Surfactant Protein-G in Wildtype and 3xTg-AD Mice: Localization in the Forebrain, Age-Dependent Hippocampal Dot-like Deposits and Brain Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Brain Tissue Preparation
2.3. Fluorescence Labeling
2.4. Microscopy and Image Processing
2.5. Biochemical Analyses
2.6. Statistical Analysis
3. Results
3.1. Neurons, SP-G and Vessels
3.2. Oligodendroglia, SP-G and Vessels
3.3. Microglia, SP-G and Vessels
3.4. Astroglial Markers, SP-G and Vessels
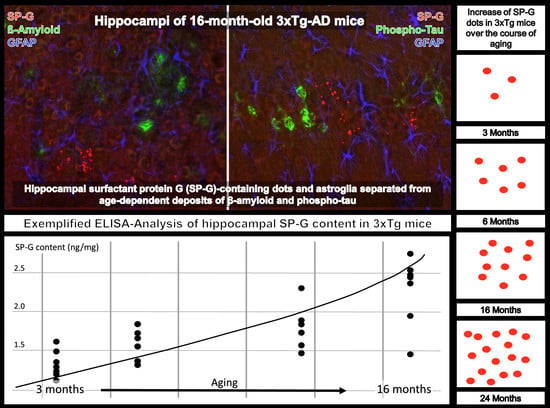
3.5. Clustered SP-G Dots, β-Amyloid Deposits, and Hyperphosphorylated Tau
3.6. SP-G and Reelin-Containing Dots in the Hippocampus
3.7. Technical Considerations
3.8. Computer-Based Semiquantification of SP-G-Containing Dots
3.9. Compartment-Specific Biochemical Analyses of SP-G and Reelin
4. Discussion
4.1. SP-G-Immunoreactivity within the Neurovascular Unit
4.2. 3xTg Mice as Animal Model with Multifaceted Age-Dependent Deposits
4.3. SP-G and Further Proteins in Age-Dependent Hippocampal Reelin Deposits
4.4. Possible Role of SP-G in the (G)lymphatic System—Outlook
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Griese, M. Pulmonary surfactant in health and human lung diseases: State of the art. Eur. Respir. J. 1999, 13, 1455–1476. [Google Scholar] [CrossRef]
- Weiskirchen, R. Severity of Coronavirus Disease 2019 (COVID-19): Does Surfactant Matter? Front. Microbiol. 2020, 11, 1905. [Google Scholar] [CrossRef]
- Sunde, M.; Pham, C.L.L.; Kwan, A.H. Molecular Characteristics and Biological Functions of Surface-Active and Surfactant Proteins. Annu. Rev. Biochem. 2017, 86, 585–608. [Google Scholar] [CrossRef]
- Schob, S.; Schicht, M.; Sel, S.; Stiller, D.; Kekulé, A.S.; Kekulé, A.; Paulsen, F.; Maronde, E.; Bräuer, L. The detection of surfactant proteins A, B, C and D in the human brain and their regulation in cerebral infarction, autoimmune conditions and infections of the CNS. PLoS ONE 2013, 8, e74412. [Google Scholar] [CrossRef]
- Schob, S.; Dieckow, J.; Fehrenbach, M.; Peukert, N.; Weiss, A.; Kluth, D.; Thome, U.; Quäschling, U.; Lacher, M.; Preuß, M. Occurrence and colocalization of surfactant proteins A, B, C and D in the developing and adult rat brain. Ann. Anat. 2017, 210, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Weiß, A.; Krause, M.; Stockert, A.; Richter, C.; Puchta, J.; Bhogal, P.; Hoffmann, K.-T.; Emmer, A.; Quäschling, U.; Scherlach, C.; et al. Rheologically Essential Surfactant Proteins of the CSF Interacting with Periventricular White Matter Changes in Hydrocephalus Patients Implications for CSF Dynamics and the Glymphatic System. Mol. Neurobiol. 2019, 56, 7863–7871. [Google Scholar] [CrossRef]
- Schob, S.; Weiß, A.; Surov, A.; Dieckow, J.; Richter, C.; Pirlich, M.; Horvath-Rizea, D.; Härtig, W.; Hoffmann, K.-T.; Krause, M.; et al. Elevated Surfactant Protein Levels and Increased Flow of Cerebrospinal Fluid in Cranial Magnetic Resonance Imaging. Mol. Neurobiol. 2018, 55, 6227–6236. [Google Scholar] [CrossRef]
