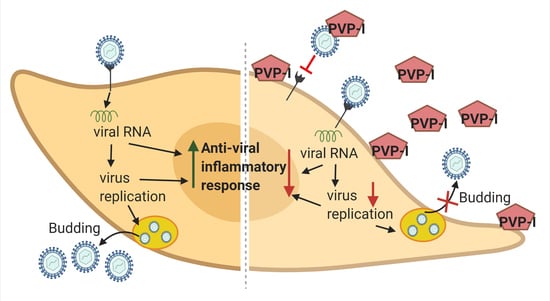
Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Culture Conditions
2.2. Virus Strains and Infection
2.3. Plaque Assay
2.4. Immunofluorescence Staining
2.5. Real-Time PCR
2.6. Cellular Toxicity Assay
2.7. Statistical Analysis
3. Results
3.1. PVP-I Directly Inactivates Enveloped RNA Viruses
3.2. PVP-I Treatment Attenuates Viral Replication in Human Retinal Pigment Epithelial Cells
3.3. PVP-I Exposure Attenuated CHIKV Replication in Corneal Epithelial Cells
3.4. PVP-I Attenuates CHIKV-Induced Inflammatory Response in Corneal Epithelial Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Osterholm, M.T.; Moore, K.A.; Kelley, N.S.; Brosseau, L.M.; Wong, G.; Murphy, F.A.; Peters, C.J.; LeDuc, J.W.; Russell, P.K.; Van Herp, M.; et al. Transmission of Ebola viruses: What we know and what we do not know. mBio 2015, 6, e00137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Dias, J.R.; Ventura, C.V.; Borba, P.D.; de Paula Freitas, B.; Pierroti, L.C.; do Nascimento, A.P.; de Moraes, N.S.B.; Maia, C.; Belfort, R., Jr. Infants with Congenital Zika Syndrome and Ocular Findings from São Paulo, Brazil: Spread of Infection. Retin. Cases Brief Rep. 2018, 12, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Merle, H.; Donnio, A.; Jean-Charles, A.; Guyomarch, J.; Hage, R.; Najioullah, F.; Césaire, R.; Cabié, A. Ocular manifestations of emerging arboviruses: Dengue fever, Chikungunya, Zika virus, West Nile virus, and yellow fever. J. Fr. d’Ophtalmol. 2018, 41, e235–e243. [Google Scholar] [CrossRef]
- Oliver, G.F.; Carr, J.M.; Smith, J.R. Emerging infectious uveitis: Chikungunya, dengue, Zika and Ebola: A review. Clin. Exp. Ophthalmol. 2019, 47, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Farr, D.; Kumar, A. Ocular Manifestations of Emerging Flaviviruses and the Blood-Retinal Barrier. Viruses 2018, 10, 530. [Google Scholar] [CrossRef]
- Singh, P.K.; Khatri, I.; Jha, A.; Pretto, C.D.; Spindler, K.R.; Arumugaswami, V.; Giri, S.; Kumar, A.; Bhasin, M.K. Determination of system level alterations in host transcriptome due to Zika virus (ZIKV) Infection in retinal pigment epithelium. Sci. Rep. 2018, 8, 11209. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kasetti, R.B.; Zode, G.S.; Goyal, A.; Juzych, M.S.; Kumar, A. Zika Virus Infects Trabecular Meshwork and Causes Trabeculitis and Glaucomatous Pathology in Mouse Eyes. mSphere 2019, 4, e00173-19. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Guest, J.-M.; Kanwar, M.; Boss, J.; Gao, N.; Juzych, M.S.; Abrams, G.W.; Yu, F.-S.; Kumar, A. Zika virus infects cells lining the blood-retinal barrier and causes chorioretinal atrophy in mouse eyes. JCI Insight 2017, 2, e92340. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, S.; Farr, D.; Kumar, A. Interferon-stimulated gene 15 (ISG15) restricts Zika virus replication in primary human corneal epithelial cells. Ocul. Surf. 2019, 17, 551–559. [Google Scholar] [CrossRef]
- Couderc, T.; Gangneux, N.; Caro, V.; Ducloux, B.; Tolou, H.; Chrétien, F.; Le Luong, T.; Lecuit, M.; Grandadam, M. Chikungunya Virus Infection of Corneal Grafts. J. Infect. Dis. 2012, 206, 851–859. [Google Scholar] [CrossRef]
