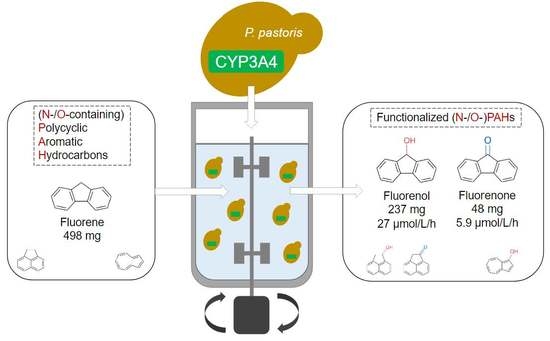
Preparative Production of Functionalized (N- and O-Heterocyclic) Polycyclic Aromatic Hydrocarbons by Human Cytochrome P450 3A4 in a Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Broad PAH Substrate Screening and HPLC
2.2. Bioreactor Biotransformations
2.3. NMR
3. Results and Discussion
3.1. Broad PAH Substrate Screening
3.2. Bioreactor Biotransformation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takikawa, H.; Nishii, A.; Sakai, T.; Suzuki, K. Aryne-Based Strategy in the Total Synthesis of Naturally Occurring Polycyclic Compounds. Chem. Soc. Rev. 2018, 47, 8030–8056. [Google Scholar] [CrossRef] [PubMed]
- Hussein, E.M.; Alsantali, R.I.; Abd El-Galil, S.M.; Obaid, R.J.; Alharbi, A.; Abourehab, M.A.S.; Ahmed, S.A. Bioactive Fluorenes. Part I. Synthesis, Pharmacological Study and Molecular Docking of Novel Dihydrofolate Reductase Inhibitors Based-2,7-Dichlorofluorene. Heliyon 2019, 5, e01982. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Gao, S. Recent Advances of Synthesis of Fluorenone and Fluorene Containing Natural Products. Tetrahedron 2016, 72, 1717–1735. [Google Scholar] [CrossRef]
- Tóth, B.; Hohmann, J.; Vasas, A. Phenanthrenes: A Promising Group of Plant Secondary Metabolites. J. Nat. Prod. 2018, 81, 661–678. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Han, L.; Sun, L.; Zheng, D.; Liu, J.; Fu, Y.; Huang, X.; Wang, Z. Synthesis and Antitumor Activities of Phenanthrene-Based Alkaloids. Molecules 2009, 14, 5042–5053. [Google Scholar] [CrossRef] [Green Version]
- Badria, F.A.; Ibrahim, A.S. Evaluation of Natural Anthracene-Derived Compounds as Antimitotic Agents. Drug Discov. Ther. 2013, 7, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debbab, A.; Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Pretsch, A.; Pescitelli, G.; Kurtan, T.; Proksch, P. New Anthracene Derivatives-Structure Elucidation and Antimicrobial Activity. Eur. J. Org. Chem. 2012, 2012, 1351–1359. [Google Scholar] [CrossRef]
- Lasák, P.; Motyka, K.; Kryštof, V.; Stýskala, J. Synthesis, Bacteriostatic and Anticancer Activity of Novel Phenanthridines Structurally Similar to Benzo[c]Phenanthridine Alkaloids. Molecules 2018, 23, 2155. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, L.R.; Wallace, S.; Haigh, D.; Patton, E.E.; Hulme, A.N. Rapid Synthesis and Zebrafish Evaluation of a Phenanthridine-Based Small Molecule Library. Org. Biomol. Chem. 2011, 9, 2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aumaitre, C.; Morin, J. Polycyclic Aromatic Hydrocarbons as Potential Building Blocks for Organic Solar Cells. Chem. Rec. 2019, 19, 1142–1154. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Koh, C.W.; Zhou, X.; Sun, H.; Yu, J.; Yang, K.; Wang, H.; Liao, Q.; Woo, H.Y.; et al. Facile Synthesis of Polycyclic Aromatic Hydrocarbon (PAH)–Based Acceptors with Fine-Tuned Optoelectronic Properties: Toward Efficient Additive-Free Nonfullerene Organic Solar Cells. Adv. Energy Mater. 2019, 9, 1803976. [Google Scholar] [CrossRef]
- Lo, P.-Y.; Lee, G.-Y.; Zheng, J.-H.; Huang, J.-H.; Cho, E.-C.; Lee, K.-C. Intercalating Pyrene with Polypeptide as a Novel Self-Assembly Nano-Carrier for Colon Cancer Suppression In Vitro and In Vivo. Mater. Sci. Eng. C 2020, 109, 110593. [Google Scholar] [CrossRef] [PubMed]
