Implication of Opioid Receptors in the Antihypertensive Effect of a Novel Chicken Foot-Derived Peptide
Abstract
:1. Introduction
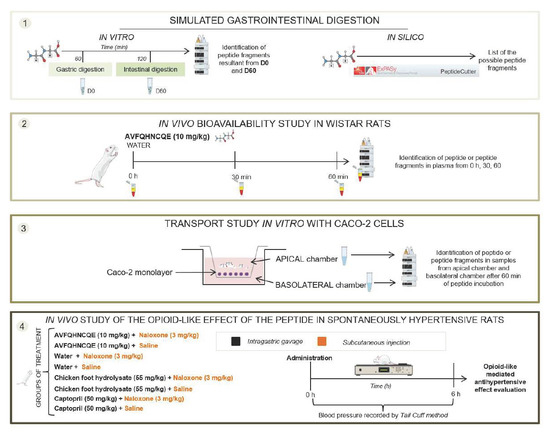
2. Materials and Methods
2.1. Materials
2.2. In Silico and In Vitro Simulated Digestion
2.3. Peptide Bioavailability Studies
2.3.1. In Vivo Experiment
2.3.2. In Vitro Experiment
2.4. Peptide Analysis by UHPLC-MS/MS and UHPLC-HRMS
2.4.1. Optimization of Peptide Extraction from Plasma
2.4.2. Peptide Extractions from In Vitro Digestions and Caco-2 Monolayers
2.4.3. Analysis by UHPLC-MS/MS and UHPLC-HRMS
2.5. Evaluation of the Opioid-Like Activity of AVFQHNCQE in SHR
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoshikawa, M. Bioactive peptides derived from natural proteins with respect to diversity of their receptors and physiological effects. Peptides 2015, 72, 208–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Singh, R.; Rana, S. Bioactive peptides: A review. Int. J. Bioautomation 2011, 15, 223–250. [Google Scholar]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–49. [Google Scholar] [CrossRef]
- Hong, F.; Ming, L.; Yi, S.; Zhanxia, L.; Yongquan, W.; Chi, L. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides 2008, 29, 1062–1071. [Google Scholar] [CrossRef]
- Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Natural angiotensin converting enzyme (ACE) inhibitors with antihypetensive properties. In Natural Products Targeting Clinically Relevant Enzymes; Andrade, P., Valentao, P., Pereira, D.M., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2017; pp. 45–67. [Google Scholar]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cífková, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Prim. 2018, 4, 18014. [Google Scholar] [CrossRef] [Green Version]
- Sica, D.A. Angiotensin-converting enzyme inhibitors side effects—physiologic and non-physiologic considerations. J. Clin. Hypertens. 2004, 6, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Mohan, A. Mechanisms of food protein-derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Barba de la Rosa, A.P.; Barba Montoya, A.; Martínez-Cuevas, P.; Hernández-Ledesma, B.; León-Galván, M.F.; De León-Rodríguez, A.; González, C. Tryptic amaranth glutelin digests induce endothelial nitric oxide production through inhibition of ACE: Antihypertensive role of amaranth peptides. Nitric Oxide Biol. Chem. 2010, 23, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Wu, J. Bioactive peptides on endothelial function. Food Sci. Hum. Wellness 2016, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Vermeirssen, V.; Camp, J.V.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357. [Google Scholar] [CrossRef]
- Miguel, M.; Dávalos, A.; Manso, M.A.; Peæa, G.D.; Lasunción, M.A.; López-fandiæo, R. Transepithelial transport across Caco-2 cell monolayers of antihypertensive egg-derived peptides. PepT1-mediated flux of Tyr-Pro-Ile. Mol. Nutr. Food Res. 2008, 52, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Del Mar Contreras, M.; Recio, I. Antihypertensive peptides: Production, bioavailability and incorporation into foods. Adv. Colloid Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Rivera, L.; Ares, I.; Miralles, B.; Gómez-Ruiz, J.Á.; Recio, I.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Bioavailability and kinetics of the antihypertensive casein-derived peptide HLPLP in rats. J. Agric. Food Chem. 2014, 62, 11869–11875. [Google Scholar] [CrossRef] [PubMed]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are intact peptides absorbed from the healthy gut in the adult human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef] [Green Version]
