Why the Ala-His-His Peptide Is an Appropriate Scaffold to Remove and Redox Silence Copper Ions from the Alzheimer’s-Related Aβ Peptide
Abstract
:1. Introduction
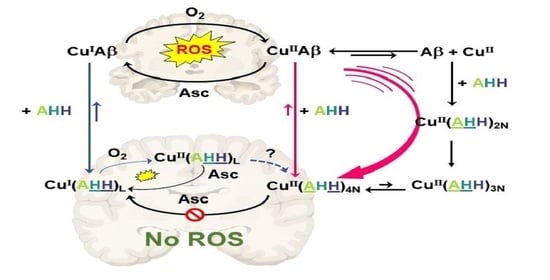
2. Results and Discussion
2.1. Effect on Cu- and Cu(Aβ)-Induced ROS Production
2.2. Kinetics of Cu and Cu(Aβ) Capture by the Peptides
2.3. Effect of ZnII on Cu(Aβ)-Induced ROS Production
3. Concluding Remarks
4. Experimental Section
4.1. Chemicals
4.2. Peptides
4.3. Synthesis of Peptides
4.4. UV–Visible Spectroscopy
4.5. Ascorbate Consumption Assay
4.6. Stopped-Flow Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/ (accessed on 18 August 2022).
- Available online: https://www.brightfocus.org/alzheimers/article/alzheimers-disease-facts-figures (accessed on 18 August 2022).
- Available online: https://aaic.alz.org/releases_2021/global-prevalence.asp (accessed on 18 August 2022).
- Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 18 August 2022).
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Padhi, D.; Govindaraju, T.J. Mechanistic Insights for Drug Repurposing and the Design of Hybrid Drugs for Alzheimer’s Disease. Med. Chem. 2022, 65, 7088–7105. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.X.; Dai, C.L.; Liu, F.; Iqbal, K. Multi-Targets: An Unconventional Drug Development Strategy for Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 837649. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.E.Z.N.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12295. [Google Scholar] [CrossRef] [PubMed]
- Athar, T.; Al Balushi, K.; Khan, S.A. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve the bioavailability. Mol. Biol. Rep. 2021, 48, 5629–5645. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cong, L.; Han, C.; Li, B.; Dai, R. Recent Progress in the Drug Development for the Treatment of Alzheimer’s Disease Especially on Inhibition of Amyloid-peptide Aggregation. Mini-Rev. Med. Chem. 2021, 21, 969–990. [Google Scholar] [CrossRef] [PubMed]
- Priya, K.; Siddesha, J.M.; Dharini, S.; Shashanka, K.P. Interacting Models of Amyloid-β and Tau Proteins: An Approach to Identify Drug Targets in Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2021, 5, 405–411. [Google Scholar] [CrossRef] [PubMed]
- West, S.; Bhugra, P. Emerging drug targets for Aβ and tau in Alzheimer’s disease: A systematic review. Br. J. Clin. Pharmacol. 2015, 80, 221–234. [Google Scholar] [CrossRef]
- Singh, S.K.; Balendra, V.; Obaid, A.A.; Esposto, J.; Tikhonova, M.A.; Gautam, N.K.; Poeggeler, B. Copper-mediated β-amyloid toxicity and its chelation therapy in Alzheimer’s disease. Metallomics 2022, 14, mfac018. [Google Scholar] [CrossRef]
- Fasae, K.D.; Abolaji, A.O.; Faloye, T.R.; Odunsi, A.Y.; Oyetayo, B.O.; Enya, J.I.; Rotimi, J.A.; Akinyemi, R.O.; Whitworth, A.J.; Aschner, M.J. Metallobiology and therapeutic chelation of biometals (copper, zinc and iron) in Alzheimer’s disease: Limitations, and current and future perspectives. Trace Elem. Med. Biol. 2021, 67, 126779. [Google Scholar] [CrossRef]
