Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives
Abstract
:1. Introduction
2. Materials and Methods
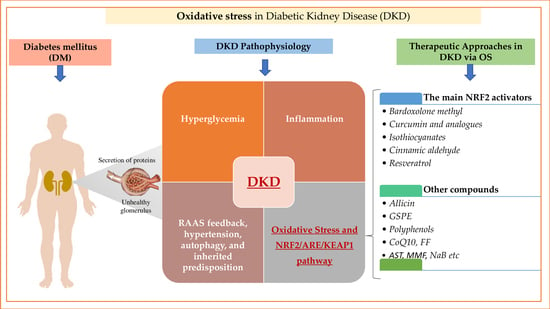
3. Mechanism and Pathophysiology of Diabetic Kidney Disease
3.1. The Involvement of Hyperglycemia and Inflammation
3.2. The Key Role of Oxidative Stress in Diabetic Kidney Disease
4. NRF2/ARE/KEAP1 Pathway: Components and Functions
5. Therapeutic Approaches in Diabetic Kidney Disease via Oxidative Stress
5.1. The Main NRF2 Activators
5.1.1. Bardoxolone Methyl
5.1.2. Curcumin and Analogues
5.1.3. Isothiocyanates and the Main Representatives: Sulforaphane, Moringa Isothiocyanate
5.1.4. Cinnamic Aldehyde
5.1.5. Resveratrol
5.2. Other Compounds That Modulate the NRF2/KEAP1/ARE Pathway
5.2.1. Allicin
5.2.2. Grape Seed Proanthocyanidins Extract and Eucommia Ulmoides
5.2.3. Other Polyphenols
5.2.4. Antioxidant Compounds Targeting Mitophagy
5.2.5. Astaxanthin (AST)
5.2.6. Drugs with NRF2 Activity
5.2.7. Less-Known NRF2 Activators
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dey, P.; Saha, M.R.; Roy Choudhuri, S.; Sarkar, I.; Halder, B.; Poddar-Sarkar, M.; Sen, A.; Chaudhuri, T.K. Oleander Stem and Root Standardized Extracts Mitigate Acute Hyperglycaemia by Limiting Systemic Oxidative Stress Response in Diabetic Mice. Adv. Pharmacol. Sci. 2019, 2019, 7865359. [Google Scholar] [CrossRef]
- International Diabetes Federation Atlas 10th Edition 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 1 August 2022).
- American Diabetes Association. Standards of Medical Care in Diabetes—2022 Abridged for Primary Care Providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Buendía, A.S.; Castañeda-Lara, L.G.; Loredo-Mendoza, M.L.; García-Arroyo, F.E.; Rojas-Morales, P.; Argüello-García, R.; Juárez-Rojas, J.G.; Tapia, E.; Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; et al. Effects of Allicin on Pathophysiological Mechanisms during the Progression of Nephropathy Associated to Diabetes. Antioxidants 2020, 9, 1134. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.M.; Jha, J.C. Oxidative Stress and Inflammation in Renal and Cardiovascular Complications of Diabetes. Biology 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Yang, R.; Liu, X.; Ma, S.; Wang, L. Genistein Attenuates Renal Fibrosis in Streptozotocin-induced Diabetic Rats. Mol. Med. Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.; Al-Waili, H.; Al-Waili, T.; Salom, K. Natural Antioxidants in the Treatment and Prevention of Diabetic Nephropathy; a Potential Approach That Warrants Clinical Trials. Redox Rep. 2017, 22, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Civantos, E.; Bosch, E.; Ramírez Bustillo, E.; Zhenyukh, O.; Egido, J.; Lorenzo, O.; Mas, S. Sitagliptin Ameliorates Oxidative Stress in Experimental Diabetic Nephropathy by Diminishing the MiR-200a/Keap-1/Nrf2 Antioxidant Pathway. Diabetes Metab. Syndr. Obes. 2017, 10, 207–222. [Google Scholar] [CrossRef]
- Landstra, C.P.; de Koning, E.J.P. COVID-19 and Diabetes: Understanding the Interrelationship and Risks for a Severe Course. Front. Endocrinol. 2021, 12, 649525. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Ferrannini, E. Anti-Inflammatory Properties of Antidiabetic Drugs: A “Promised Land” in the COVID-19 Era? J. Diabetes Its Complicat. 2020, 34, 107723. [Google Scholar] [CrossRef]
- Yamazaki, T.; Mimura, I.; Tanaka, T.; Nangaku, M. Treatment of Diabetic Kidney Disease: Current and Future. Diabetes Metab. J. 2021, 45, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Sakashita, M.; Tanaka, T.; Inagi, R. Metabolic Changes and Oxidative Stress in Diabetic Kidney Disease. Antioxidants 2021, 10, 1143. [Google Scholar] [CrossRef]
- Cui, W.; Min, X.; Xu, X.; Du, B.; Luo, P. Role of Nuclear Factor Erythroid 2-Related Factor 2 in Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 3797802. [Google Scholar] [CrossRef] [PubMed]
