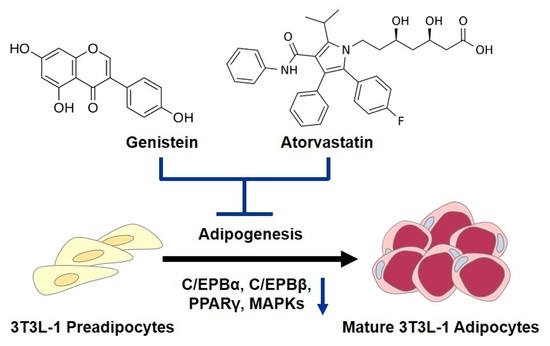
Combined Beneficial Effect of Genistein and Atorvastatin on Adipogenesis in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Materials
2.2. Cell Culture and Adipogenic Differentiation
2.3. Measurement of Cell Viability
2.4. Oil Red O Staining
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
3.1. Inhibitory Effects of Genistein and Atorvastatin on Adipogenesis in 3T3-L1 Preadipocytes
3.2. Effect of Genistein and Atorvastatin on the Expression of Proteins Involved in Adipogenesis in Differentiated 3T3L-1 Cells
4. Discussions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gold, E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. N. Am. 2011, 38, 425–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, S.-H.; Kim, H.-S. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-Q.; Brown, T.R.; Russo, J. Regulation of energy metabolism pathways by estrogens and estrogenic chemicals and potential implications in obesity associated with increased exposure to endocrine disruptors. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 1128–1143. [Google Scholar] [CrossRef] [Green Version]
- Hinney, A.; Volckmar, A.-L.; Knoll, N. Melanocortin-4 receptor in energy homeostasis and obesity pathogenesis. Prog. Mol. Biol. Transl. Sci. 2013, 114, 147–191. [Google Scholar] [PubMed]
- Lizcano, F.; Guzmán, G. Estrogen deficiency and the origin of obesity during menopause. BioMed Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Liu, E.Y.; Xu, M.L.; Jin, Y.; Wu, Q.; Dong, T.T.; Tsim, K.W. Genistein, a phytoestrogen in soybean, induces the expression of acetylcholinesterase via G protein-coupled receptor 30 in PC12 cells. Front. Mol. Neurosci. 2018, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S. Effect of genistein on in vitro and in vivo models of cancer. J. Nutr. 1995, 125, 777S–783S. [Google Scholar]
- Bang, O.Y.; Hong, H.S.; Kim, D.H.; Kim, H.; Boo, J.H.; Huh, K.; Mook-Jung, I. Neuroprotective effect of genistein against beta amyloid-induced neurotoxicity. Neurobiol. Dis. 2004, 16, 21–28. [Google Scholar] [CrossRef]
- Ji, G.; Yang, Q.; Hao, J.; Guo, L.; Chen, X.; Hu, J.; Leng, L.; Jiang, Z. Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int. Immunopharmacol. 2011, 11, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Wang, D.; Xie, Y.; Wang, S.; Zhao, W. Pharmacological studies of the large-scaled purified genistein from Huaijiao (Sophora japonica–Leguminosae) on anti-osteoporosis. Phytomedicine 2006, 13, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Kim, H.S.; Song, Y.-S. Genistein as a potential anticancer agent against ovarian cancer. J. Tradit. Complementary Med. 2012, 2, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Pummoung, S.; Werawatganon, D.; Chayanupatkul, M.; Klaikeaw, N.; Siriviriyakul, P. Genistein Modulated Lipid Metabolism, Hepatic PPARγ, and Adiponectin Expression in Bilateral Ovariectomized Rats with Nonalcoholic Steatohepatitis (NASH). Antioxidants 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Papageorgiou, A.A.; Mercouris, B.R.; Athyrou, V.V.; Symeonidis, A.N.; Basayannis, E.O.; Demitriadis, D.S.; Kontopoulos, A.G. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus’ usual’care in secondary coronary heart disease prevention. Curr. Med. Res. Opin. 2002, 18, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Han, S.; Lee, H.; Park, C.H.; Chung, Y.M.; Shin, K.; Lee, H.G.; Ye, S.-K. Metformin enhances the anti-adipogenic effects of atorvastatin via modulation of STAT3 and TGF-β/Smad3 signaling. Biochem. Biophys. Res. Commun. 2015, 456, 173–178. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Uzzan, B.; Cohen, R. Metformin and digestive disorders. Diabetes Metab. 2011, 37, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Kang, H.; Yoo, M.J.; Yu, J.S.; Lee, S.; Yi, S.A.; Beemelmanns, C.; Lee, J.; Kim, K.H. Anti-adipogenic pregnane steroid from a Hydractinia-associated fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Kim, H.; Choi, P.; Kim, T.; Kim, Y.; Song, B.G.; Park, Y.-T.; Choi, S.-J.; Yoon, C.H.; Lim, W.-C.; Ko, H. Ginsenosides Rk1 and Rg5 inhibit transforming growth factor-β1-induced epithelial-mesenchymal transition and suppress migration, invasion, anoikis resistance, and development of stem-like features in lung cancer. J. Ginseng Res. 2021, 45, 134–148. [Google Scholar] [CrossRef]
- Iqbal, H.; Kim, S.-K.; Cha, K.-M.; Jeong, M.-S.; Ghosh, P.; Rhee, D.-K. Korean Red Ginseng alleviates neuroinflammation and promotes cell survival in the intermittent heat stress-induced rat brain by suppressing oxidative stress via estrogen receptor beta and brain-derived neurotrophic factor upregulation. J. Ginseng Res. 2020, 44, 593–602. [Google Scholar] [CrossRef]