- Hayek, S.; Rousseau, A.; Bouthry, E.; Prat, C.M.; Labetoulle, M. Chikungunya Virus Infection and Bilateral Stromal Keratouveitis. JAMA Ophthalmol. 2015, 133, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Mahendradas, P.; Ranganna, S.K.; Shetty, R.; Balu, R.; Narayana, K.M.; Babu, R.B.; Shetty, B.K. Ocular Manifestations Associated with Chikungunya. Ophthalmology 2008, 115, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Mahendradas, P.; Shetty, R.; Malathi, J.; Madhavan, H.N. Chikungunya virus iridocyclitis in Fuchs′ heterochromic iridocyclitis. Indian J. Ophthalmol. 2010, 58, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Sawant, O.B.; Singh, S.; Wright, R.E., III; Jones, K.M.; Titus, M.S.; Dennis, E.; Hicks, E.; Majmudar, P.A.; Kumar, A.; Mian, S.I. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul. Surf. 2020, 19, 322–329. [Google Scholar] [CrossRef]
- Feng, Y.; Armenti, S.T.; Mian, S.I. COVID-19 and the Eye: A Comprehensive Review of the Literature. Int. Ophthalmol. Clin. 2021, 61, 1–14. [Google Scholar] [CrossRef]
- Gopinathan, U.; Reddy, M.K.; Nadkarni, M.S.; Dasari, S.; Rao, G.N. Antimicrobial Effect of Ciprofloxacin, Povidone—Iodine, and Gentamicin in the Decontamination of Human Donor Globes. Cornea 1998, 17, 57. [Google Scholar] [CrossRef]
- Sperling, S.; Sørensen, I.G. Decontamination of Cadaver Corneas. Acta Ophthalmol. 2009, 59, 126–133. [Google Scholar] [CrossRef]
- Pels, E.; Vrensen, G.F.J.M. Microbial decontamination of human donor eyes with povidone-iodine: Penetration, toxicity, and effectiveness. Br. J. Ophthalmol. 1999, 83, 1019–1026. [Google Scholar] [CrossRef] [Green Version]
- Atum, M.; Boz, A.A.E.; Cakir, B.; Karabay, O.; Koroglu, M.; Ogutlu, A.; Alagöz, G. Evaluation of Conjunctival Swab PCR Results in Patients with SARS-CoV-2 Infection. Ocul. Immunol. Inflamm. 2020, 28, 745–748. [Google Scholar] [CrossRef]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; El-harakeh, A.; Bognanni, A.; Lotfi, T.; Loeb, M.; et al. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: A systematic review and meta-analysis. Lancet 2020, 395, 1973–1987. [Google Scholar] [CrossRef]
- Güemes-Villahoz, N.; Burgos-Blasco, B.; Arribi-Vilela, A.; Arriola-Villalobos, P.; Vidal-Villegas, B.; Mendez-Fernandez, R.; Delgado-Iribarren, A.; Garcia-Feijoo, J. SARS-CoV-2 RNA detection in tears and conjunctival secretions of COVID-19 patients with conjunctivitis. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Arabi, A.; Shahraki, T.; Safi, S. Detection of severe acute respiratory syndrome Coronavirus-2 in the tears of patients with Coronavirus disease 2019. Eye 2020, 34, 1220–1223. [Google Scholar] [CrossRef]
- Seah, I.; Agrawal, R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020, 28, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Ruan, F.; Huang, M.; Liang, L.; Huang, H.; Hong, Z.; Yu, J.; Kang, M.; Song, Y.; Xia, J.; et al. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020, 382, 1177–1179. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.M.; Hauser, J.; Mueller, S.; Bosse, B.; Hopp, M. Efficacy of Conventional and Liposomal Povidone–Iodine in Infected Mesh Skin Grafts: An Exploratory Study. Infect. Dis. Ther. 2017, 6, 545–555. [Google Scholar] [CrossRef]
- Eggers, M.; Koburger-Janssen, T.; Eickmann, M.; Zorn, J. In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash against Respiratory and Oral Tract Pathogens. Infect. Dis. Ther. 2018, 7, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Vergara-Buenaventura, A.; Castro-Ruiz, C. Use of mouthwashes against COVID-19 in dentistry. Br. J. Oral Maxillofac. Surg. 2020, 58, 924–927. [Google Scholar] [CrossRef]