- Tümay, S.O.; Haddad Irani-nezhad, M.; Khataee, A. Design of Novel Anthracene-Based Fluorescence Sensor for Sensitive and Selective Determination of Iron in Real Samples. J. Photochem. Photobiol. A Chem. 2020, 402, 112819. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Moradi, R.; Lashgari, N.; Kruger, H.G. Fluorene Dyes. In Metal-Free Synthetic Organic Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–164. ISBN 9780128156476. [Google Scholar]
- Van Damme, J.; Du Prez, F. Anthracene-Containing Polymers toward High-End Applications. Prog. Polym. Sci. 2018, 82, 92–119. [Google Scholar] [CrossRef]
- Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Vachal, P.; Krska, S.W. The Medicinal Chemist’s Toolbox for Late Stage Functionalization of Drug-like Molecules. Chem. Soc. Rev. 2016, 45, 546–576. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Singh, P.K.; Silakari, O. The Current Status of O-Heterocycles: A Synthetic and Medicinal Overview. ChemMedChem 2018, 13, 1071–1087. [Google Scholar] [CrossRef]
- Fessner, N.D. P450 Monooxygenases Enable Rapid Late-Stage Diversification of Natural Products via C−H Bond Activation. ChemCatChem 2019, 11, 2226–2242. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Feng, X. Bottom-Up Synthesis of Nitrogen-Doped Polycyclic Aromatic Hydrocarbons. Synlett 2020, 31, 211–222. [Google Scholar] [CrossRef]
- Hagui, W.; Doucet, H.; Soulé, J.F. Application of Palladium-Catalyzed C(Sp2)–H Bond Arylation to the Synthesis of Polycyclic (Hetero)Aromatics. Chem 2019, 5, 2006–2078. [Google Scholar] [CrossRef]
- Guengerich, F.P. Intersection of the Roles of Cytochrome P450 Enzymes with Xenobiotic and Endogenous Substrates: Relevance to Toxicity and Drug Interactions. Chem. Res. Toxicol. 2017, 30, 2–12. [Google Scholar] [CrossRef]
- Rendic, S.; Guengerich, F.P. Contributions of Human Enzymes in Carcinogen Metabolism. Chem. Res. Toxicol. 2012, 25, 1316–1383. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.; Guengerich, F.P. Survey of Human Oxidoreductases and Cytochrome P450 Enzymes Involved in the Metabolism of Xenobiotic and Natural Chemicals. Chem. Res. Toxicol. 2015, 28, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, T.; Murayama, N.; Kakimoto, K.; Takenaka, S.; Lim, Y.-R.; Yeom, S.; Kim, D.; Yamazaki, H.; Guengerich, F.P.; Komori, M. Oxidation of 1-Chloropyrene by Human CYP1 Family and CYP2A Subfamily Cytochrome P450 Enzymes: Catalytic Roles of Two CYP1B1 and Five CYP2A13 Allelic Variants. Xenobiotica 2017, 48, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Takenaka, S.; Kakimoto, K.; Murayama, N.; Lim, Y.-R.; Kim, D.; Foroozesh, M.K.; Yamazaki, H.; Guengerich, F.P.; Komori, M. Structure–Function Studies of Naphthalene, Phenanthrene, Biphenyl, and Their Derivatives in Interaction with and Oxidation by Cytochromes P450 2A13 and 2A6. Chem. Res. Toxicol. 2016, 29, 1029–1040. [Google Scholar] [CrossRef] [Green Version]
- Shimada, T.; Takenaka, S.; Murayama, N.; Yamazaki, H.; Kim, J.-H.; Kim, D.; Yoshimoto, F.K.; Guengerich, F.P.; Komori, M. Oxidation of Acenaphthene and Acenaphthylene by Human Cytochrome P450 Enzymes. Chem. Res. Toxicol. 2015, 28, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Guengerich, F.P. 2017 Human P450 Xenobiotics Toxicity. Chem. Res. Toxicol. 2017, 30, 2–12. [Google Scholar] [CrossRef]
