A Chitosan–Agarose Polysaccharide-Based Hydrogel for Biomimetic Remineralization of Dental Enamel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tooth Slice Preparation
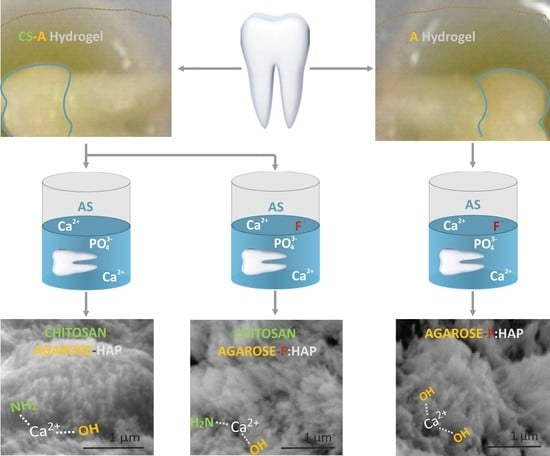
2.2. Preparation of Remineralized Coatings
2.3. Morphological and Compositional Characterization
2.4. Structural Analysis
2.5. Mechanical Characterization
3. Results
3.1. Morphologic and Structural Characterization of Biomimetic Remineralized Enamel
3.2. Mechanical Characterization of Biomimetic Remineralized Enamel
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic Systems for Hydroxyapatite Mineralization Inspired by Bone and Enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsharkawy, S.; Mata, A. Hierarchical Biomineralization: From Nature’s Designs to Synthetic Materials for Regenerative Medicine and Dentistry. Adv. Healthc. Mater. 2018, 1800178. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Mei, M.L.; Li, Q.L.; Lo, E.C.; Chu, C.H. Enamel prism-like tissue regeneration using enamel matrix derivative. J. Dent. 2014, 42, 1535–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, V.; Srinivasan, A.; Nikolajeff, F.; Kumar, S. Biomineralization process in hard tissues: The interaction complexity within protein and inorganic counterparts. Acta Biomater. 2021, 120, 20–37. [Google Scholar] [CrossRef]
- Aliaga, I.J.; Vera, V.; Paz, J.F.; Garcia, A.E.; Mohamad, M.S. Modelling the Longevity of Dental Restorations by means of a CBR System. BioMed Res. Int. 2015, 540306. [Google Scholar] [CrossRef] [Green Version]
- Tezvergil-Mutluay, A.; Pashley, D.; Mutluay, M.M. Long-Term Durability of Dental Adhesives. Curr. Oral Health Rep. 2015, 2, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Askar, H.; Krois, J.; Göstemeyer, G.; Bottenberg, P.; Zero, D.; Banerjee, A.; Schwendicke1, F. Secondary caries: What is it, and how it can be controlled, detected, and managed? Clin. Oral Investig. 2020, 24, 1869–1876. [Google Scholar] [CrossRef]
- Scholz, k.J.; Bittner, A.; Cieplik, F.; Hiller, K.-A.; Schmalz, G.; Buchalla, W.; Federlin, M. Micromorphology of the Adhesive Interface of Self-Adhesive Resin Cements to Enamel and Dentin. Materials 2021, 14, 492. [Google Scholar] [CrossRef]
- Kamal, D.; Hassanein, H.; Elkassas, D.; Hamza, H. Complementary remineralizing effect of self-assembling peptide (P11-4) with CPP-ACPF or fluoride: An in vitro study. J. Clin. Exp. Dent. 2020, 12, e161. [Google Scholar] [CrossRef]
- Li, Y.; Fok, A.; Aparicio, C. Nonsurgical Techniques for Restoring Tooth Enamel. U.S. Patent 10,709,733 B2, 14 July 2020. [Google Scholar]
- Cao, C.Y.; Mei, M.L.; Li, Q.-I.; Lo, E.C.M.; Chu, C.H. Methods for Biomimetic Mineralisation of Human Enamel: A Systematic Review. Materials 2015, 8, 2873–2886. [Google Scholar] [CrossRef] [Green Version]
- Pandya, M.; Diekwisch, T.G.H. Enamel biomimetics—Fiction or future of dentistry. Int. J. Oral Sci. 2019, 11, 8. [Google Scholar] [CrossRef] [Green Version]