- Ejaz, H.W.; Wang, W.; Lang, M. Copper toxicity links to pathogenesis of Alzheimer’s disease and therapeutics approaches. Int. J. Mol. Sci. 2020, 21, 7660. [Google Scholar] [CrossRef]
- Esmieu, C.; Guettas, D.; Conte-Daban, A.; Sabater, L.; Faller, P.; Hureau, C. Copper-Targeting Approaches in Alzheimer’s Disease: How To Improve the Fallouts Obtained from in Vitro Studies. Inorg. Chem. 2019, 58, 13509–13527. [Google Scholar] [CrossRef]
- Savelieff, M.G.; Nam, G.; Kang, J.; Lee, H.J.; Lee, M.; Lim, M.H. Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev. 2019, 119, 1221–1322. [Google Scholar] [CrossRef]
- Robert, A.; Liu, Y.; Nguyen, M.; Meunier, B. Regulation of copper and iron homeostasis by metal chelators: A possible chemotherapy for Alzheimer’s disease. Acc. Chem. Res. 2015, 48, 1332–1339. [Google Scholar] [CrossRef]
- Weibull, M.G.M.; Simonsen, S.; Oksbjerg, C.R.; Tiwari, M.K.; Hemmingsen, L. Effects of Cu(II) on the aggregation of amyloid-β. J. Biol. Inorg. Chem. 2019, 24, 1197–1215. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Huat, T.J.; Camats-Perna, J.; Newcombe, E.A.; Valmas, N.; Kitazawa, M.; Medeiros, R.J. Metal Toxicity Links to Alzheimer’s Disease and Neuroinflammation. Mol. Biol. 2019, 431, 1843–1868. [Google Scholar] [CrossRef]
- Reybier, K.; Ayala, S.; Alies, B.; Bustos Rodriguez, S.; Rodrigues, J.; Gomes, C.; La Penna, G.; Collin, F.; Hureau, C.; Faller, P. Free superoxide is an intermediate in the production of H2O2 by Cu(I)-amyloid-β and O2. Angew. Chem. Int. Ed. 2016, 55, 1085–1089. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Guilloreau, L.; Combalbert, S.; Sournia-Saquet, A.; Marzaguil, H.; Faller, P. Redox Chemistry of Copper-Amyloid-beta: The Generation of Hydroxyl Radical in the Presence of Ascorbate is Linked to Redox-Potentials and Aggregation State. ChemBioChem 2007, 8, 1317–1325. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Vitek, M.P.; Mason, R.P. Cupric-amyloid beta peptide complex stimulates oxidation of ascorbate and generation of hydroxyl radical. Free Radic. Biol. Med. 2004, 36, 340–347. [Google Scholar] [CrossRef]
- Bellotti, D.; Remelli, M. Lights and Shadows on the Therapeutic Use of Antimicrobial Peptides. Molecules 2022, 27, 4584. [Google Scholar] [CrossRef]
- Li Petri, G.; Di Martino, S.; De Rosa, M.J. Peptidomimetics: An Overview of Recent Medicinal Chemistry Efforts toward the Discovery of Novel Small Molecule Inhibitors. Med. Chem. 2022, 65, 7438–7475. [Google Scholar] [CrossRef]
- Lai, X.; Tang, J.; ElSayed, M.E.H. Recent advances in proteolytic stability for peptide, protein, and antibody drug discovery. Exp. Opin. Drug. Discov. 2021, 16, 1467–1482. [Google Scholar] [CrossRef]
- Wątły, J.; Miller, A.; Kozłowski, H.; Rowińska-Żyrek, M.J. Peptidomimetics – An infinite reservoir of metal binding motifs in metabolically stable and biologically active molecules. Inorg. Biochem. 2021, 217, 111386. [Google Scholar] [CrossRef]