- Alaofi, A.L. Sinapic Acid Ameliorates the Progression of Streptozotocin (STZ)-Induced Diabetic Nephropathy in Rats via NRF2/HO-1 Mediated Pathways. Front. Pharmacol. 2020, 11, 1119. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, X.; Ma, F.; Sun, W.; Wang, W.; Yu, J.; Shi, Y.; Cai, L.; Xu, Z. The Role of Akt2 in the Protective Effect of Fenofibrate against Diabetic Nephropathy. Int. J. Biol. Sci. 2020, 16, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, R.D.; Dissard, R.; Faivre, A.; Ino, F.; Delitsikou, V.; Jaquet, V.; Cagarelli, T.; Lindenmeyer, M.; Jansen-Duerr, P.; Cohen, C.; et al. Tubular NOX4 Expression Decreases in Chronic Kidney Disease but Does Not Modify Fibrosis Evolution. Redox Biol. 2019, 26, 101234. [Google Scholar] [CrossRef]
- Gong, D.-J.; Wang, L.; Yang, Y.-Y.; Zhang, J.-J.; Liu, X.-H. Diabetes Aggravates Renal Ischemia and Reperfusion Injury in Rats by Exacerbating Oxidative Stress, Inflammation, and Apoptosis. Ren. Fail. 2019, 41, 750–761. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, H.; Wang, X.; Gao, Q.; Li, Z.; Huang, H. CoQ10 Ameliorates Mitochondrial Dysfunction in Diabetic Nephropathy through Mitophagy. J. Endocrinol. 2019, 240, 445–465. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Sharma, E.; Kumar, A.; Grover, M.; Bungau, S. Unfolding Nrf2 in Diabetes Mellitus. Mol. Biol. Rep. 2021, 48, 927–939. [Google Scholar] [CrossRef]
- Jung, S.W.; Moon, J.-Y. The Role of Inflammation in Diabetic Kidney Disease. Korean J. Intern. Med. 2021, 36, 753–766. [Google Scholar] [CrossRef]
- Martini, S.; Nair, V.; Keller, B.J.; Eichinger, F.; Hawkins, J.J.; Randolph, A.; Böger, C.A.; Gadegbeku, C.A.; Fox, C.S.; Cohen, C.D.; et al. Integrative Biology Identifies Shared Transcriptional Networks in CKD. J. Am. Soc. Nephrol. 2014, 25, 2559–2572. [Google Scholar] [CrossRef] [Green Version]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Lim, J.H.; Kim, M.Y.; Kim, Y.; Park, H.S.; Kim, H.W.; Choi, B.S.; Chang, Y.S.; Kim, H.W.; Kim, T.-Y.; et al. Extracellular Superoxide Dismutase Attenuates Renal Oxidative Stress Through the Activation of Adenosine Monophosphate-Activated Protein Kinase in Diabetic Nephropathy. Antioxid. Redox Signal. 2018, 28, 1543–1561. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of Diabetic Kidney Disease: Impact of SGLT2 Inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Pak, W.L.W.; Tanaka, T.; Tang, S.C.W.; Nangaku, M. Update on Diagnosis, Pathophysiology, and Management of Diabetic Kidney Disease. Nephrology 2021, 26, 491–500. [Google Scholar] [CrossRef]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.-H.; Hsu, Y.-C.; Chen, T.-H.; Lin, C.-L. Recent Advances in Diabetic Kidney Diseases: From Kidney Injury to Kidney Fibrosis. Int. J. Mol. Sci. 2021, 22, 11857. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Alicic, R.Z. Glycemic Variability and KIM-1–Induced Inflammation in the Diabetic Kidney. Diabetes 2021, 70, 1617–1619. [Google Scholar] [CrossRef]
- Khan, N.U.; Lin, J.; Liu, X.; Li, H.; Lu, W.; Zhong, Z.; Zhang, H.; Waqas, M.; Shen, L. Insights into Predicting Diabetic Nephropathy Using Urinary Biomarkers. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1868, 140475. [Google Scholar] [CrossRef]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Targeting Redox Imbalance as an Approach for Diabetic Kidney Disease. Biomedicines 2020, 8, 40. [Google Scholar] [CrossRef]
- Wei, P.Z.; Szeto, C.C. Mitochondrial Dysfunction in Diabetic Kidney Disease. Clin. Chim. Acta 2019, 496, 108–116. [Google Scholar] [CrossRef]
- Do, M.H.; Lee, J.H.; Cho, K.; Kang, M.C.; Subedi, L.; Parveen, A.; Kim, S.Y. Therapeutic Potential of Lespedeza Bicolor to Prevent Methylglyoxal-Induced Glucotoxicity in Familiar Diabetic Nephropathy. J. Clin. Med. 2019, 8, 1138. [Google Scholar] [CrossRef] [PubMed]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kanazawa, Y.; Kawanami, D.; Yokota, T.; Utsunomiya, K.; Nishimura, R. ROCK Inhibition May Stop Diabetic Kidney Disease. JMA J. 2020, 3, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Matoba, K.; Takeda, Y.; Nagai, Y.; Kawanami, D.; Utsunomiya, K.; Nishimura, R. Unraveling the Role of Inflammation in the Pathogenesis of Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3393. [Google Scholar] [CrossRef]