- Ryu, Y.-S.; Hyun, J.-W.; Chung, H.-S. Fucoidan Induces Apoptosis in A2058 Cells through ROS-exposed Activation of MAPKs Signaling Pathway. Nat. Prod. Sci. 2020, 26, 191–199. [Google Scholar]
- Nakata, M.; Nagasaka, S.; Kusaka, I.; Matsuoka, H.; Ishibashi, S.; Yada, T. Effects of statins on the adipocyte maturation and expression of glucose transporter 4 (SLC2A4): Implications in glycaemic control. Diabetologia 2006, 49, 1881–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.R.; Shim, J.; Kim, M.J. Genistin: A Novel Potent Anti-Adipogenic and Anti-Lipogenic Agent. Molecules 2020, 25, 2042. [Google Scholar] [CrossRef]
- Aziz, S.A.; Wakeling, L.A.; Miwa, S.; Alberdi, G.; Hesketh, J.E.; Ford, D. Metabolic programming of a beige adipocyte phenotype by genistein. Mol. Nutr. Food Res. 2017, 61, 1600574. [Google Scholar] [CrossRef] [PubMed]
- Heward, E.; Lau, A. Epistaxis and atorvastatin: Is there an association and are clinicians aware? A retrospective audit of 100 patients. J. Laryngol. Otol. 2020, 134, 138–140. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.W.; Patel, Y.M.; Harp, J.B. Genistein inhibits CCAAT/enhancer-binding protein β (C/EBPβ) activity and 3T3-L1 adipogenesis by increasing C/EBP homologous protein expression. Biochem. J. 2002, 367, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Kassouf, T.; Sumara, G. Impact of Conventional and Atypical MAPKs on the Development of Metabolic Diseases. Biomolecules 2020, 10, 1256. [Google Scholar] [CrossRef]
- Leiva, M.; Matesanz, N.; Pulgarín-Alfaro, M.; Nikolic, I.; Sabio, G. Uncovering the role of p38 family members in adipose tissue physiology. Front. Endocrinol. 2020, 11, 572089. [Google Scholar] [CrossRef] [PubMed]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A complex interplay of multiple molecular determinants and pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, J.W.; Grønborg, M.; Urlaub, H.; Lane, M.D.; Tang, Q.-Q. Role of cdk2 in the sequential phosphorylation/activation of C/EBPβ during adipocyte differentiation. Proc. Natl. Acad. Sci. USA 2007, 104, 11597–11602. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.A.; Harris, C.A.; Wang, J.-C. Glucocorticoid receptor and adipocyte biology. Nucl. Recept. Res. 2018, 5, 101373. [Google Scholar] [CrossRef]
- Payne, V.A.; Au, W.-S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2010, 425, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Li, X.; Tang, Q.-Q. Transcriptional regulation of adipocyte differentiation: A central role for CCAAT/enhancer-binding protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camp, H.S.; Tafuri, S.R.; Leff, T. c-Jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-γ1 and negatively regulates its transcriptional activity. Endocrinology 1999, 140, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, E.; Lee, J.; Huh, S.; Kim, Y.-S.; Kim, Y.-W.; Kim, Y.S.; Park, D. Anti-adipogenesis by 6-thioinosine is mediated by downregulation of PPAR γ through JNK-dependent upregulation of iNOS. Cell. Mol. Life Sci. 2010, 67, 467–481. [Google Scholar] [CrossRef]
- Rosen, E.D.; Hsu, C.-H.; Wang, X.; Sakai, S.; Freeman, M.W.; Gonzalez, F.J.; Spiegelman, B.M. C/EBPα induces adipogenesis through PPARγ: A unified pathway. Genes Dev. 2002, 16, 22–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Zhang, Y.; Steger, D.J.; Schupp, M.; Schug, J.; Cristancho, A.; Feng, D.; Zhuo, D.; Stoeckert, C.J.; Liu, X.S. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008, 22, 2941–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenkranz, B.; Fasinu, P.; Bouic, P. An overview of the evidence and mechanisms of herb–drug interactions. Front. Pharmacol. 2012, 3, 69. [Google Scholar]
- Zha, W. Transporter-mediated natural product–drug interactions for the treatment of cardiovascular diseases. J. Food Drug Anal. 2018, 26, S32–S44. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, J.-Y.; Kim, H.-W.; Yoo, J.-E.; Kang, K.S. Combined Beneficial Effect of Genistein and Atorvastatin on Adipogenesis in 3T3-L1 Adipocytes. Biomolecules 2021, 11, 1052. https://doi.org/10.3390/biom11071052
Lee D, Kim J-Y, Kim H-W, Yoo J-E, Kang KS. Combined Beneficial Effect of Genistein and Atorvastatin on Adipogenesis in 3T3-L1 Adipocytes. Biomolecules. 2021; 11(7):1052. https://doi.org/10.3390/biom11071052
Chicago/Turabian StyleLee, Dahae, Ji-Youn Kim, Hae-Won Kim, Jeong-Eun Yoo, and Ki Sung Kang. 2021. "Combined Beneficial Effect of Genistein and Atorvastatin on Adipogenesis in 3T3-L1 Adipocytes" Biomolecules 11, no. 7: 1052. https://doi.org/10.3390/biom11071052
APA StyleLee, D., Kim, J. -Y., Kim, H. -W., Yoo, J. -E., & Kang, K. S. (2021). Combined Beneficial Effect of Genistein and Atorvastatin on Adipogenesis in 3T3-L1 Adipocytes. Biomolecules, 11(7), 1052. https://doi.org/10.3390/biom11071052