- Fowler, C.E.; Li, M.; Mann, S.; Margolis, H.C. Influence of surfactant assembly on the formation of calcium phosphate materials—A model for dental enamel formation. J. Mater. Chem. 2005, 15, 3317–3325. [Google Scholar] [CrossRef]
- Fang, Z.; Guo, M.; Zhou, Q.; Li, Q.; Wong, H.M.; Cao, C.Y. Enamel-like tissue regeneration by using biomimetic enamel matrix proteins. Int. J. Biol. Macromol. 2021, 183, 2131–2141. [Google Scholar] [CrossRef]
- Fan, Y.; Sun, Z.; Moradian-Oldak, J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials 2009, 30, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Dogan, S.; Fong, H.; Yucesoy, D.T.; Cousin, T.; Gresswell, C.; Dag, S.; Huang, G.; Sarikaya, M. Biomimetic Tooth Repair: Amelogenin-derived peptide enables in vitro remineralization of human enamel. ACS Biomater. Sci. Eng. 2018, 4, 1788–1796. [Google Scholar] [CrossRef]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.C.; Brookes, S.J.; Aggeli, A. Self-assembling Peptide Scaffolds Promote Enamel Remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Khurshid, Z.; Najeeb, S.; Zafar, M.S.; Sefat, F. Calcium orthophosphates as dental regenerative material. Adv. Dent. Biomater. 2019. [Google Scholar] [CrossRef]
- Ding, L.; Han, S.; Wang, K.; Zheng, S.; Zheng, W.; Peng, X.; Niu, Y.; Li, W.; Zhang, L. Remineralization of enamel caries by an amelogenin-derived peptide and fluoride in vitro. Regen. Biomater. 2020, 7, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Mei, M.L.; Li, Q.L.; Lo, E.C.M.; Chu, C.H. Agarose Hydrogel Biomimetic Mineralization Model for the Regeneration of Enamel Prism like Tissue. ACS Appl. Mater. Interfaces 2014, 6, 410–420. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based Biomaterials for Tissue Engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Önnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.-X.; Ran, J.-B.; Chen, S.; Jiang, P.; Shen, X.-Y.; Tong, H. Carboxylated Agarose (CA)-Silk Fibroin (SF) Dual Confluent Matrices Containing Oriented Hydroxyapatite (HA) Crystals: Biomimetic Organic/Inorganic Composites for Tibia Repair. Biomacromolecules 2016, 17, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Campos, F.; Bonhame-Espinosa, A.B.; Vizcaino, G.; Rodriguez, I.A.; Durand-Herrera, D.; Lopez-Lopez, M.T.; Sánchez-Montesinos, I.; Alaminos, M.; Sánchez-Quevedo, M.C.; Carriel, V. Generation of genipin cross-linked fibrin-agarose hydrogels tissue-like models for tissue engineering applications. Biomed. Mater. 2018, 13, 025021. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Li, Q.-L.; Cao, Y.; Fang, H.; Xia, R.; Zhang, Z.H. In vivo remineralization of dentin using an agarose hydrogel biomimetic mineralization system. Sci. Rep. 2017, 7, 41955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raafat, D.; Sahl, H.G. Chitosan and Its Antimicrobial Potential-A Critical Literature Survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fariasa, J.M.; Stamford, T.C.M.; Resende, A.H.M.; Aguiard, J.S.; Rufino, R.D.; Luna, J.M.; Sarubbo, L.A. Mouthwash containing a biosurfactant and chitosan: An eco-sustainable option for the control of cariogenic microorganisms. Int. J. Biol. Macromol. 