- Cerbaro, A.F.; Rodrigues, V.S.B.; Rigotti, M.; Branco, C.S.; Rech, G.; de Oliveira, D.L.; Salvador, M. Grape Seed Proanthocyanidins Improves Mitochondrial Function and Reduces Oxidative Stress through an Increase in Sirtuin 3 Expression in EA.Hy926 Cells in High Glucose Condition. Mol. Biol. Rep. 2020, 47, 3319–3330. [Google Scholar] [CrossRef] [PubMed]
- Winiarska, A.; Knysak, M.; Nabrdalik, K.; Gumprecht, J.; Stompór, T. Inflammation and Oxidative Stress in Diabetic Kidney Disease: The Targets for SGLT2 Inhibitors and GLP-1 Receptor Agonists. Int. J. Mol. Sci. 2021, 22, 10822. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Johnson, E.J.; Tuttle, K.R. Inflammatory Mechanisms as New Biomarkers and Therapeutic Targets for Diabetic Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 181–191. [Google Scholar] [CrossRef]
- Pichler, R.; Afkarian, M.; Dieter, B.P.; Tuttle, K.R. Immunity and Inflammation in Diabetic Kidney Disease: Translating Mechanisms to Biomarkers and Treatment Targets. Am. J. Physiol. Ren. Physiol. 2017, 312, F716–F731. [Google Scholar] [CrossRef]
- Dragoș, D.; Manea, M.M.; Timofte, D.; Ionescu, D. Mechanisms of Herbal Nephroprotection in Diabetes Mellitus. J. Diabetes Res. 2020, 2020, 5710513. [Google Scholar] [CrossRef]
- Su, L.; Cao, P.; Wang, H. Tetrandrine Mediates Renal Function and Redox Homeostasis in a Streptozotocin-Induced Diabetic Nephropathy Rat Model through Nrf2/HO-1 Reactivation. Ann. Transl. Med. 2020, 8, 990. [Google Scholar] [CrossRef]
- Wang, D.; Jin, M.; Zhao, X.; Zhao, T.; Lin, W.; He, Z.; Fan, M.; Jin, W.; Zhou, J.; Jin, L.; et al. FGF1ΔHBS Ameliorates Chronic Kidney Disease via PI3K/AKT Mediated Suppression of Oxidative Stress and Inflammation. Cell Death Dis. 2019, 10, 464. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Sámano, M.Á.; Grajales-Gómez, M.; Zuarth-Vázquez, J.M.; Navarro-Flores, M.F.; Martínez-Saavedra, M.; Juárez-León, Ó.A.; Morales-García, M.G.; Enríquez-Estrada, V.M.; Gómez-Pérez, F.J.; Cuevas-Ramos, D. Fibroblast Growth Factor 21 and Its Novel Association with Oxidative Stress. Redox Biol. 2017, 11, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Alabi, T.D.; Brooks, N.L.; Oguntibeju, O.O. Leaf Extracts of Anchomanes Difformis Ameliorated Kidney and Pancreatic Damage in Type 2 Diabetes. Plants 2021, 10, 300. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Park, C.W. Catalytic Antioxidants in the Kidney. Antioxidants 2021, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Chung, S.; Kim, S.; Son, M.; Kim, M.; Koh, E.; Shin, S.; Park, C.; Kim, H.-S. Inhibition of P300/CBP-Associated Factor Attenuates Renal Tubulointerstitial Fibrosis through Modulation of NF-KB and Nrf2. Int. J. Mol. Sci. 2019, 20, 1554. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A. Molecular Interactions Between Reactive Oxygen Species and Autophagy in Kidney Disease. Int. J. Mol. Sci. 2019, 20, 3791. [Google Scholar] [CrossRef]
- Nezu, M.; Suzuki, N. Roles of Nrf2 in Protecting the Kidney from Oxidative Damage. Int. J. Mol. Sci. 2020, 21, 2951. [Google Scholar] [CrossRef]
- Shi, S.; Lei, S.; Tang, C.; Wang, K.; Xia, Z. Melatonin Attenuates Acute Kidney Ischemia/Reperfusion Injury in Diabetic Rats by Activation of the SIRT1/Nrf2/HO-1 Signaling Pathway. Biosci. Rep. 2019, 39, BSR20181614. [Google Scholar] [CrossRef]