- Aileen Funke, S.; van Groen, T.; Kadish, I.; Bartnik, D.; Nagel-Steger, L.; Brener, O.; Sehl, T.; Batra-Safferling, R.; Moriscot, C.; Schoehn, G.; et al. Oral treatment with the d-enantiomeric peptide D3 improves the pathology and behavior of Alzheimer’s Disease transgenic mice. ACS Chem. Neurosci. 2010, 1, 639–648. [Google Scholar] [CrossRef]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve the bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef]
- Covarrubias-Zambrano, O.; Yu, J.; Bossmann, H.S. Nano-Inspired Technologies for Peptide Delivery. Curr. Protein Pept. Sci. 2020, 21, 379–400. [Google Scholar] [CrossRef]
- Makowski, M.; Silva, Í.C.; Pais do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in lipid and metal nanoparticles for antimicrobial peptide delivery. Pharmaceutics 2019, 11, 588. [Google Scholar] [CrossRef]
- Tang, Y.; Varyambath, A.; Ding, Y.; Chen, B.; Huang, X.; Zhang, Y.; Yu, D.-g.; Kim, I.; Song, W. Porous organic polymers for drug delivery: Hierarchical pore structures, variable morphologies, and biological properties. Biomater. Sci. 2022. [Google Scholar] [CrossRef]
- Reissmann, S.; Filatova, M.P. New generation of cell-penetrating peptides: Functionality and potential clinical application. J. Pept. Sci. 2021, 27, e3300. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Smith, Q.R.; Liu, X. Brain penetrating peptides and peptide–drug conjugates to overcome the blood–brain barrier and target CNS diseases. WIREs Nanomed. Nanobiotechnology 2021, 13, e1695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kim, I.; Lu, Y.; Xu, Y.; Yu, D.-G.; Song, W. Intelligent poly(L-histidine)-based nanovehicles for controlled drug delivery. J. Control. Release 2022, 349, 963–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly(L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Atrian-Blasco, E.; Conte-Daban, A.; Hureau, C. Mutual interference of Cu and Zn ions in Alzheimer’s disease: Perspectives at the molecular level. Dalton Trans. 2017, 46, 12750–12759. [Google Scholar] [CrossRef]
- Atrian-Blasco, E.; Gonzalez, P.; Santoro, A.; Alies, B.; Faller, P.; Hureau, C. Cu and Zn coordination to amyloid peptides: From fascinating chemistry to debated pathological relevance. Coord. Chem. Rev. 2018, 375, 38–55. [Google Scholar] [CrossRef]
- Rana, M.; Sharma, A.K. Cu and Zn interactions with Aβ peptides: Consequence of coordination on aggregation and formation of neurotoxic soluble Aβ oligomers. Metallomics 2019, 11, 64–84. [Google Scholar] [CrossRef]
- Dorlet, P.; Gambarelli, S.; Faller, P.; Hureau, C. Pulse EPR Spectroscopy Reveals the Coordination Sphere of Copper(II) Ions in the 1–16 Amyloid-β Peptide: A Key Role of the First Two N-Terminus Residues. Angew. Chem. Int. Ed. Engl. 2009, 48, 9273–9276. [Google Scholar] [CrossRef]
- Alies, B.; Conte-Daban, A.; Sayen, S.; Collin, F.; Kieffer, I.; Guillon, E.; Faller, P.; Hureau, C. Zinc(II) Binding Site to the Amyloid-β Peptide: Insights from Spectroscopic Studies with a Wide Series of Modified Peptides. Inorg. Chem. 2016, 55, 10499–10509. [Google Scholar] [CrossRef]
- De Gregorio, G.; Biasotto, F.; Hecel, A.; Luczkowski, M.; Kozlowski, H.; Valensin, D. Structural analysis of copper(I) interaction with amyloid β peptide. J. Inorg. Biochem. 2019, 195, 31–38. [Google Scholar] [CrossRef]