2019, 129, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Persadmehr, A.; Torneck, C.D.; Cvitkovitch, D.G.; Pinto, V.; Talior, I.; Kazembe, M.; Shrestha, S.; Mcculloch, C.A.; Kishen, A. Bioactive chitosan nanoparticles and photodynamic therapy inhibit collagen degradation in vitro. J. Endod. 2014, 40, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Q.; Zhang, Y.; Yang, X.; Nutt, S.; Moradian-Oldak, J. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013, 9, 7289–7297. [Google Scholar] [CrossRef] [Green Version]
- Zaharia, A.; Muşat, V.; Anghel, E.M.; Atkinson, I.; Mocioiu, O.C.; Buşilă, M.; Pleşcan, V.G. Biomimetic chitosan-hydroxyapatite hybrid biocoatings for enamel remineralization. Ceram. Int. 2017, 43, 11390–11402. [Google Scholar] [CrossRef]
- Simeonov, M.; Gussiyska, A.; Mironova, J.; Nikolova, D.; Apostolova, A.; Sezanova, K.; Dyulgerova, E.; Vassileva, E. Novel hybrid chitosan/calcium phosphates microgels for remineralization of demineralized enamel—A model study. Eur. Polym. J. 2019, 119, 14–21. [Google Scholar] [CrossRef]
- Garakani, S.S.; Khanmohammadi, M.; Atoufi, Z.; Kamrava, S.K.; Setayeshmehr, M.; Alizadeh, R.; Faghihi, F.; Bagher, Z.; Davachi, S.M.; Abbaspourrad, A. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int. J. Biol. Macromol. 2020, 159, 11–21. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Palka, K.; Przekora, A. Development and Optimization of the Novel Fabrication Method of Highly Macroporous Chitosan/Agarose/Nanohydroxyapatite Bone Scaffold for Potential Regenerative Medicine Applications. Biomolecules 2019, 9, 434. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Mascaraque, L.G.; Méndez, J.A.; Fernández-Gutiérrez, M.; Vázquez, B.; Román, J.S. Oxidized dextrins as alternative crosslinking agents for polysaccharides: Application to hydrogels of agarose-chitosan. Acta Biomater. 2014, 10, 798–811. [Google Scholar] [CrossRef]
- Zaharia, A.; Ghisman Plescan, V.; Anghel, E.M.; Musat, V. Human Dentine Remineralization Under Non-colagen Materials Action. Rev. Chim. 2017, 68, 928–932. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar] [CrossRef]
- Tingting, X.; Wanqian, L.; Li, Y. A review of gradient stiffness hydrogels used in tissue engineering and regenerative medicine. J. Biomed. Mater. Res. A 2017, 105, 1799–17812. [Google Scholar] [CrossRef]
- Coelho, A.; Amaro, I.; Apolónio, A.; Paula, A.; Saraiva, J.; Ferreira, M.M.; Marto, C.M.; Carrilho, E. Effect of Cavity Disinfectants on Adhesion to Primary Teeth—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 4398. [Google Scholar] [CrossRef]
- Fletcher, J.; Walsh, D.; Fowler, C.E.; Mann, S. Electrospun mats of PVP/ACP nanofibres for remineralization of enamel tooth surfaces. CrystEngComm 2011, 13, 3692–3697. [Google Scholar] [CrossRef]
- Dorvee, J.R.; Boskey, A.L.; Estroff, L.A. Rediscovering hydrogel-based double-diffusion systems for studying biomineralization. CrystEngComm 2012, 14, 5681–5700. [Google Scholar] [CrossRef] [Green Version]