- Ito, M.; Tanaka, T.; Nangaku, M. Nuclear Factor Erythroid 2-Related Factor 2 as a Treatment Target of Kidney Diseases. Curr. Opin. Nephrol. Hypertens. 2020, 29, 128–135. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 Signaling in Oxidative and Reductive Stress. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Ulasov, A.V.; Rosenkranz, A.A.; Georgiev, G.P.; Sobolev, A.S. Nrf2/Keap1/ARE Signaling: Towards Specific Regulation. Life Sci. 2022, 291, 120111. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Sundarraj, K.; Nagarajan, R.; Arfuso, F.; Bian, J.; Kumar, A.P.; Sethi, G.; Perumal, E. Antioxidant Response Elements: Discovery, Classes, Regulation and Potential Applications. Redox Biol. 2018, 17, 297–314. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; Rayego-Mateos, S.; Vázquez-Carballo, C.; Palomino-Antolín, A.; García-Caballero, C.; Opazo-Rios, L.; Morgado-Pascual, J.L.; Herencia, C.; Mas, S.; Ortiz, A.; et al. Protective Role of Nrf2 in Renal Disease. Antioxidants 2020, 10, 39. [Google Scholar] [CrossRef]
- Kopacz, A.; Kloska, D.; Forman, H.J.; Jozkowicz, A.; Grochot-Przeczek, A. Beyond Repression of Nrf2: An Update on Keap1. Free Radic. Biol. Med. 2020, 157, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Kanlaya, R.; Thongboonkerd, V. Molecular Mechanisms of Epigallocatechin-3-Gallate for Prevention of Chronic Kidney Disease and Renal Fibrosis: Preclinical Evidence. Curr. Dev. Nutr. 2019, 3, nzz101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, Z.; Gong, W.; Zou, Y.; Xu, F.; Chen, L.; Huang, H. Paeonol Ameliorates Diabetic Renal Fibrosis Through Promoting the Activation of the Nrf2/ARE Pathway via Up-Regulating Sirt1. Front. Pharmacol. 2018, 9, 512. [Google Scholar] [CrossRef]
- Li, W.; Yu, S.-W.; Kong, A.-N.T. Nrf2 Possesses a Redox-Sensitive Nuclear Exporting Signal in the Neh5 Transactivation Domain. J. Biol. Chem. 2006, 281, 27251–27263. [Google Scholar] [CrossRef]
- Arellano-Buendía, A.S.; Tostado-González, M.; García-Arroyo, F.E.; Cristóbal-García, M.; Loredo-Mendoza, M.L.; Tapia, E.; Sánchez-Lozada, L.-G.; Osorio-Alonso, H. Anti-Inflammatory Therapy Modulates Nrf2-Keap1 in Kidney from Rats with Diabetes. Oxidative Med. Cell. Longev. 2016, 2016, 4693801. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Gao, L.; Su, S.; Sargsyan, D.; Wu, R.; Raskin, I.; Kong, A.-N. Moringa Isothiocyanate Activates Nrf2: Potential Role in Diabetic Nephropathy. AAPS J. 2019, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.A.d.S.; Santos, R.d.S.; Cruz, A.H.d.S.; da Silva, E.G.; da Cruz, A.D.; Pedrino, G.R. The Effect of Nrf2 on Diabetic Complications. In A Master Regulator of Oxidative Stress—The Transcription Factor Nrf2; Morales-Gonzalez, J.A., Morales-Gonzalez, A., Madrigal-Santillan, E.O., Eds.; InTech: London, UK, 2016; ISBN 978-953-51-2837-3. [Google Scholar]
- Bonner, R.; Albajrami, O.; Hudspeth, J.; Upadhyay, A. Diabetic Kidney Disease. Prim. Care Clin. Off. Pract. 2020, 47, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Mahn, A.; Castillo, A. Potential of Sulforaphane as a Natural Immune System Enhancer: A Review. Molecules 2021, 26, 752. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, Y.; Masuda, K. A Novel Nrf2 Activator from Microbial Transformation Inhibits Radiation-Induced Dermatitis in Mice. J. Radiat. Res. 2016, 57, 567–571. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Zhe, H.; Zhao, R. Preclinical Evidences toward the Use of Triterpenoid CDDO-Me for Solid Cancer Prevention and Treatment. Mol. Cancer 2014, 13, 30. [Google Scholar] [CrossRef]
- Zhou, S.-F.; Wang, Y.-Y.; Zhe, H.; Yang, Y.; He, Z. Bardoxolone Methyl (CDDO-Me) as a Therapeutic Agent: An Update on Its Pharmacokinetic and Pharmacodynamic Properties. Drug Des. Dev. Ther. 2014, 2075. [Google Scholar] [CrossRef]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone Methyl and Kidney Function in CKD with Type 2 Diabetes. N. Engl. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef]
- Nagaraj, S.; Youn, J.-I.; Weber, H.; Iclozan, C.; Lu, L.; Cotter, M.J.; Meyer, C.; Becerra, C.R.; Fishman, M.; Antonia, S.; et al. Anti-Inflammatory Triterpenoid Blocks Immune Suppressive Function of MDSCs and Improves Immune Response in Cancer. Clin. Cancer Res. 2010, 16, 1812–1823. [Google Scholar] [CrossRef]
- Hong, D.S.; Kurzrock, R.; Supko, J.G.; He, X.; Naing, A.; Wheler, J.; Lawrence, D.; Eder, J.P.; Meyer, C.J.; Ferguson, D.A.; et al. A Phase I First-in-Human Trial of Bardoxolone Methyl in Patients with Advanced Solid Tumors and Lymphomas. Clin. Cancer Res. 2012, 18, 3396–3406. [Google Scholar] [CrossRef]