- Pelletier, J.S.; Tessema, B.; Frank, S.; Westover, J.B.; Brown, S.M.; Capriotti, J.A. Efficacy of Povidone-Iodine Nasal and Oral Antiseptic Preparations against Severe Acute Respiratory Syndrome-Coronavirus 2 (SARS-CoV-2). Ear Nose Throat J. 2020, 100, 192S–196S. [Google Scholar] [CrossRef]
- Kawana, R.; Kitamura, T.; Nakagomi, O.; Matsumoto, I.; Arita, M.; Yoshihara, N.; Yanagi, K.; Yamada, A.; Morita, O.; Yoshida, Y.; et al. Inactivation of Human Viruses by Povidone-Iodine in Comparison with Other Antiseptics. Dermatology 1997, 195, 29–35. [Google Scholar] [CrossRef]
- Kariwa, H.; Fujii, N.; Takashima, I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions and chemical reagents. Dermatology 2006, 212 (Suppl. 1), 119–123. [Google Scholar] [CrossRef] [Green Version]
- Eggers, M.; Eickmann, M.; Zorn, J. Rapid and Effective Virucidal Activity of Povidone-Iodine Products Against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA). Infect. Dis. Ther. 2015, 4, 491–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.E.; Sivalingam, V.; Kang, A.E.; Ananthanarayanan, A.; Arumugam, H.; Jenkins, T.M.; Hadjiat, Y.; Eggers, M. Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Bidra, A.S.; Pelletier, J.S.; Westover, J.B.; Frank, S.; Brown, S.M.; Tessema, B. Comparison of In Vitro Inactivation of SARS CoV-2 with Hydrogen Peroxide and Povidone-Iodine Oral Antiseptic Rinses. J. Prosthodont. 2020, 29, 599–603. [Google Scholar] [CrossRef]
- Liang, B.; Yuan, X.; Wei, G.; Wang, W.; Zhang, M.; Peng, H.; Javer, A.; Mendenhall, M.; Julander, J.; Huang, S.; et al. In-Vivo Toxicity Studies and In-Vitro Inactivation of SARS-CoV-2 by Povidone-iodine In-situ Gel Forming Formulations. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wutzler, P.; Sauerbrei, A.; Klöcking, R.; Burkhardt, J.; Schacke, M.; Thust, R.; Fleischer, W.; Reimer, K. Virucidal and Chlamydicidal Activities of Eye Drops with Povidone-Iodine Liposome Complex. Ophthalmic Res. 2000, 32, 118–125. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, C.N.; Glybina, I.V.; Mahmoud, T.H.; Yu, F.S. Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J. Infect. Dis. 2010, 201, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.V.; Nagineni, C.N.; Chin, M.S.; Hooks, J.J.; Detrick, B. Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J. Neuroimmunol. 2004, 153, 7–15. [Google Scholar] [CrossRef]
- Singh, S.; Singh, P.K.; Suhail, H.; Arumugaswami, V.; Pellett, P.E.; Giri, S.; Kumar, A. AMP-Activated Protein Kinase Restricts Zika Virus Replication in Endothelial Cells by Potentiating Innate Antiviral Responses and Inhibiting Glycolysis. J. Immunol. 2020, 204, 1810–1824. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.; Singh, S.; Nair, S.R.; Hulyalkar, N.V.; Surendran, A.; Jaleel, A.; Sreekumar, E. Nucleophosmin (NPM1)/B23 in the Proteome of Human Astrocytic Cells Restricts Chikungunya Virus Replication. J. Proteome Res. 2017, 16, 4144–4155. [Google Scholar] [CrossRef]
- Kumar, S.; Jaffar-Bandjee, M.-C.; Giry, C.; De Kerillis, L.C.; Merits, A.; Gasque, P.; Hoarau, J.-J. Mouse macrophage innate immune response to chikungunya virus infection. Virol. J. 2012, 9, 313. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Agarwal, A.; Mahendradas, P.; Lee, C.S.; Gupta, V.; Pavesio, C.E.; Agrawal, R. Viral posterior uveitis. Surv. Ophthalmol. 2017, 62, 404–445. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, G.C.; Ventura, C.V.; Mello Filho, P.A.; Maia, M.; Vianello, S.; Rodrigues, E.B. Arboviruses and the eye. Int. J. Retina Vitr. 2017, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Roehrich, H.; Yuan, C.; Hou, J.H. Immunohistochemical Study of SARS-CoV-2 Viral Entry Factors in the Cornea and Ocular Surface. Cornea 2020, 39, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Schreier, H.; Erdos, G.; Reimer, K.; König, B.; König, W.; Fleischer, W. Molecular Effects of Povidone-Iodine on Relevant Microorganisms: An Electron-Microscopic and Biochemical Study. Dermatology 1997, 195, 111–116. [Google Scholar] [CrossRef]