- Fleet, M. Carbonated Hydroxyapatite, Materials, Synthesis and Application; Jenny Stanford Publishing: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Person, A.; Bocherens, H.; Saliege, J.F.; Paris, F.; Zeitoun, V.; Gerard, M. Early diagenetic evolution of bone phosphate: An X-ray diffractometry analysis. J. Archaeol. Sci. 1995, 22, 211–221. [Google Scholar] [CrossRef]
- Attin, T.; Wegehaupt, F.J. Methods for Assessment of Dental Erosion. Monogr. Oral Sci. 2014, 25, 123–142. [Google Scholar] [CrossRef] [Green Version]
- El Moshy, S.; Abbass, M.M.S.; El-Motayam, A.M. Biomimetic remineralization of acid etched enamel using agarose hydrogel model. F1000Research 2018, 7, 1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, C.; Jin, B.; Mu, Z.; Lu, H.; Zhao, Y.; Wu, Z.; Yan, L.; Zhang, Z.; Zhou, Y.; Pan, H.; et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci. Adv. 2019, 5, eaaw9569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz, K.J.; Federlin, M.; Hiller, K.-A.; Ebensberger, H.; Ferstl, G.; Buchalla, W. EDX-analysis of fluoride precipitation on human enamel. Sci. Rep. 2019, 9, 13442. [Google Scholar] [CrossRef] [Green Version]
- Hutchens, S.A.; Benson, R.S.; Evans, B.R.; O’Neill, H.M.; Rawn, C.J. Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 2006, 27, 4661–4670. [Google Scholar] [CrossRef]
- Grove, C.A.; Judd, G.; Ansell, G.S. Determination of Hydroxyapatite Crystallite Size in Human Dental Enamel by Dark-Field Electron Microscopy. J. Dent. Res. 1972, 51, 22–29. [Google Scholar] [CrossRef]
- Lamonier, C.; Lamonier, J.F.; Aellach, B.; Ezzamarty, A.; Leglise, A. Specific tuning of acid/base sites in apatite materials to enhance their methanol thiolation catalytic performances. Catal. Today 2011, 164, 124–130. [Google Scholar] [CrossRef]
- Leventouri, T.; Antonakos, A.; Kyriacou, A.; Venturelli, R.; Liarokapis, E.; Perdikatsis, V. Crystal Structure Studies of Human Dental Apatite as a Function of Age. Int. J. Biomater. 2009, 2009, 698547. [Google Scholar] [CrossRef] [Green Version]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Witzler, M.; Ottensmeyer, P.F.; Gericke, M.; Heinze, T.; Tobiasch, E.; Schulze, M. Non-Cytotoxic Agarose/Hydroxyapatite Composite Scaffolds for Drug Release. Int. J. Mol. Sci. 2019, 20, 3565. [Google Scholar] [CrossRef] [Green Version]
- Sivashankari, P.R.; Prabaharan, M. Three-dimensional porous scaffolds based on agarose/chitosan/graphene oxide composite for tissue engineering. Int. J. Biol. Macromol. 2020, 146, 222–231. [Google Scholar] [CrossRef]
- Ramakrishnaiah, R.; Rehman, G.; Basavarajappa, S.; Khuraif, A.A.A.; Durgesh, B.H.; Khan, A.S.; Rehman, I. Applications of Raman Spectroscopy in Dentistry: Analysis of Tooth Structure. Appl. Spectrosc. Rev. 2015, 50, 332–350. [Google Scholar] [CrossRef]
- Taube, F.; Marczewski, M.; Noren, J.G. Deviations of inorganic and organic carbon content in hypomineralised enamel. J. Dent. 2015, 43, 269–278. [Google Scholar] [CrossRef]