- Pergola, P.E.; Krauth, M.; Huff, J.W.; Ferguson, D.A.; Ruiz, S.; Meyer, C.J.; Warnock, D.G. Effect of Bardoxolone Methyl on Kidney Function in Patients with T2D and Stage 3b–4 CKD. Am. J. Nephrol. 2011, 33, 469–476. [Google Scholar] [CrossRef]
- De Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Lambers Heerspink, H.J.; et al. Bardoxolone Methyl in Type 2 Diabetes and Stage 4 Chronic Kidney Disease. N. Engl. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Tayek, J.A.; Kalantar-Zadeh, K. The Extinguished BEACON of Bardoxolone: Not a Monday Morning Quarterback Story. Am. J. Nephrol. 2013, 37, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Vanholder, R. Bardoxolone Methyl, Chronic Kidney Disease, and Type 2 Diabetes. N. Engl. J. Med. 2011, 365, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Zoja, C.; Benigni, A.; Remuzzi, G. The Nrf2 Pathway in the Progression of Renal Disease. Nephrol. Dial. Transplant. 2014, 29, i19–i24. [Google Scholar] [CrossRef]
- Nangaku, M.; Kanda, H.; Takama, H.; Ichikawa, T.; Hase, H.; Akizawa, T. Randomized Clinical Trial on the Effect of Bardoxolone Methyl on GFR in Diabetic Kidney Disease Patients (TSUBAKI Study). Kidney Int. Rep. 2020, 5, 879–890. [Google Scholar] [CrossRef]
- Kyowa Kirin Co., Ltd. RTA 402 Phase 3 Clinical Trial (A Randomized, Double-Blind, Placebo-Controlled Clinical Trial in Patients with Diabetic Kidney Disease). 2021. Available online: https://www.kyowakirin.com/media_center/news_releases/2021/e20210106_01 (accessed on 1 August 2022).
- Reata Pharmaceuticals, Inc. An Extended Access Program to Assess Long Term Safety of Bardoxolone Methyl in Patients with Chronic Kidney Disease. 2022. Available online: https://clinicaltrials.gov (accessed on 1 August 2022).
- Chertow, G.M.; Appel, G.B.; Andreoli, S.; Bangalore, S.; Block, G.A.; Chapman, A.B.; Chin, M.P.; Gibson, K.L.; Goldsberry, A.; Iijima, K.; et al. Study Design and Baseline Characteristics of the CARDINAL Trial: A Phase 3 Study of Bardoxolone Methyl in Patients with Alport Syndrome. Am. J. Nephrol. 2021, 52, 180–189. [Google Scholar] [CrossRef]
- Reata Pharmaceuticals, Inc. A Phase 2/3 Trial of the Efficacy and Safety of Bardoxolone Methyl in Patients with Alport Syndrome. 2022. Available online: https://clinicaltrials.gov (accessed on 1 August 2022).
- Reata Pharmaceuticals, Inc. A Phase 3 Trial of Bardoxolone Methyl in Patients with Autosomal Dominant Polycystic Kidney Disease. 2022. Available online: https://clinicaltrials.gov (accessed on 1 August 2022).
- Dang, Y.-Y.; Luo, H.; Li, Y.-M.; Zhou, Y.; Luo, X.; Lin, S.-M.; Liu, S.-P.; Lee, S.M.-Y.; Li, C.-W.; Dai, X.-Y. Curcumin Prevents As3+-Induced Carcinogenesis through Regulation of GSK3β/Nrf2. Chin. Med. 2021, 16, 116. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Chao, M.; Jun, A.; Wei, S.; LiFeng, M. Effect of Curcumin on Diabetic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized, Double-Blind, Placebo-Controlled Clinical Trials. Evid. Based Complementary Altern. Med. 2021, 2021, 6109406. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef]
- Di Tu, Q.; Jin, J.; Hu, X.; Ren, Y.; Zhao, L.; He, Q. Curcumin Improves the Renal Autophagy in Rat Experimental Membranous Nephropathy via Regulating the PI3K/AKT/MTOR and Nrf2/HO-1 Signaling Pathways. BioMed Res. Int. 2020, 2020, 7069052. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Sohn, H.-Y.; Koh, Y.H.; Jo, C. Curcumin Activates Nrf2 through PKCδ-Mediated P62 Phosphorylation at Ser351. Sci. Rep. 2021, 11, 8430. [Google Scholar] [CrossRef]
- He, H.-J. Curcumin Attenuates Nrf2 Signaling Defect, Oxidative Stress in Muscle and Glucose Intolerance in High Fat Diet-Fed Mice. World J. Diabetes 2012, 3, 94. [Google Scholar] [CrossRef]