- Hureau, C.; Balland, V.; Coppel, Y.; Solari, P.L.; Fonda, E.; Faller, P. Importance of dynamical processes in the coordination chemistry and redox conversion of copper amyloid-β complexes. J. Biol. Inorg. Chem. 2009, 14, 995–1000. [Google Scholar] [CrossRef]
- Alies, B.; Renaglia, E.; Rozga, M.; Bal, W.; Faller, P.; Hureau, C. Cu(II) affinity for the Alzheimer’s Peptide: Tyrosine fluorescence studies revisited. Anal. Chem. 2013, 85, 1501–1508. [Google Scholar] [CrossRef]
- Zawisza, I.; Rozga, M.; Bal, W. Affinity of peptides (Aβ, APP, α-synuclein, PrP) for metal ions (Cu, Zn). Coord. Chem. Rev. 2012, 256, 2297–2307. [Google Scholar] [CrossRef]
- Alies, B.; Badei, B.; Faller, P.; Hureau, C. Reevaluation of Copper(I) affinity for amyloid-β peptides by competition with Ferrozine—An unusual Copper(I) indicator. Chem. Eur. J. 2012, 18, 1161–1167. [Google Scholar] [CrossRef]
- Young, T.R.; Kirchner, A.; Wedd, A.G.; Xiao, Z. An integrated study of the affinities of the Aβ16 peptide for Cu(I) and Cu(II): Implications for the catalytic production of reactive oxygen species. Metallomics 2014, 6, 505–517. [Google Scholar] [CrossRef]
- Xiao, Z.; Gottschlich, L.; van der Meulen, R.; Udagedara, S.R.; Wedd, A.G. Evaluation of quantitative probes for weaker Cu(i) binding sites completes a set of four capable of detecting Cu(I) affinities from nanomolar to attomolar. Metallomics 2013, 5, 501–513. [Google Scholar] [CrossRef]
- Gonzalez, P.; Vileno, B.; Bossak, K.; El Khoury, Y.; Hellwig, P.; Bal, W.; Hureau, C.; Faller, P. Cu(II)-binding to the peptide Ala-His-His, a chimera of the canonical Cu(II)-binding motifs Xxx-His and Xxx-Zzz-His (ATCUN). Inorg. Chem. 2017, 56, 14870–14879. [Google Scholar] [CrossRef]
- Pickart, L.; Margolina, A. Regenerative and protective actions of the GHK-Cu peptide in the light of the new gene data. Int J. Mol. Sci. 2018, 19, 1987. [Google Scholar] [CrossRef]
- Ma, W.H.; Li, M.; Ma, H.F.; Li, W.; Liu, L.; Yin, Y.; Zhou, X.M.; Hou, G. Protective effects of GHK-Cu in bleomycin-induced pulmonary fibrosis via anti-oxidative stress and anti-inflammation pathways. Life Sci. 2020, 241, 117139. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Xu, Q.; Sun, H.; Shi, M.; Wang, D.; Guo, M.; Yu, J.; Zhao, C.; Feng, B. GHK-Cu-liposomes accelerate scald wound healing in mice by promoting cell proliferation and angiogenesis. Wound Repair Regen. 2017, 25, 270–278. [Google Scholar] [CrossRef]
- Hureau, C.; Eury, H.; Guillot, R.; Bijani, C.; Sayen, S.; Solari, P.L.; Guillon, E.; Faller, P.; Dorlet, P. X-Ray and solution structures of CuGHK and CuDAHK complexes. Influence on their redox properties. Chem. Eur. J. 2011, 17, 10151–10160. [Google Scholar] [CrossRef]
- Gonzalez, P.; Bossak, K.; Stefaniak, E.; Hureau, C.; Raibaut, L.; Bal, W.; Faller, P. N-terminal Cu Binding Motifs Xxx-Zzz-His (ATCUN) and Xxx-His and their derivatives: Chemistry, Biology and Medicinal Applications. Chem. Eur. J. 2018, 24, 8029–8041. [Google Scholar] [CrossRef]