- Holzer, P. Opioid receptors in the gastrointestinal tract. Regul. Pept. 2009, 155, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Moughan, P.J.; Rutherfurd, S.M.; Montoya, C.A.; Dave, L.A. Food-derived bioactive peptides—A new paradigm. Nutr. Res. Rev. 2014, 27, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Pihlanto-leppa, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2001, 11, 347–356. [Google Scholar] [CrossRef]
- Nurminen, M.L.; Sipola, M.; Kaarto, H.; Pihlanto-Leppälä, A.; Piilola, K.; Korpela, R.; Tossavainen, O.; Korhonen, H.; Vapaatalo, H. Alpha-lactorphin lowers blood pressure measured by radiotelemetry in normotensive and spontaneously hypertensive rats. Life Sci. 2000, 66, 1535–1543. [Google Scholar] [CrossRef]
- Levin, E.R.; Mills, S.; Weber, M.A. Endogenous opioids and opiate antagonists modulate the blood pressure of the spontaneously hypertensive rat. Peptides 1986, 7, 977–981. [Google Scholar] [CrossRef]
- Sipola, M.; Finckenberg, P.; Vapaatalo, H.; Pihlanto-Leppälä, A.; Korhonen, H.; Korpela, R.; Nurminen, M.L. Alpha-lactorphin and beta-lactorphin improve arterial function in spontaneously hypertensive rats. Life Sci. 2002, 71, 1245–1253. [Google Scholar] [CrossRef]
- Bravo, F.I.; Arola, L.; Muguerza, B. Procedure for Obtaining a Hydrolyzate Claw Chicken Leg with Antihypertensive Activity, and Peptides Obtained Hydrolyzate Containing. Spain Patent ES2606954A1, 18 December 2017. [Google Scholar]
- Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Aragonès, G.; Aleixandre, A.; Bravo, F.I.; Muguerza, B. Evidence that Nitric Oxide is Involved in the Blood Pressure Lowering Effect of the Peptide AVFQHNCQE in Spontaneously Hypertensive Rats. Nutrients 2019, 11, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas-Capdevila, A.; Pons, Z.; Aleixandre, A.; Bravo, F.I.; Muguerza, B. Dose-Related Antihypertensive Properties and the Corresponding Mechanisms of a Chicken Foot Hydrolysate in Hypertensive Rats. Nutrients 2018, 10, 1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Fandiño, R.; Martos, G.; Contreras, P.; Molina, E. Egg white ovalbumin digestion mimicking physiological conditions. J. Agric. Food Chem. 2010, 58, 5640–5648. [Google Scholar]
- Buñag, R.D.; Butterfield, J. Tail-cuff blood pressure measurement without external preheating in awake rats. Hypertension 1982, 4, 898–903. [Google Scholar] [CrossRef] [Green Version]
- Aluko, R.E. Antihypertensive Peptides from Food Proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- Li, G.H.; Le, G.W.; Shi, Y.H.; Shrestha, S. Angiotensin I—Converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I.; Hernández-Ledesma, B. Antihypertensive peptides from food proteins: A review. Food Funct. 2012, 3, 350–361. [Google Scholar] [CrossRef]
- Li, M.; Zhou, L.; Ma, G.; Cao, S.; Dong, S. The cardiovascular effects of a chimeric opioid peptide based on morphiceptin and PFRTic-NH2. Peptides 2013, 39, 89–94. [Google Scholar] [CrossRef]
- Ijäs, H.; Collin, M.; Finckenberg, P.; Pihlanto-Leppälä, A.; Korhonen, H.; Korpela, R.; Vapaatalo, H.; Nurminen, M.L. Antihypertensive opioid-like milk peptide α-lactorphin: Lack of effect on behavioural tests in mice. Int. Dairy J. 2004, 14, 201–205. [Google Scholar] [CrossRef]
- Reid, J.L.; Rubin, P.C.; Petty, M.A. Opioid Peptides and Central Control of Blood Pressure. Clin. Exp. Hypertens. 1984, 6, 107–120. [Google Scholar] [CrossRef]
- Toda, N.; Kishioka, S.; Hatano, Y.; Toda, H. Modulation of Opioid Actions by Nitric Oxide Signaling. Anesthesiology 2009, 110, 166–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Atkinson, A.B.; Robertson, J.I.S. Captopril in the Treatment of Clinical Hypertension and Cardiac Failure. Lancet 1979, 314, 836–839. [Google Scholar] [CrossRef]