- Zhang, Z.; Henzel, W.J. Signal peptide prediction based on analysis of experimentally verified cleavage sites. Protein Sci. 2004, 13, 2819–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rausch, F.; Schicht, M.; Paulsen, F.; Ngueya, I.; Bräuer, L.; Brandt, W. SP-G, a putative new surfactant protein-tissue localization and 3D structure. PLoS ONE 2012, 7, e47789. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.A.; Hammel, M.; Schwarz, J.; Heschl, K.M.; Bretschneider, N.; Flemmer, A.W.; Herber-Jonat, S.; Königshoff, M.; Eickelberg, O.; Holzinger, A. SFTA2--A novel secretory peptide highly expressed in the lung--is modulated by lipopolysaccharide but not hyperoxia. PLoS ONE 2012, 7, e40011. [Google Scholar] [CrossRef] [Green Version]
- Krause, M.; Peukert, N.; Härtig, W.; Emmer, A.; Mahr, C.V.; Richter, C.; Dieckow, J.; Puchta, J.; Pirlich, M.; Hoffmann, K.-T.; et al. Localization, Occurrence, and CSF Changes of SP-G, a New Surface Active Protein with Assumable Immunoregulatory Functions in the CNS. Mol. Neurobiol. 2019, 56, 2433–2439. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Härtig, W.; Mahr, C.V.; Richter, C.; Schob, J.; Puchta, J.; Hoffmann, K.-T.; Nestler, U.; Thome, U.; Knüpfer, M.; et al. CSF Surfactant Protein Changes in Preterm Infants after Intraventricular Hemorrhage. Front. Pediatr. 2020, 8, 572851. [Google Scholar] [CrossRef] [PubMed]
- Hofstein, R.; Hesse, G.; Shashoua, V.E. Proteins of the extracellular fluid of mouse brain: Extraction and partial characterization. J. Neurochem. 1983, 40, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Schob, S.; Puchta, J.; Härtig, W. Surfactant protein C is associated with perineuronal nets and shows age-dependent changes of brain content and hippocampal deposits in wildtype and 3xTg mice. J. Chem. Neuroanat. 2021, 118, 102036. [Google Scholar] [CrossRef] [PubMed]
- Hoe, H.-S.; Lee, K.J.; Carney, R.S.E.; Lee, J.; Markova, A.; Lee, J.-Y.; Howell, B.W.; Hyman, B.T.; Pak, D.T.S.; Bu, G.; et al. Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J. Neurosci. 2009, 29, 7459–7473. [Google Scholar] [CrossRef]
- Kobro-Flatmoen, A.; Nagelhus, A.; Witter, M.P. Reelin-immunoreactive neurons in entorhinal cortex layer II selectively express intracellular amyloid in early Alzheimer’s disease. Neurobiol. Dis. 2016, 93, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Kocherhans, S.; Madhusudan, A.; Doehner, J.; Breu, K.S.; Nitsch, R.M.; Fritschy, J.-M.; Knuesel, I. Reduced Reelin expression accelerates amyloid-beta plaque formation and tau pathology in transgenic Alzheimer’s disease mice. J. Neurosci. 2010, 30, 9228–9240. [Google Scholar] [CrossRef] [Green Version]
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 126, 238–292. [Google Scholar] [CrossRef]
- Wirths, O.; Multhaup, G.; Bayer, T.A. A modified beta-amyloid hypothesis: Intraneuronal accumula-tion of the beta-amyloid peptide—The first step of a fatal cascade. J. Neurochem. 2004, 91, 513–520. [Google Scholar] [CrossRef]
- Walker, L.C. Aβ Plaques. Free Neuropathol. 2020, 1, 31. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Kittler, J.; Illingworth, J. Minimum error thresholding. Pattern Recognit. 1986, 19, 41–47. [Google Scholar] [CrossRef]
- GIMP. The GIMP Development Team. 2019. Available online: https://www.gimp.org (accessed on 20 June 2021).