- Maiti, B.K.; Govil, N.; Kundu, T.; Moura, J.J.G. Designed Metal-ATCUN Derivatives: Redox- and Non-redox-Based Applications Relevant for Chemistry, Biology, and Medicine. iScience 2020, 23, 101792. [Google Scholar] [CrossRef]
- Harford, C.; Sarkar, B. Amino Terminal Cu(II)- and Ni(II)-Binding (ATCUN) Motif of Proteins and Peptides: MetalBindi ng, DNA Cleavage, and Other Properties. Acc. Chem. Res. 1997, 30, 123–130. [Google Scholar] [CrossRef]
- Bal, W.; Sokołowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Gonzalez, P.; Bossak-Ahmad, K.; Vileno, B.; Wezynfeld, N.E.; El Khoury, Y.; Hellwig, P.; Hureau, C.; Bal, W.; Faller, P. Triggering Cu-coordination change in Cu(ii)-Ala-His-His by external ligands. Chem. Commun. 2019, 55, 8110–8113. [Google Scholar] [CrossRef]
- Trapaidze, A.; Hureau, C.; Bal, W.; Winterhalter, M.; Faller, P. Thermodynamic study of Cu2+ binding to the DAHK and GHK peptides by isothermal titration calorimetry (ITC) with the weaker competitor glycine. J. Biol. Inorg. Chem. 2012, 17, 37–47. [Google Scholar] [CrossRef]
- Bossak-Ahmad, K.; Wiśniewska, M.D.; Bal, W.; Drew, S.C.; Frączyk, T. Ternary Cu(II) Complex with GHK Peptide and Cis-Urocanic Acid as a Potential Physiologically Functional Copper Chelate. Int. J. Mol. Sci. 2020, 21, 6190. [Google Scholar] [CrossRef]
- Sóvágó, I.; Kállay, C.; Várnagy, K. Peptides as complexing agents. Factors influencing the structure and thermodynamic stability of peptide complexes. Coord. Chem. Rev. 2012, 256, 2225–2233. [Google Scholar] [CrossRef]
- Hureau, C. Coordination of redox active metal ions to the APP and to the amyloid-β peptides involved in AD. Part 1: An overview. Coord. Chem. Rev. 2012, 256, 2164–2174. [Google Scholar] [CrossRef]
- Portelinha, J.; Duay, S.S.; Yu, S.I.; Heilemann, K.; Libardo, M.D.J.; Juliano, S.A.; Klassen, J.L.; Angeles-Boza, A.M. Antimicrobial Peptides and Copper(II) Ions: Novel Therapeutic Opportunities. Chem. Rev. 2021, 121, 2648–2712. [Google Scholar] [CrossRef] [PubMed]
- Agbale, C.M.; Cardoso, M.H.; Galyuon, I.K.; Franco, O.L. Designing metallodrugs with nuclease and protease activity. Metallomics 2016, 8, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Folk, D.S.; Franz, K.J. A prochelator activated by beta-secretase inhibits Aβ aggregation and suppresses copper-induced reactive oxygen species formation. J. Am. Chem. Soc. 2010, 132, 4994–4995. [Google Scholar] [CrossRef] [PubMed]
- Caballero, A.B.; Terol-Ordaz, L.; Espargaró, A.; Vázquez, G.; Nicolás, E.; Sabaté, R.; Gamez, P. Histidine-Rich Oligopeptides To Lessen Copper-Mediated Amyloid-β Toxicity. Chem. Eur. J. 2016, 22, 7268–7280. [Google Scholar] [CrossRef]
- Jensen, M.; Canning, A.; Chiha, S.; Bouquerel, P.; Pedersen, J.T.; Østergaard, J.; Cuvillier, O.; Sasaki, I.; Hureau, C.; Faller, P. Bi-functional peptide with β-sheet breaker and chelator moieties against Cu- amyloid-β. Chem. Eur. J. 2012, 18, 4836–4839. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Q.; Wang, W.; Yuan, Z.; Zhu, X.; Chen, B.; Chen, X. Tripeptide GGH as the Inhibitor of Copper-Amyloid-β-Mediated Redox Reaction and Toxicity. ACS Chem. Neurosci. 2016, 7, 1255–1263. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, H.; Dong, X.; Liu, F.; Sun, Y. RTHLVFFARK-NH2: A potent and selective modulator on Cu2+-mediated amyloid-β protein aggregation and cytotoxicity. J.Inorg. Biochem. 2018, 181, 56–64. [Google Scholar] [CrossRef]