- Da, A.; Lasuncio, M.A.; Quiro, A. Bioavailability of the antihypertensive peptide LHLPLP: Transepithelial flux of HLPLP. Int. Dairy J. 2008, 18, 279–286. [Google Scholar]



| Original Sequence | Enzyme | Digestion Stage | Final Sequence |
|---|---|---|---|
| AVFQHNCQE | Pepsin | Gastric | VFQHNCQE |
| AVFQHNCQE | Pepsin | Gastric | AV |
| AVFQHNCQE | Pepsin | Gastric | FQHNCQE |
| VFQHNCQE | Chymotrypsin | Duodenal | VF |
| VFQHNCQE | Chymotrypsin | Duodenal | QHNCQE |
| QHNCQE | Chymotrypsin | Duodenal | VFQH |
| QHNCQE | Chymotrypsin | Duodenal | NCQE |
| VFQHNCQE | Chymotrypsin | Duodenal | QHNCQE |
| VFQHNCQE | Chymotrypsin | Duodenal | FQHNCQE |
| FQHNCQE | Chymotrypsin | Duodenal | QHNCQE |
| FQHNCQE | Chymotrypsin | Duodenal | FQH |
| FQHNCQE | Chymotrypsin | Duodenal | NCQE |
| FQHNCQE | Chymotrypsin | Duodenal | QH |
| FQHNCQE | Chymotrypsin | Duodenal | NCQE |
| Peptide Sequence a | m/zb | Mass Monoisotopic (Da) | RT c | Charge | Area G60 d | Area D60 e |
|---|---|---|---|---|---|---|
| AVFQHNCQE | 538.233 | 1074.451 | 4.86 | 2 | 5,236,186 | 13,652 |
| AVFQHNCQE(S-S)AVFQHNCQE | 537.729 | 2146.887 | 5.24 | 4 | 605,812 | - |
| AVF | 336.191 | 335.183 | 5.83 | 1 | 200,712 | - |
| AVFQHNC | 409.682 | 817.350 | 4.72 | 2 | 220,051 | - |
| AVFQHN | 358.179 | 714.343 | 4.24 | 2 | 685,482 | - |
| AVFQHNCQE(S-S)QHNCQE | 458.436 | 1829.715 | 4.11 | 4 | 136,056 | - |
| AVFQH | 301.157 | 600.300 | 4.34 | 2 | 155,533 | - |
| AVFQHNCQE(S-S)CQE | 484.526 | 1450.554 | 4.25 | 3 | 93,694 | - |
| QHNCQE | 379.647 | 757.278 | 1.23 | 2 | 288,536 | 335,871 |
| NCQE | 493.169 | 492.161 | 1.27 | 1 | - | 57,009 |
| Peptide Sequences a | m/zb | Mass Monoisotopic (Da) | RT c | Charge | G60 % Transport d |
|---|---|---|---|---|---|
| AVFQHNCQE | 538.233 | 1074.451 | 4.86 | 2 | 0.25 |
| AVFQHNCQE(S-S)AVFQHNCQE | 537.729 | 2146.887 | 5.24 | 4 | 0.11 |
| AVF | 336.191 | 335.183 | 5.83 | 1 | 0.15 |
| AVFQHNC | 409.682 | 817.350 | 4.72 | 2 | 0.60 |
| AVFQHN | 358.179 | 714.343 | 4.24 | 2 | 0.10 |
| AVFQHNCQE(S-S)QHNCQE | 458.436 | 1829.715 | 4.11 | 4 | 0.15 |
| AVFQH | 301.157 | 600.300 | 4.34 | 2 | 0.17 |
| AVFQHNCQE(S-S)CQE | 484.526 | 1450.554 | 4.25 | 3 | 0.15 |
| QHNCQE | 379.647 | 757.278 | 1.23 | 2 | N.D |
| NCQE | 493.169 | 492.161 | 1.27 | 1 | N.D |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mas-Capdevila, A.; Iglesias-Carres, L.; Arola-Arnal, A.; Aragonès, G.; Muguerza, B.; Bravo, F.I. Implication of Opioid Receptors in the Antihypertensive Effect of a Novel Chicken Foot-Derived Peptide. Biomolecules 2020, 10, 992. https://doi.org/10.3390/biom10070992
Mas-Capdevila A, Iglesias-Carres L, Arola-Arnal A, Aragonès G, Muguerza B, Bravo FI. Implication of Opioid Receptors in the Antihypertensive Effect of a Novel Chicken Foot-Derived Peptide. Biomolecules. 2020; 10(7):992. https://doi.org/10.3390/biom10070992
Chicago/Turabian StyleMas-Capdevila, Anna, Lisard Iglesias-Carres, Anna Arola-Arnal, Gerard Aragonès, Begoña Muguerza, and Francisca Isabel Bravo. 2020. "Implication of Opioid Receptors in the Antihypertensive Effect of a Novel Chicken Foot-Derived Peptide" Biomolecules 10, no. 7: 992. https://doi.org/10.3390/biom10070992
APA StyleMas-Capdevila, A., Iglesias-Carres, L., Arola-Arnal, A., Aragonès, G., Muguerza, B., & Bravo, F. I. (2020). Implication of Opioid Receptors in the Antihypertensive Effect of a Novel Chicken Foot-Derived Peptide. Biomolecules, 10(7), 992. https://doi.org/10.3390/biom10070992