- Tapia, E.; Soto, V.; Ortiz-Vega, K.M.; Zarco-Márquez, G.; Molina-Jijón, E.; Cristóbal-García, M.; Santamaría, J.; García-Niño, W.R.; Correa, F.; Zazueta, C.; et al. Curcumin Induces Nrf2 Nuclear Translocation and Prevents Glomerular Hypertension, Hyperfiltration, Oxidant Stress, and the Decrease in Antioxidant Enzymes in 5/6 Nephrectomized Rats. Oxidative Med. Cell. Longev. 2012, 2012, 269039. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, D.; Guo, L.; Liang, W.; Jiang, Y.; Li, H.; Zhao, Y.; Lu, S.; Chi, Z.-H. Curcumin Protects Renal Tubular Epithelial Cells from High Glucose-Induced Epithelial-to-Mesenchymal Transition through Nrf2-Mediated Upregulation of Heme Oxygenase-1. Mol. Med. Rep. 2015, 12, 1347–1355. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin Attenuates Oxidative Stress in RAW264.7 Cells by Increasing the Activity of Antioxidant Enzymes and Activating the Nrf2-Keap1 Pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef]
- Shin, J.W.; Chun, K.-S.; Kim, D.-H.; Kim, S.-J.; Kim, S.H.; Cho, N.-C.; Na, H.-K.; Surh, Y.-J. Curcumin Induces Stabilization of Nrf2 Protein through Keap1 Cysteine Modification. Biochem. Pharmacol. 2020, 173, 113820. [Google Scholar] [CrossRef]
- Rahban, M.; Habibi-Rezaei, M.; Mazaheri, M.; Saso, L.; Moosavi-Movahedi, A.A. Anti-Viral Potential and Modulation of Nrf2 by Curcumin: Pharmacological Implications. Antioxidants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Huang, J.; Huang, K.; Lan, T.; Xie, X.; Shen, X.; Liu, P.; Huang, H. Curcumin Ameliorates Diabetic Nephropathy by Inhibiting the Activation of the SphK1-S1P Signaling Pathway. Mol. Cell. Endocrinol. 2013, 365, 231–240. [Google Scholar] [CrossRef]
- Li, D.; Lu, Z.; Jia, J.; Zheng, Z.; Lin, S. Curcumin Ameliorates Podocytic Adhesive Capacity Damage Under Mechanical Stress By Inhibiting MiR-124 Expression. Kidney Blood Press. Res. 2013, 38, 61–71. [Google Scholar] [CrossRef]
- Soetikno, V.; Watanabe, K.; Sari, F.R.; Harima, M.; Thandavarayan, R.A.; Veeraveedu, P.T.; Arozal, W.; Sukumaran, V.; Lakshmanan, A.P.; Arumugam, S.; et al. Curcumin Attenuates Diabetic Nephropathy by Inhibiting PKC-α and PKC-Β1 Activity in Streptozotocin-Induced Type I Diabetic Rats. Mol. Nutr. Food Res. 2011, 55, 1655–1665. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Miao, X.; Wang, S.; Adhikari, B.K.; Wang, X.; Sun, J.; Liu, Q.; Tong, Q.; Wang, Y. Novel Curcumin C66 That Protects Diabetes-Induced Aortic Damage Was Associated with Suppressing JNK2 and Upregulating Nrf2 Expression and Function. Oxidative Med. Cell. Longev. 2018, 2018, 5783239. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kong, L.; Tan, Y.; Epstein, P.N.; Zeng, J.; Gu, J.; Liang, G.; Kong, M.; Chen, X.; Miao, L.; et al. C66 Ameliorates Diabetic Nephropathy in Mice by Both Upregulating NRF2 Function via Increase in MiR-200a and Inhibiting MiR-21. Diabetologia 2016, 59, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cai, Y.; Yu, Y. Effects of a Novel Curcumin Derivative on the Functions of Kidney in Streptozotocin-Induced Type 2 Diabetic Rats. Inflammopharmacology 2018, 26, 1257–1264. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Lu, K.; Lu, C.; Zhao, Y.; Zheng, S.; Li, J.; Huang, Z.; Huang, Y.; Zhang, Y.; et al. Inhibition of High Glucose-Induced Inflammation and Fibrosis by a Novel Curcumin Derivative Prevents Renal and Heart Injury in Diabetic Mice. Toxicol. Lett. 2017, 278, 48–58. [Google Scholar] [CrossRef]
- Janczewski, Ł. Sulforaphane and Its Bifunctional Analogs: Synthesis and Biological Activity. Molecules 2022, 27, 1750. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef]
- Vanduchova, A.; Anzenbacher, P.; Anzenbacherova, E. Isothiocyanate from Broccoli, Sulforaphane, and Its Properties. J. Med. Food 2019, 22, 121–126. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Alvarenga, L.A.; Ribeiro, M.; Dai, L.; Shiels, P.G.; Stenvinkel, P.; Lindholm, B.; Mafra, D. Cruciferous Vegetables: Rationale for Exploring Potential Salutary Effects of Sulforaphane-Rich Foods in Patients with Chronic Kidney Disease. Nutr. Rev. 2021, 79, 1204–1224. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxidative Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed]