- Perrone, L.; Mothes, E.; Vignes, M.; Mockel, A.; Figueroa, C.; Miquel, M.C.; Maddelein, M.L.; Faller, P. Copper transfer from Cu-Abeta to human serum albumin inhibits aggregation, radical production and reduces Aβ toxicity. ChemBioChem 2010, 11, 110–118. [Google Scholar] [CrossRef]
- Mital, M.; Wezynfeld, N.E.; Frączyk, T.; Wiloch, M.Z.; Wawrzyniak, U.E.; Bonna, A.; Tumpach, C.; Barnham, K.J.; Haigh, C.L.; Bal, W.; et al. A Functional Role for Aβ in Metal Homeostasis? N-Truncation and High-Affinity Copper Binding. Angew. Chem. Int. Ed. 2015, 54, 10460–10464. [Google Scholar] [CrossRef]
- Pushie, M.J.; Stefaniak, E.; Sendzik, M.R.; Sokaras, D.; Kroll, T.; Haas, K.L. Using N-Terminal Coordination of Cu(II) and Ni(II) to Isolate the Coordination Environment of Cu(I) and Cu(II) Bound to His13 and His14 in Amyloid-β(4–16). Inorg. Chem. 2019, 58, 15138–15154. [Google Scholar] [CrossRef]
- Streltsov, V.A.; Ekanayake, R.S.K.; Drew, S.C.; Chantler, C.T.; Best, S.P. Structural Insight into Redox Dynamics of Copper Bound N-Truncated Amyloid-β Peptides from In Situ X-ray Absorption Spectroscopy. Inorg. Chem. 2018, 57, 11422–11435. [Google Scholar] [CrossRef]
- Alies, B.; Sasaki, I.; Proux, O.; Sayen, S.; Guillon, E.; Faller, P.; Hureau, C. Zn impacts Cu coordination to Amyloid-ß, the Alzheimer’s peptide, but not the ROS production and the associated cell toxicity. Chem. Commun. 2013, 49, 1214–1216. [Google Scholar] [CrossRef]
- Cheignon, C.; Collin, F.; Faller, P.; Hureau, C. Is ascorbate Dr Jekyll or Mr Hyde in the Cu(Aβ) mediated oxidative stress linked to Alzheimer’s Disease? Dalton Trans. 2016, 45, 12627–12631. [Google Scholar] [CrossRef]
- Chassaing, S.; Collin, F.; Dorlet, P.; Gout, J.; Hureau, C.; Faller, P. Copper and heme-mediated Aβ toxicity: Redox chemistry, Abeta oxidations and anti-ROS compounds. Curr. Top. Med. Chem. 2012, 12, 2573–2595. [Google Scholar] [CrossRef]
- Conte-Daban, A.; Day, A.; Faller, P.; Hureau, C. How Zn can impede Cu detoxification by chelating agents in Alzheimer’s disease: A proof-of-concept study. Dalton Trans. 2016, 45, 15671–15678. [Google Scholar] [CrossRef]
- Conte-Daban, A.; Beyler, M.; Tripier, R.; Hureau, C. Kinetic is crucial when targeting copper ions to fight Alzheimer’s disease: An illustration with azamacrocyclic ligands. Chem. Eur. J. 2018, 24, 8447–8452. [Google Scholar] [CrossRef]
- Kotuniak, R.; Strampraad, M.J.F.; Bossak-Ahmad, K.; Wawrzyniak, U.E.; Ufnalska, I.; Hagedoorn, P.-L.; Bal, W. Key Intermediate Species Reveal the Copper(II)-Exchange Pathway in Biorelevant ATCUN/NTS Complexes. Angew. Chem. Int. Ed. 2020, 59, 11234–11239. [Google Scholar] [CrossRef]