- Luckert, C.; Ehlers, A.; Buhrke, T.; Seidel, A.; Lampen, A.; Hessel, S. Polycyclic Aromatic Hydrocarbons Stimulate Human CYP3A4 Promoter Activity via PXR. Toxicol. Lett. 2013, 222, 180–188. [Google Scholar] [CrossRef]
- Winkler, M.; Geier, M.; Hanlon, S.P.; Nidetzky, B.; Glieder, A. Human Enzymes for Organic Synthesis. Angew. Chem. Int. Ed. 2018, 57, 13406–13423. [Google Scholar] [CrossRef] [Green Version]
- Fessner, N.D.; Grimm, C.; Srdic, M.; Weber, H.; Kroutil, W.; Schwaneberg, U.; Glieder, A. Natural Product Diversification by One-Step Biocatalysis Using Human P450 3A4. ChemCatChem 2021. [Google Scholar] [CrossRef]
- Bernhardt, R. Cytochromes P450 as Versatile Biocatalysts. J. Biotechnol. 2006, 124, 128–145. [Google Scholar] [CrossRef]
- Dong, J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic Oxidation Reactions: A Chemist’s Perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261. [Google Scholar] [CrossRef]
- Martinez, C.; Rupashinghe, S. Cytochrome P450 Bioreactors in the Pharmaceutical Industry: Challenges and Opportunities. Curr. Top. Med. Chem. 2013, 13, 1470–1490. [Google Scholar] [CrossRef]
- Zöllner, A.; Buchheit, D.; Meyer, M.R.; Maurer, H.H.; Peters, F.T.; Bureik, M. Production of Human Phase 1 and 2 Metabolites by Whole-Cell Biotransformation with Recombinant Microbes. Bioanalysis 2010, 2, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Urlacher, V.B.; Girhard, M. Cytochrome P450 Monooxygenases in Biotechnology and Synthetic Biology. Trends Biotechnol. 2019, 37, 882–897. [Google Scholar] [CrossRef]
- Bernhardt, R.; Urlacher, V.B. Cytochromes P450 as Promising Catalysts for Biotechnological Application: Chances and Limitations. Appl. Microbiol. Biotechnol. 2014, 98, 6185–6203. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Wang, Y.; Perkins, J.C.; Narayan, A.R.H. Scalable Biocatalytic C-H Oxyfunctionalization Reactions. Chem. Soc. Rev. 2020, 49, 8137–8155. [Google Scholar] [CrossRef] [PubMed]
- Tieves, F.; Tonin, F.; Fernández-Fueyo, E.; Robbins, J.M.; Bommarius, B.; Bommarius, A.S.; Alcalde, M.; Hollmann, F. Energising the E-Factor: The E+-Factor. Tetrahedron 2019, 75, 1311–1314. [Google Scholar] [CrossRef]
- Islam, R.S.; Tisi, D.; Levy, M.S.; Lye, G.J. Scale-up of Escherichia Coli Growth and Recombinant Protein Expression Conditions from Microwell to Laboratory and Pilot Scale Based on Matched k La. Biotechnol. Bioeng. 2008, 99, 1128–1139. [Google Scholar] [CrossRef]
- Marques, M.P.C.; Cabral, J.M.S.; Fernandes, P. Bioprocess Scale-up: Quest for the Parameters to Be Used as Criterion to Move from Microreactors to Lab-Scale. J. Chem. Technol. Biotechnol. 2010, 85, 1184–1198. [Google Scholar] [CrossRef]
- Geier, M.; Braun, A.; Emmerstorfer, A.; Pichler, H.; Glieder, A. Production of Human Cytochrome P450 2D6 Drug Metabolites with Recombinant Microbes—A Comparative Study. Biotechnol. J. 2012, 7, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Fessner, N.D.; Srdič, M.; Weber, H.; Schmid, C.; Schönauer, D.; Schwaneberg, U.; Glieder, A. Preparative-Scale Production of Testosterone Metabolites by Human Liver Cytochrome P450 Enzyme 3A4. Adv. Synth. Catal. 2020, 362, 2725–2738. [Google Scholar] [CrossRef]
