 9, 199–210. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Kaltschmidt, C.; Widera, D. Adult Craniofacial Stem Cells: Sources and Relation to the Neural Crest. Stem Cell Rev. Rep. 2011, 8, 658–671. [Google Scholar] [CrossRef]
- Iandolo, D.; Sheard, J.; Levy, G.K.; Pitsalidis, C.; Tan, E.; Dennis, A.; Kim, J.-S.; Markaki, A.E.; Widera, D.; Owens, R.M. Biomimetic and electroactive 3D scaffolds for human neural crest-derived stem cell expansion and osteogenic differentiation. MRS Commun. 2020, 10, 179–187. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bicer, M.; Sheard, J.; Iandolo, D.; Boateng, S.Y.; Cottrell, G.S.; Widera, D. Electrical Stimulation of Adipose-Derived Stem Cells in 3D Nanofibrillar Cellulose Increases Their Osteogenic Potential. Biomolecules 2020, 10, 1696. https://doi.org/10.3390/biom10121696
Bicer M, Sheard J, Iandolo D, Boateng SY, Cottrell GS, Widera D. Electrical Stimulation of Adipose-Derived Stem Cells in 3D Nanofibrillar Cellulose Increases Their Osteogenic Potential. Biomolecules. 2020; 10(12):1696. https://doi.org/10.3390/biom10121696
Chicago/Turabian StyleBicer, Mesude, Jonathan Sheard, Donata Iandolo, Samuel Y. Boateng, Graeme S. Cottrell, and Darius Widera. 2020. "Electrical Stimulation of Adipose-Derived Stem Cells in 3D Nanofibrillar Cellulose Increases Their Osteogenic Potential" Biomolecules 10, no. 12: 1696. https://doi.org/10.3390/biom10121696
APA StyleBicer, M., Sheard, J., Iandolo, D., Boateng, S. Y., Cottrell, G. S., & Widera, D. (2020). Electrical Stimulation of Adipose-Derived Stem Cells in 3D Nanofibrillar Cellulose Increases Their Osteogenic Potential. Biomolecules, 10(12), 1696. https://doi.org/10.3390/biom10121696