- Siddharta, A.; Pfaender, S.; Vielle, N.J.; Dijkman, R.; Friesland, M.; Becker, B.; Yang, J.; Engelmann, M.; Todt, D.; Windisch, M.P.; et al. Virucidal Activity of World Health Organization–Recommended Formulations Against Enveloped Viruses, Including Zika, Ebola, and Emerging Coronaviruses. J. Infect. Dis. 2017, 215, 902–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, S.; Brown, S.M.; Capriotti, J.A.; Westover, J.B.; Pelletier, J.S.; Tessema, B. In Vitro Efficacy of a Povidone-Iodine Nasal Antiseptic for Rapid Inactivation of SARS-CoV-2. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 1054–1058. [Google Scholar] [CrossRef]
- Peng, X.; Xu, X.; Li, Y.; Cheng, L.; Zhou, X.; Ren, B. Transmission routes of 2019-nCoV and controls in dental practice. Int. J. Oral Sci. 2020, 12, 1–6. [Google Scholar] [CrossRef]
- Sriwilaijaroen, N.; Wilairat, P.; Hiramatsu, H.; Takahashi, T.; Suzuki, T.; Ito, M.; Ito, Y.; Tashiro, M.; Suzuki, Y. Mechanisms of the action of povidone-iodine against human and avian influenza A viruses: Its effects on hemagglutination and sialidase activities. Virol. J. 2009, 6, 124. [Google Scholar] [CrossRef] [Green Version]
- Eggers, M. Correction to: Infectious Disease Management and Control with Povidone Iodine. Infect. Dis. Ther. 2019, 8, 595. [Google Scholar] [CrossRef] [Green Version]
- Beukelman, C.; Berg, A.V.D.; Hoekstra, M.; Uhl, R.; Reimer, K.; Mueller, S. Anti-inflammatory properties of a liposomal hydrogel with povidone-iodine (Repithel®) for wound healing in vitro. Burns 2008, 34, 845–855. [Google Scholar] [CrossRef]
- Bilal, M.Y.; Dambaeva, S.; Kwak-Kim, J.; Gilman-Sachs, A.; Beaman, K.D. A Role for Iodide and Thyroglobulin in Modulating the Function of Human Immune Cells. Front. Immunol. 2017, 8, 1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, B.; Reimer, K.; Fleischer, W.; König, W. Effects of Betaisodona® on Parameters of Host Defense. Dermatology 1997, 195, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Thomas, A.; Harding, K.G. Iodine released from the wound dressing Iodosorb modulates the secretion of cytokines by human macrophages responding to bacterial lipopolysaccharide. Int. J. Biochem. Cell Biol. 1997, 29, 163–171. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.; Sawant, O.B.; Mian, S.I.; Kumar, A. Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses. Biomolecules 2021, 11, 753. https://doi.org/10.3390/biom11050753
Singh S, Sawant OB, Mian SI, Kumar A. Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses. Biomolecules. 2021; 11(5):753. https://doi.org/10.3390/biom11050753
Chicago/Turabian StyleSingh, Sneha, Onkar B. Sawant, Shahzad I. Mian, and Ashok Kumar. 2021. "Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses" Biomolecules 11, no. 5: 753. https://doi.org/10.3390/biom11050753
APA StyleSingh, S., Sawant, O. B., Mian, S. I., & Kumar, A. (2021). Povidone-Iodine Attenuates Viral Replication in Ocular Cells: Implications for Ocular Transmission of RNA Viruses. Biomolecules, 11(5), 753. https://doi.org/10.3390/biom11050753