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A Review of Its Therapeutic Potentials, Advances in Its Nanodelivery, Recent Patents, and Clinical Trials. Phytother. Res. 2021, 35, 5440–5458. [Google Scholar] [CrossRef] [PubMed]
- Paunkov, A.; Chartoumpekis, D.V.; Ziros, P.G.; Sykiotis, G.P. A Bibliometric Review of the Keap1/Nrf2 Pathway and Its Related Antioxidant Compounds. Antioxidants 2019, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Pre-Clinical Neuroprotective Evidences and Plausible Mechanisms of Sulforaphane in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2929. [Google Scholar] [CrossRef] [PubMed]
- Yuanfeng, W.; Chengzhi, L.; Ligen, Z.; Juan, S.; Xinjie, S.; Yao, Z.; Jianwei, M. Approaches for Enhancing the Stability and Formation of Sulforaphane. Food Chem. 2021, 345, 128771. [Google Scholar] [CrossRef]
- Kamal, M.M.; Akter, S.; Lin, C.-N.; Nazzal, S. Sulforaphane as an Anticancer Molecule: Mechanisms of Action, Synergistic Effects, Enhancement of Drug Safety, and Delivery Systems. Arch. Pharm. Res. 2020, 43, 371–384. [Google Scholar] [CrossRef]
- Elkashty, O.A.; Tran, S.D. Sulforaphane as a Promising Natural Molecule for Cancer Prevention and Treatment. Curr. Med. Sci. 2021, 41, 250–269. [Google Scholar] [CrossRef]
- Kuran, D.; Pogorzelska, A.; Wiktorska, K. Breast Cancer Prevention-Is There a Future for Sulforaphane and Its Analogs? Nutrients 2020, 12, 1559. [Google Scholar] [CrossRef]
- Ishida, K.; Kaji, K.; Sato, S.; Ogawa, H.; Takagi, H.; Takaya, H.; Kawaratani, H.; Moriya, K.; Namisaki, T.; Akahane, T.; et al. Sulforaphane Ameliorates Ethanol plus Carbon Tetrachloride-Induced Liver Fibrosis in Mice through the Nrf2-Mediated Antioxidant Response and Acetaldehyde Metabolization with Inhibition of the LPS/TLR4 Signaling Pathway. J. Nutr. Biochem. 2021, 89, 108573. [Google Scholar] [CrossRef]
- Isaacson, R.H.; Beier, J.I.; Khoo, N.K.; Freeman, B.A.; Freyberg, Z.; Arteel, G.E. Olanzapine-Induced Liver Injury in Mice: Aggravation by High-Fat Diet and Protection with Sulforaphane. J. Nutr. Biochem. 2020, 81, 108399. [Google Scholar] [CrossRef]
- Schepici, G.; Bramanti, P.; Mazzon, E. Efficacy of Sulforaphane in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8637. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Zhou, H.; Cui, K. Sulforaphane Alleviates LPS-Induced Inflammatory Injury in ARPE-19 Cells by Repressing the PWRN2/NF-KB Pathway. Immunopharmacol. Immunotoxicol. 2022, 0, 1–9. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Zhou, W.; Men, H.; Bao, T.; Sun, Y.; Wang, Q.; Tan, Y.; Keller, B.B.; Tong, Q.; et al. Ferroptosis Is Essential for Diabetic Cardiomyopathy and Is Prevented by Sulforaphane via AMPK/NRF2 Pathways. Acta Pharm. Sin. B 2022, 12, 708–722. [Google Scholar] [CrossRef]
- Su, X.; Wang, S.; Zhang, H.; Yang, G.; Bai, Y.; Liu, P.; Meng, L.; Jiang, X.; Xin, Y. Sulforaphane Prevents Angiotensin II-Induced Cardiomyopathy by Activation of Nrf2 through Epigenetic Modification. J. Cell. Mol. Med. 2021, 25, 4408–4419. [Google Scholar] [CrossRef]
- Wu, H.; Kong, L.; Cheng, Y.; Zhang, Z.; Wang, Y.; Luo, M.; Tan, Y.; Chen, X.; Miao, L.; Cai, L. Metallothionein Plays a Prominent Role in the Prevention of Diabetic Nephropathy by Sulforaphane via Up-Regulation of Nrf2. Free Radic. Biol. Med. 2015, 89, 431–442. [Google Scholar] [CrossRef]
- Shang, G.; Tang, X.; Gao, P.; Guo, F.; Liu, H.; Zhao, Z.; Chen, Q.; Jiang, T.; Zhang, N.; Li, H. Sulforaphane Attenuation of Experimental Diabetic Nephropathy Involves GSK-3 Beta/Fyn/Nrf2 Signaling Pathway. J. Nutr. Biochem. 2015, 26, 596–606. [Google Scholar] [CrossRef]
- Liebman, S.E.; Le, T.H. Eat Your Broccoli: Oxidative Stress, NRF2, and Sulforaphane in Chronic Kidney Disease. Nutrients 2021, 13, 266. [Google Scholar] [CrossRef]