- Fessner, N.D.; Nelson, D.R.; Glieder, A. Evolution and Enrichment of CYP5035 in Polyporales: Functionality of an Understudied P450 Family. Appl. Microbiol. Biotechnol. 2021, 105, 6779–6792. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Santos, G.; Dhoke, G.V.; Davari, M.D.; Ruff, A.J.; Schwaneberg, U. Directed Evolution of P450 BM3 towards Functionalization of Aromatic O-Heterocycles. Int. J. Mol. Sci. 2019, 20, 3353. [Google Scholar] [CrossRef] [Green Version]
- Sideri, A.; Goyal, A.; Di Nardo, G.; Tsotsou, G.E.; Gilardi, G. Hydroxylation of Non-Substituted Polycyclic Aromatic Hydrocarbons by Cytochrome P450 BM3 Engineered by Directed Evolution. J. Inorg. Biochem. 2013, 120, 1–7. [Google Scholar] [CrossRef]
- Sulistyaningdyah, W.T.; Ogawa, J.; Li, Q.-S.; Maeda, C.; Yano, Y.; Schmid, R.D.; Shimizu, S. Hydroxylation Activity of P450 BM-3 Mutant F87V towards Aromatic Compounds and Its Application to the Synthesis of Hydroquinone Derivatives from Phenolic Compounds. Appl. Microbiol. Biotechnol. 2005, 67, 556–562. [Google Scholar] [CrossRef]
- Li, Q.-S.; Ogawa, J.; Schmid, R.D.; Shimizu, S. Engineering Cytochrome P450 BM-3 for Oxidation of Polycyclic Aromatic Hydrocarbons. Appl. Environ. Microbiol. 2001, 67, 5735–5739. [Google Scholar] [CrossRef] [Green Version]
- Urlacher, V.; Schmid, R.D. Biotransformations Using Prokaryotic P450 Monooxygenases. Curr. Opin. Biotechnol. 2002, 13, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Syed, K.; Porollo, A.; Miller, D.; Yadav, J.S. Rational Engineering of the Fungal P450 Monooxygenase CYP5136A3 to Improve Its Oxidizing Activity toward Polycyclic Aromatic Hydrocarbons. Protein Eng. Des. Sel. 2013, 26, 553–557. [Google Scholar] [CrossRef]
- Syed, K.; Porollo, A.; Lam, Y.W.; Grimmett, P.E.; Yadav, J.S. CYP63A2, a Catalytically Versatile Fungal P450 Monooxygenase Capable of Oxidizing Higher-Molecular-Weight Polycyclic Aromatic Hydrocarbons, Alkylphenols, and Alkanes. Appl. Environ. Microbiol. 2013, 79, 2692–2702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, K.; Porollo, A.; Lam, Y.W.; Yadav, J.S. A Fungal P450 (CYP5136A3) Capable of Oxidizing Polycyclic Aromatic Hydrocarbons and Endocrine Disrupting Alkylphenols: Role of Trp129 and Leu324. PLoS ONE 2011, 6, e28286. [Google Scholar] [CrossRef] [Green Version]
- Syed, K.; Doddapaneni, H.; Subramanian, V.; Lam, Y.W.; Yadav, J.S. Genome-to-Function Characterization of Novel Fungal P450 Monooxygenases Oxidizing Polycyclic Aromatic Hydrocarbons (PAHs). Biochem. Biophys. Res. Commun. 2010, 399, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Torres, E.; Hayen, H.; Niemeyer, C.M. Evaluation of Cytochrome P450BSβ Reactivity against Polycyclic Aromatic Hydrocarbons and Drugs. Biochem. Biophys. Res. Commun. 2007, 355, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Fessner, N.D.; Grimm, C.; Kroutil, W.; Glieder, A. Late-Stage Functionalisation of Polycyclic (N-Hetero-) Aromatic Hydrocarbons by Detoxifying CYP5035S7 Monooxygenase of the White-Rot Fungus Polyporus Arcularius. Biomolecules 2021, 11, 1708. [Google Scholar] [CrossRef]
- Invitrogen. Pichia Fermentation Process Guidelines Overview; Invitrogen: 2002. 2021. Available online: http://tools.thermofisher.com/content/sfs/manuals/pichiaferm_prot.pdf (accessed on 15 November 2021).