- Ngoka, L.C.M. Sample prep for proteomics of breast cancer: Proteomics and gene ontology reveal dramatic differences in protein solubilization preferences of radioimmunoprecipitation assay and urea lysis buffers. Proteome Sci. 2008, 6, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.C.; Wojtkiewicz, G.; Linnoila, J.J.; Chen, J.W. Measuring myeloperoxidase activity in biological samples. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knuesel, I.; Nyffeler, M.; Mormède, C.; Muhia, M.; Meyer, U.; Pietropaolo, S.; Yee, B.K.; Pryce, C.R.; LaFerla, F.M.; Marighetto, A.; et al. Age-related accumulation of Reelin in amyloid-like deposits. Neurobiol. Aging 2009, 30, 697–716. [Google Scholar] [CrossRef] [Green Version]
- Jucker, M.; Walker, L.C.; Kuo, H.; Tian, M.; Ingram, D.K. Age-related fibrillar deposits in brains of C57BL/6 mice. A review of localization, staining characteristics, and strain specificity. Mol. Neurobiol. 1994, 9, 125–133. [Google Scholar] [CrossRef]
- Jucker, M.; Walker, L.C.; Martin, L.J.; Kitt, C.A.; Kleinman, H.K.; Ingram, D.K.; Price, D.L. Age-associated inclusions in normal and transgenic mouse brain. Science 1992, 255, 1443–1445. [Google Scholar] [CrossRef] [Green Version]
- Allen Institute for Brain Science. In Situ Hybridization of SFTA2 in Mus Musculus. Available online: https://mouse.brain-map.org/experiment/show?id=72076509 (accessed on 5 December 2021).
- Vigh-Teichmann, I.; Vigh, B. The infundibular cerebrospinal-fluid contacting neurons. Adv. Anat. Embryol. Cell Biol. 1974, 50, 1–91. [Google Scholar] [CrossRef] [PubMed]
- Dale, N.; Roberts, A.; Ottersen, O.P.; Storm-Mathisen, J. The morphology and distribution of ‘Kolmer-Agduhr cells’, a class of cerebrospinal-fluid-contacting neurons revealed in the frog embryo spinal cord by GABA immunocytochemistry. Proc. R. Soc. Lond. B Biol. Sci. 1987, 232, 193–203. [Google Scholar] [CrossRef]
- Vígh, B.; Manzano e Silva, M.J.; Frank, C.L.; Vincze, C.; Czirok, S.J.; Szabó, A.; Lukáts, A.; Szél, A. The system of cerebrospinal fluid-contacting neurons. Its supposed role in the nonsynaptic signal transmission of the brain. Histol. Histopathol. 2004, 19, 607–628. [Google Scholar] [CrossRef]
- Kolmer, W. Über das Sagittalorgan, ein zentrales Sinnesorgan der Wirbeltiere, insbesondere beim Affen. Z. Für Zellforsch. Und Mikrosk. Anat. 1931, 13, 236–248. [Google Scholar] [CrossRef]
- Kolmer, W. Das „Sagittalorgan“ der Wirbeltiere. Z. Anat. Entwickl. Gesch. 1921, 60, 652–717. [Google Scholar] [CrossRef]
- Agduhr, E. Über ein zentrales Sinnesorgan (?) bei den Vertebraten. Z. Anat. Entwickl. Gesch. 1922, 66, 223–360. [Google Scholar] [CrossRef]
- Vigh-Teichmann, I.; Vigh, B. The cerebrospinal fluid-contacting neuron: A peculiar cell type of the central nervous system. Immunocytochemical aspects. Arch. Histol. Cytol. 1989, 52, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Orts-Del’Immagine, A.; Wyart, C. Cerebrospinal-fluid-contacting neurons. Curr. Biol. 2017, 27, R1198–R1200. [Google Scholar] [CrossRef] [Green Version]
- Schwarze, E.W. Proceedings: Cytology and cytochemistry of (Kolmer’s) epiplexus cells, demonstrated on the telencephalic choroid plexus of man. Verh. Dtsch. Ges. Pathol. 1974, 58, 562. [Google Scholar] [PubMed]
- Oemichen, M. Mononuclear phagocytes in the central nervous system. Origin, mode of distribution, and function of progressive microglia, perivascular cells of intracerebral vessels, free subarachnoidal cells, and epiplexus cells. Schriftenr. Neurol. 1978, 21, 1–167. [Google Scholar]