- Świetlicka, I.; Arczewska, M.; Muszyński, S.; Tomaszewska, E.; Świetlicki, M.; Kuc, D.; Mielnik-Błaszczak, M.; Gołacki, K.; Cieślak, K. Surface analysis of etched enamel modified during the prenatal period. Spectrochim. Acta Mol. Biomol. Spectrosc. 2019, 222, 117271. [Google Scholar] [CrossRef]
- Tsuda, H.; Arends, J. Micro-Raman Spectroscopy, HAP Single Crystals, and Human Enamel. J. Dent. Res. 1994, 73, 1703–1710. [Google Scholar] [CrossRef]
- Xu, C.; Reed, R.; Gorski, G.P.; Wang, Y.; Walker, M.P. The Distribution of Carbonate in Enamel and its Correlation with Structure and Mechanical Properties. J. Mater. Sci. 2012, 47, 8035–8043. [Google Scholar] [CrossRef]
- Zhang, J.; Boye, V.; Festy, F.; Lynch, R.J.M.; Watson, T.F.; Banerjee, A. In-vitro subsurface remineralisation of artificial enamel white spot lesions pre-treated with chitosan. Dent. Mater. 2018, 34, 1154–1167. [Google Scholar] [CrossRef] [Green Version]
- Pezzotti, G.; Adachi, T.; Gasparutti, I.; Vincini, G.; Zhu, W.; Boffelli, M.; Rondinella, A.; Marin, E.; Ichioka, H.; Yamamoto, T.; et al. Vibrational monitor of early demineralization in tooth enamel after in vitro exposure to phosphoridic liquid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 173, 19–33. [Google Scholar] [CrossRef]
- Lopez-Berganza, J.A.; Fu, B.; Lee, C.-W.J.; Rogers, S.A.; Espinosa-Marzal, R.M. Mediating the Enhanced Interaction Between Hydroxyapatite and Agarose through Amorphous Calcium Carbonate. Cryst. Growth Des. 2020, 20, 6917–6929. [Google Scholar] [CrossRef]
- Rogina, A.; Ressler, A.; Matić, I.; Ferrer, G.G.; Marijanović, I.; Ivanković, M.; Ivanković, H. Cellular hydrogels based on pH-responsive chitosan-hydroxyapatite system. Carbohydr. Polym. 2017, 166, 173–182. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Z.; Hu, Q. Chitosan Hydrogel Structure Modulated by Metal Ions. Sci. Rep. 2016, 6, 36005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swietlicka, I.; Kuc, D.; Swietlicki, M.; Arczewska, M.; Muszynski, S.; Tomaszewska, E.; Prószynski, A.; Gołacki, K.; Błaszczak, J.; Cieslak, K.; et al. Near-Surface Studies of the Changes to the Structure and Mechanical Properties of Human Enamel under the Action of Fluoride Varnish Containing CPP–ACP Compound. Biomolecules 2020, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Wang, R.; Zhang, D. Role of crystal arrangement on the mechanical performance of enamel. Acta Biomater. 2012, 8, 3784–3793. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Swain, M.V. Understanding the mechanical behaviour of human enamel from its structural and compositional characteristics. Mech. Behav. Biomed. Mater. 2008, 1, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Lowenstam, H. Organization of extracellularly mineralized tissues: A comparative study of biological crystal growth. CRC Crit. Rev. Biochem. 1986, 20, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Z.; Cao, Y.; Liu, W.; Chu, C.H.; Li, Q.-L. Polydopamine-induced tooth remineralization. ACS Appl. Mater. Interfaces 2012, 4, 6901–6910. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.; Rietzler, B.; Pham, T.; Bechtold, T. Multivalent Ions as Reactive Crosslinkers for Biopolymers-A Review. Molecules 2020, 25, 1840. [Google Scholar] [CrossRef]