- Schiavini, P.; Cheong, K.J.; Moitessier, N.; Auclair, K. Active Site Crowding of Cytochrome P450 3A4 as a Strategy To Alter Its Selectivity. ChemBioChem 2017, 18, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.C. Membrane-Embedded Substrate Recognition by Cytochrome P450 3A4. J. Biol. Chem. 2018, 293, 4037–4046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekroos, M.; Sjögren, T. Structural Basis for Ligand Promiscuity in Cytochrome P450 3A4. Proc. Natl. Acad. Sci. USA 2006, 103, 13682–13687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paloncýová, M.; Navrátilová, V.; Berka, K.; Laio, A.; Otyepka, M. Role of Enzyme Flexibility in Ligand Access and Egress to Active Site: Bias-Exchange Metadynamics Study of 1,3,7-Trimethyluric Acid in Cytochrome P450 3A4. J. Chem. Theory Comput. 2016, 12, 2101–2109. [Google Scholar] [CrossRef]
- Donato, M.T.; Jiménez, N.; Castell, J.V.; Gómez-Lechón, M.J. Fluorescence-Based Assays for Screening Nine Cytochrome P450 (P450) Activities in Intact Cells Expressing Individual Human P450 Enzymes. Drug Metab. Dispos. 2004, 32, 699–706. [Google Scholar] [CrossRef] [Green Version]
- Rudroff, F. Whole-Cell Based Synthetic Enzyme Cascades—Light and Shadow of a Promising Technology. Curr. Opin. Chem. Biol. 2019, 49, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, Z. Whole-Cell Cascade Biotransformations for One-Pot Multistep Organic Synthesis. ChemCatChem 2018, 10, 2164–2178. [Google Scholar] [CrossRef]
- Weißhoff, H.; Preiß, A.; Nehls, I.; Win, T.; Mügge, C. Development of an HPLC-NMR Method for the Determination of PAHs in Soil Samples—A Comparison with Conventional Methods. Anal. Bioanal. Chem. 2002, 373, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.-P. Performance Metrics for Sustainable Catalysis in Industry. Nat. Catal. 2021, 4, 186–192. [Google Scholar] [CrossRef]
- Vail, R.B.; Homann, M.J.; Hanna, I.; Zaks, A. Preparative Synthesis of Drug Metabolites Using Human Cytochrome P450s 3A4, 2C9 and 1A2 with NADPH-P450 Reductase Expressed in Escherichia Coli. J. Ind. Microbiol. Biotechnol. 2005, 32, 67–74. [Google Scholar] [CrossRef]




| 0 min (mol%) | 420 min (mol%) | 1560 min (mol%) | |
|---|---|---|---|
| Fluorene (1) | 97.1 | 52.9 | 42.7 |
| Fluorenol (2) | 2.9 | 38.8 | 47.6 |
| Fluorenone (3) | 0.0 | 8.3 | 9.7 |
| Sampled weight in 200 mL | 15.2 mg | 14.7 mg | 15.4 mg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srdič, M.; Fessner, N.D.; Yildiz, D.; Glieder, A.; Spiertz, M.; Schwaneberg, U. Preparative Production of Functionalized (N- and O-Heterocyclic) Polycyclic Aromatic Hydrocarbons by Human Cytochrome P450 3A4 in a Bioreactor. Biomolecules 2022, 12, 153. https://doi.org/10.3390/biom12020153
Srdič M, Fessner ND, Yildiz D, Glieder A, Spiertz M, Schwaneberg U. Preparative Production of Functionalized (N- and O-Heterocyclic) Polycyclic Aromatic Hydrocarbons by Human Cytochrome P450 3A4 in a Bioreactor. Biomolecules. 2022; 12(2):153. https://doi.org/10.3390/biom12020153
Chicago/Turabian StyleSrdič, Matic, Nico D. Fessner, Deniz Yildiz, Anton Glieder, Markus Spiertz, and Ulrich Schwaneberg. 2022. "Preparative Production of Functionalized (N- and O-Heterocyclic) Polycyclic Aromatic Hydrocarbons by Human Cytochrome P450 3A4 in a Bioreactor" Biomolecules 12, no. 2: 153. https://doi.org/10.3390/biom12020153
APA StyleSrdič, M., Fessner, N. D., Yildiz, D., Glieder, A., Spiertz, M., & Schwaneberg, U. (2022). Preparative Production of Functionalized (N- and O-Heterocyclic) Polycyclic Aromatic Hydrocarbons by Human Cytochrome P450 3A4 in a Bioreactor. Biomolecules, 12(2), 153. https://doi.org/10.3390/biom12020153