- Nakamura, S.; Koga, N.; Moriyasu, N. Epiplexus cell (Kolmer cell) and its reaction against foreign bodies. No Shinkei 1982, 34, 895–907. [Google Scholar]
- Wan, Y.; Hua, Y.; Garton, H.J.L.; Novakovic, N.; Keep, R.F.; Xi, G. Activation of epiplexus macrophag-es in hydrocephalus caused by subarachnoid hemorrhage and thrombin. CNS Neurosci. Ther. 2019, 25, 1134–1141. [Google Scholar] [CrossRef]
- Palaniyar, N. Antibody equivalent molecules of the innate immune system: Parallels between innate and adaptive immune proteins. Innate Immun. 2010, 16, 131–137. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Kitazawa, M.; Tseng, B.P.; LaFerla, F.M. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol. Aging 2003, 24, 1063–1070. [Google Scholar] [CrossRef]
- Mastrangelo, M.A.; Bowers, W.J. Detailed immunohistochemical characterization of temporal and spatial progression of Alzheimer’s disease-related pathologies in male triple-transgenic mice. BMC Neurosci. 2008, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- LaFerla, F.M.; Green, K.N.; Oddo, S. Intracellular amyloid-beta in Alzheimer’s disease. Nat. Rev. Neurosci. 2007, 8, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Gouras, G.K.; Tsai, J.; Naslund, J.; Vincent, B.; Edgar, M.; Checler, F.; Greenfield, J.P.; Haroutunian, V.; Buxbaum, J.D.; Xu, H.; et al. Intraneuronal Aβ42 Accumulation in Human Brain. Am. J. Pathol. 2000, 156, 15–20. [Google Scholar] [CrossRef]
- Wirths, O.; Multhaup, G.; Czech, C.; Feldmann, N.; Blanchard, V.; Tremp, G.; Beyreuther, K.; Pradier, L.; Bayer, T.A. Intraneuronal APP/A beta trafficking and plaque formation in beta-amyloid precursor protein and presenilin-1 transgenic mice. Brain Pathol. 2002, 12, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Bayer, T.A.; Wirths, O. Intracellular accumulation of amyloid-Beta—A predictor for synaptic dysfunction and neuron loss in Alzheimer’s disease. Front. Aging Neurosci. 2010, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Oh, K.-J.; Perez, S.E.; Lagalwar, S.; Vana, L.; Binder, L.; Mufson, E.J. Staging of Alzheimer’s pathology in triple transgenic mice: A light and electron microscopic analysis. Int. J. Alzheimers. Dis. 2010, 2010, 780102. [Google Scholar] [CrossRef] [Green Version]
- Härtig, W.; Goldhammer, S.; Bauer, U.; Wegner, F.; Wirths, O.; Bayer, T.A.; Grosche, J. Concomitant detection of beta-amyloid peptides with N-terminal truncation and different C-terminal endings in cortical plaques from cases with Alzheimer’s disease, senile monkeys and triple transgenic mice. J. Chem. Neuroanat. 2010, 40, 82–92. [Google Scholar] [CrossRef]
- Härtig, W.; Saul, A.; Kacza, J.; Grosche, J.; Goldhammer, S.; Michalski, D.; Wirths, O. Immunolesion-induced loss of cholinergic projection neurones promotes β-amyloidosis and tau hyperphosphorylation in the hippocampus of triple-transgenic mice. Neuropathol. Appl. Neurobiol. 2014, 40, 106–120. [Google Scholar] [CrossRef]
- Butkevich, E.; Härtig, W.; Nikolov, M.; Erck, C.; Grosche, J.; Urlaub, H.; Schmidt, C.F.; Klopfenstein, D.R.; Chua, J.J.E. Phosphorylation of FEZ1 by Microtubule Affinity Regulating Kinases regulates its function in presynaptic protein trafficking. Sci. Rep. 2016, 6, 26965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways during Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef]