| Sample | 2θ (deg) (002) | FWHM (deg) | Ca/P (EDX Data) (at %/wt %) | I1070/I960 (Raman Data) | Crystallite Size (002) (nm) | χc (002) | Lattice Parameters # | Microhardness (GPa) | |
|---|---|---|---|---|---|---|---|---|---|
| A = b(Å) | c(Å) | ||||||||
| Remineralized Samples in the Presence of Chitosan (CS–Agarose Hydrogel) | |||||||||
| CS-A-4 | 25.844 | 0.3413 | 1.75/2.26 | 0.0593 | 24.9(0.3) | 0.34 | 9.408(1) | 6.891(1) | 2.50 |
| CS-A-7 | 25.968 | 0.3319 | 1.64/2.12 | 0.0474 | 25.6 (0.4) | 0.38 | 9.406(1) | 6.891(1) | 2.26 |
| CS-A-10 | 25.958 | 0.3359 | 1.62/2.07 | 0.0457 | 25.3 (0.5) | 0.36 | 9.439(2) | 6.883(1) | 1.92 |
| Remineralized Samples without Chitosan (Agarose Hydrogel) | |||||||||
| A-4 | 25.947 | 0.3487 | 1.81/2.31 | 0.0442 | 24.4 (0.3) | 0.33 | 9.407(1) | 6.897(1) | 2.71 |
| A-7 | 26.02 | 0.3222 | 1.29/1.61 | 0.0699 | 27.6 (1.6) | 0.40 | 9.400(3) | 6.893(2) | 2.39 |
| A-10 | 25.958 | 0.3455 | 1.35/1.73 | 0.0424 | 24.6 (0.6) | 0.34 | 9.409(1) | 6.895(1) | 2.21 |
| Remineralized Samples in Fluoride-Free Artificial Saliva under Agarose and Chitosan Hydrogels | |||||||||
| A-0F-4 | 25.9439 | 0.3447 | 0.0569 | 24.7 (1.7) | 0.33 | 9.270(2) | 6.910(16) | ||
| A-0F-7 | 25.8797 | 0.3058 | 0.0589 | 27.8 (3.8) | 0.47 | 9.30 (3) | 6.884 (3) | ||
| CS-A-0F-4 | 25.8851 | 0.3661 | 0.0562 | 23.2 (1.3) | 0.28 | 9.23(18) | 6.912(5) | ||
| CS-A-0F-7 | 25.9634 | 0.3618 | 0.0574 | 23.5 9 (2) | 0.29 | 9.365(17) | 6.905(9) | ||
| Reference Samples—Native Enamel (as Selected or Acid Etched) | |||||||||
| R-01 | - | 0.3128 | 0.0463 | 27.2(0.5) | 0.46 | 2.92 | |||
| R-02 | 25.96 | 0.3755 | 0.0425 | 22.7(1.0) | 0.28 | 1.68 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muşat, V.; Anghel, E.M.; Zaharia, A.; Atkinson, I.; Mocioiu, O.C.; Buşilă, M.; Alexandru, P. A Chitosan–Agarose Polysaccharide-Based Hydrogel for Biomimetic Remineralization of Dental Enamel. Biomolecules 2021, 11, 1137. https://doi.org/10.3390/biom11081137
Muşat V, Anghel EM, Zaharia A, Atkinson I, Mocioiu OC, Buşilă M, Alexandru P. A Chitosan–Agarose Polysaccharide-Based Hydrogel for Biomimetic Remineralization of Dental Enamel. Biomolecules. 2021; 11(8):1137. https://doi.org/10.3390/biom11081137
Chicago/Turabian StyleMuşat, Viorica, Elena Maria Anghel, Agripina Zaharia, Irina Atkinson, Oana Cătălina Mocioiu, Mariana Buşilă, and Petrică Alexandru. 2021. "A Chitosan–Agarose Polysaccharide-Based Hydrogel for Biomimetic Remineralization of Dental Enamel" Biomolecules 11, no. 8: 1137. https://doi.org/10.3390/biom11081137
APA StyleMuşat, V., Anghel, E. M., Zaharia, A., Atkinson, I., Mocioiu, O. C., Buşilă, M., & Alexandru, P. (2021). A Chitosan–Agarose Polysaccharide-Based Hydrogel for Biomimetic Remineralization of Dental Enamel. Biomolecules, 11(8), 1137. https://doi.org/10.3390/biom11081137