- Beuning, C.N.; Zocchi, L.J.; Malikidogo, K.P.; Esmieu, C.; Dorlet, P.; Crans, D.C.; Hureau, C. Measurement of Interpeptidic CuII Exchange Rate Constants of CuII-Amyloid-β Complexes to Small Peptide Motifs by Tryptophan Fluorescence Quenching. Inorg. Chem. 2021, 60, 7650–7659. [Google Scholar] [CrossRef]
- Beuning, C.N.; Mestre-Voegtlé, B.; Faller, P.; Hureau, C.; Crans, D.C. Measurement of Interpeptidic Cu(II) Exchange Rate Constants by Static Fluorescence Quenching of Tryptophan. Inorg. Chem. 2018, 57, 4791–4794. [Google Scholar] [CrossRef]
- Kotuniak, R.; Bal, W. Kinetics of Cu(ii) complexation by ATCUN/NTS and related peptides: A gold mine of novel ideas for copper biology. Dalton Trans. 2022, 51, 14–26. [Google Scholar] [CrossRef]
- Teng, X.; Stefaniak, E.; Girvan, P.; Kotuniak, R.; Płonka, D.; Bal, W.; Ying, L. Hierarchical binding of copperII to N-truncated Aβ4–16 peptide. Metallomics 2020, 12, 470–473. [Google Scholar] [CrossRef]
- Esmieu, C.; Ferrand, G.; Borghesani, V.; Hureau, C. Impact of N-Truncated Aβ Peptides on Cu- and Cu(Aβ)-Generated ROS: Cu(I) Matters! Chem. A Eur. J. 2021, 27, 1777–1786. [Google Scholar] [CrossRef]
- Santoro, A.; Walke, G.; Vileno, B.; Kulkarni, P.P.; Raibaut, L.; Faller, P. Low catalytic activity of the Cu(II)-binding motif (Xxx-Zzz-His; ATCUN) in reactive oxygen species production and inhibition by the Cu(I)-chelator BCS. Chem. Commun. 2018, 54, 11945–11948. [Google Scholar] [CrossRef]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef]
- Rice, M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000, 23, 209–216. [Google Scholar] [CrossRef]
- Conte-Daban, A.; Boff, B.; Candido Matias, A.; Montes Aparicio, C.N.; Gateau, C.; Lebrun, C.; Cerchiaro, G.; Kieffer, I.; Sayen, S.; Guillon, E.; et al. A trishistidine pseudopeptide with ability to remove both Cu(I) and Cu(II) from the Alzheimer’s peptide and to stop the associated ROS formation. Chem. Eur. J. 2017, 23, 17078–17088. [Google Scholar] [CrossRef]
- Conte-Daban, A.; Beyler, M.; Tripier, R.; Hureau, C. Corrigendum: Kinetics Are Crucial When Targeting Copper Ions to Fight Alzheimer’s Disease: An Illustration with Azamacrocyclic Ligands. Chem. Eur. J. 2018, 24, 13058. [Google Scholar] [CrossRef]
- Stefaniak, E.; Bal, W. Cu(II) Binding Properties of N-Truncated Aβ Peptides: In Search of Biological Function. Inorg. Chem. 2019, 58, 13561–13577. [Google Scholar] [CrossRef]
- Rostagno, A.; Cabrera, E.; Lashley, T.; Ghiso, J. N-terminally truncated Aβ4-x proteoforms and their relevance for Alzheimer’s pathophysiology. Transl. Neurodegener. 2022, 11, 30. [Google Scholar] [CrossRef]
- Borghesani, V.; Alies, B.; Hureau, C. Cu(II) binding to various forms of amyloid-β peptides. Are they friends or foes ? Eur. J. Inorg. Chem. 2018, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Domingo, G.; Benussi, L.; Saraceno, C.; Bertuzzi, M.; Nicsanu, R.; Longobardi, A.; Bellini, S.; Cagnotto, A.; Salmona, M.; Binetti, G.; et al. N-Terminally Truncated and Pyroglutamate-Modified Aβ Forms Are Measurable in Human Cerebrospinal Fluid and Are Potential Markers of Disease Progression in Alzheimer’s Disease. Front. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Frączyk, T.; Cieplak, P. Neglected N-Truncated Amyloid-β Peptide and Its Mixed Cu–Zn Complexes. Protein J. 2022, 41, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Wezynfeld, N.E.; Tobolska, A.; Mital, M.; Wawrzyniak, U.E.; Wiloch, M.Z.; Płonka, D.; Bossak-Ahmad, K.; Wróblewski, W.; Bal, W. Aβ5–x Peptides: N-Terminal Truncation Yields Tunable Cu(II) Complexes. Inorg. Chem. 2020, 59, 14000–14011. [Google Scholar] [CrossRef] [PubMed]