- Doehner, J.; Genoud, C.; Imhof, C.; Krstic, D.; Knuesel, I. Extrusion of misfolded and aggregated proteins—A protective strategy of aging neurons? Eur. J. Neurosci. 2012, 35, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Notter, T.; Knuesel, I. Reelin immunoreactivity in neuritic varicosities in the human hippocampal formation of non-demented subjects and Alzheimer’s disease patients. Acta Neuropathol. Commun. 2013, 1, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Carare, R.O.; Bernardes-Silva, M.; Newman, T.A.; Page, A.M.; Nicoll, J.A.R.; Perry, V.H.; Weller, R.O. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: Significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol. Appl. Neurobiol. 2008, 34, 131–144. [Google Scholar] [CrossRef]
- Weller, R.O.; Djuanda, E.; Yow, H.-Y.; Carare, R.O. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009, 117, 1. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.N.T.P.; Bacskai, B.J.; Arbel-Ornath, M.; Aldea, R.; Bedussi, B.; Morris, A.W.J.; Weller, R.O.; Carare, R.O. Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cell. Mol. Neurobiol. 2016, 36, 181–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, R.O.; Subash, M.; Preston, S.D.; Mazanti, I.; Carare, R.O. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008, 18, 253–266. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [Green Version]
- Iliff, J.J.; Goldman, S.A.; Nedergaard, M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015, 14, 977–979. [Google Scholar] [CrossRef] [Green Version]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Tarasoff-Conway, J.M.; Carare, R.O.; Osorio, R.S.; Glodzik, L.; Butler, T.; Fieremans, E.; Axel, L.; Rusinek, H.; Nicholson, C.; Zlokovic, B.V.; et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 2015, 11, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Natale, G.; Limanaqi, F.; Busceti, C.L.; Mastroiacovo, F.; Nicoletti, F.; Puglisi-Allegra, S.; Fornai, F. Glymphatic System as a Gateway to Connect Neurodegeneration from Periphery to CNS. Front. Neurosci. 2021, 15, 639140. [Google Scholar] [CrossRef] [PubMed]
- Mestre, H.; Mori, Y.; Nedergaard, M. The Brain’s Glymphatic System: Current Controversies. Trends Neurosci. 2020, 43, 458–466. [Google Scholar] [CrossRef] [PubMed]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meinicke, A.; Härtig, W.; Winter, K.; Puchta, J.; Mages, B.; Michalski, D.; Emmer, A.; Otto, M.; Hoffmann, K.-T.; Reimann, W.; et al. Surfactant Protein-G in Wildtype and 3xTg-AD Mice: Localization in the Forebrain, Age-Dependent Hippocampal Dot-like Deposits and Brain Content. Biomolecules 2022, 12, 96. https://doi.org/10.3390/biom12010096
Meinicke A, Härtig W, Winter K, Puchta J, Mages B, Michalski D, Emmer A, Otto M, Hoffmann K-T, Reimann W, et al. Surfactant Protein-G in Wildtype and 3xTg-AD Mice: Localization in the Forebrain, Age-Dependent Hippocampal Dot-like Deposits and Brain Content. Biomolecules. 2022; 12(1):96. https://doi.org/10.3390/biom12010096
Chicago/Turabian StyleMeinicke, Anton, Wolfgang Härtig, Karsten Winter, Joana Puchta, Bianca Mages, Dominik Michalski, Alexander Emmer, Markus Otto, Karl-Titus Hoffmann, Willi Reimann, and et al. 2022. "Surfactant Protein-G in Wildtype and 3xTg-AD Mice: Localization in the Forebrain, Age-Dependent Hippocampal Dot-like Deposits and Brain Content" Biomolecules 12, no. 1: 96. https://doi.org/10.3390/biom12010096
APA StyleMeinicke, A., Härtig, W., Winter, K., Puchta, J., Mages, B., Michalski, D., Emmer, A., Otto, M., Hoffmann, K. -T., Reimann, W., Krause, M., & Schob, S. (2022). Surfactant Protein-G in Wildtype and 3xTg-AD Mice: Localization in the Forebrain, Age-Dependent Hippocampal Dot-like Deposits and Brain Content. Biomolecules, 12(1), 96. https://doi.org/10.3390/biom12010096