- Tobolska, A.; Wezynfeld, N.E.; Wawrzyniak, U.E.; Bal, W.; Wróblewski, W. Copper(II) complex of N-truncated amyloid-β peptide bearing a His-2 motif as a potential receptor for phosphate anions. Dalton Trans. 2021, 50, 2726–2730. [Google Scholar] [CrossRef]
- Bossak-Ahmad, K.; Mital, M.; Płonka, D.; Drew, S.C.; Bal, W. Oligopeptides Generated by Neprilysin Degradation of β-Amyloid Have the Highest Cu(II) Affinity in the Whole Aβ Family. Inorg. Chem. 2019, 58, 932–943. [Google Scholar] [CrossRef]
- Crouch, P.J.; Barnham, K.J. Therapeutic redistribution of metal ions to treat Alzheimer’s disease. Acc. Chem. Res. 2012, 45, 1604–1611. [Google Scholar] [CrossRef]
- Crouch, P.J.; Savva, M.S.; Hung, L.W.; Donnelly, P.S.; Mot, A.I.; Parker, S.J.; Greenough, M.A.; Volitakis, I.; Adlard, P.A.; Cherny, R.A.; et al. The Alzheimer’s therapeutic PBT2 promotes amyloid-β degradation and GSK3 phosphorylation via a metal chaperone activity. J. Neurochem. 2011, 119, 220–230. [Google Scholar] [CrossRef]
- Filiz, G.; Price, K.A.; Caragounis, A.; Du, T.; Crouch, P.J.; White, A.R. The role of metals in modulating metalloprotease activity in the AD brain. Eur. Biophys. J. 2008, 37, 315–321. [Google Scholar] [CrossRef]







| Conditions [a] | t1/2 [ms] | Corresponding Mainly to |
|---|---|---|
| AH + Cu | <5 | / |
| AAH + Cu | 150 ± 20 | 2N to 4N conversion |
| AHH + Cu | 18 ± 20 | 2N and/or 3N to 4N conversion |
| AHH + Cu(Aβ) | 250 ± 50 | Formation of 2N and/or 3N intermediates |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez, P.; Sabater, L.; Mathieu, E.; Faller, P.; Hureau, C. Why the Ala-His-His Peptide Is an Appropriate Scaffold to Remove and Redox Silence Copper Ions from the Alzheimer’s-Related Aβ Peptide. Biomolecules 2022, 12, 1327. https://doi.org/10.3390/biom12101327
Gonzalez P, Sabater L, Mathieu E, Faller P, Hureau C. Why the Ala-His-His Peptide Is an Appropriate Scaffold to Remove and Redox Silence Copper Ions from the Alzheimer’s-Related Aβ Peptide. Biomolecules. 2022; 12(10):1327. https://doi.org/10.3390/biom12101327
Chicago/Turabian StyleGonzalez, Paulina, Laurent Sabater, Emilie Mathieu, Peter Faller, and Christelle Hureau. 2022. "Why the Ala-His-His Peptide Is an Appropriate Scaffold to Remove and Redox Silence Copper Ions from the Alzheimer’s-Related Aβ Peptide" Biomolecules 12, no. 10: 1327. https://doi.org/10.3390/biom12101327
APA StyleGonzalez, P., Sabater, L., Mathieu, E., Faller, P., & Hureau, C. (2022). Why the Ala-His-His Peptide Is an Appropriate Scaffold to Remove and Redox Silence Copper Ions from the Alzheimer’s-Related Aβ Peptide. Biomolecules, 12(10), 1327. https://doi.org/10.3390/biom12101327