- Ghazizadeh-Hashemi, F.; Bagheri, S.; Ashraf-Ganjouei, A.; Moradi, K.; Shahmansouri, N.; Mehrpooya, M.; Noorbala, A.-A.; Akhondzadeh, S. Efficacy and Safety of Sulforaphane for Treatment of Mild to Moderate Depression in Patients with History of Cardiac Interventions: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Psychiatry Clin. Neurosci. 2021, 75, 250–255. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Hosseinpanah, F.; Keyzad, A.; Azizi, F. Effects of Broccoli Sprout with High Sulforaphane Concentration on Inflammatory Markers in Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Funct. Foods 2012, 4, 837–841. [Google Scholar] [CrossRef]
- Traka, M.H.; Melchini, A.; Coode-Bate, J.; Al Kadhi, O.; Saha, S.; Defernez, M.; Troncoso-Rey, P.; Kibblewhite, H.; O’Neill, C.M.; Bernuzzi, F.; et al. Transcriptional Changes in Prostate of Men on Active Surveillance after a 12-Mo Glucoraphanin-Rich Broccoli Intervention-Results from the Effect of Sulforaphane on Prostate CAncer PrEvention (ESCAPE) Randomized Controlled Trial. Am. J. Clin. Nutr. 2019, 109, 1133–1144. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Wu, R.; Yin, R.; Sargsyan, D.; Raskin, I.; Kong, A.-N. Epigenome and Transcriptome Study of Moringa Isothiocyanate in Mouse Kidney Mesangial Cells Induced by High Glucose, a Potential Model for Diabetic-Induced Nephropathy. AAPS J. 2020, 22, 8. [Google Scholar] [CrossRef]
- Amiya, E. Nrf-2: The Target of Vascular Dysfunction in Diabetes. Am. J. Hypertens. 2020, 33, 597–598. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Rajaguru, P.; Paulmurugan, R.; Ramkumar, K.M. The Emerging Role of Redox-Sensitive Nrf2–Keap1 Pathway in Diabetes. Pharmacol. Res. 2015, 91, 104–114. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, H.; Duan, M.; Liu, R.; Zhu, Q.; Zhang, K.; Wang, L. Cinnamaldehyde Improves Metabolic Functions in Streptozotocin-Induced Diabetic Mice by Regulating Gut Microbiota. Drug Des. Dev. Ther. 2021, 15, 2339–2355. [Google Scholar] [CrossRef]
- Zheng, H.; Whitman, S.A.; Wu, W.; Wondrak, G.T.; Wong, P.K.; Fang, D.; Zhang, D.D. Therapeutic Potential of Nrf2 Activators in Streptozotocin-Induced Diabetic Nephropathy. Diabetes 2011, 60, 3055–3066. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D.; et al. Cinnamaldehyde in Diabetes: A Review of Pharmacology, Pharmacokinetics and Safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef]
- Chao, L.K.; Chang, W.-T.; Shih, Y.-W.; Huang, J.-S. Cinnamaldehyde Impairs High Glucose-Induced Hypertrophy in Renal Interstitial Fibroblasts. Toxicol. Appl. Pharmacol. 2010, 244, 174–180. [Google Scholar] [CrossRef]
- Wang, F.; Pu, C.; Zhou, P.; Wang, P.; Liang, D.; Wang, Q.; Hu, Y.; Li, B.; Hao, X. Cinnamaldehyde Prevents Endothelial Dysfunction Induced by High Glucose by Activating Nrf2. Cell. Physiol. Biochem. 2015, 36, 315–324. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Fahmy, A.; Badawy, D. Cinnamaldehyde Protects from the Hypertension Associated with Diabetes. Food Chem. Toxicol. 2011, 49, 3007–3012. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Wang, D.; Yang, Q.; Wan, J.; Liu, S.; Zhou, P.; Yang, Y. Cinnamaldehyde Ameliorates Vascular Dysfunction in Diabetic Mice by Activating Nrf2. Am. J. Hypertens. 2020, 33, 610–619. [Google Scholar] [CrossRef]
- Huang, D.-D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A Review on the Potential of Resveratrol in Prevention and Therapy of Diabetes and Diabetic Complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.-T.; Ye, X.-L.; Li, R.-R.; Chen, H.; Wang, Y.-Y.; Yong, H.-J.; Pan, M.-L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-X.; Ji, M.-J.; Sun, H.-J. An Updated Pharmacological Insight of Resveratrol in the Treatment of Diabetic Nephropathy. Gene 2021, 780, 145532. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, Y.; Cui, W.; Yuan, H.; Sun, J.; Wu, M.; Guo, Q.; Kong, L.; Wu, H.; Miao, L. Resveratrol Prevention of Diabetic Nephropathy Is Associated with the Suppression of Renal Inflammation and Mesangial Cell Proliferation: Possible Roles of Akt/NF- κ B Pathway. Int. J. Endocrinol. 2014, 2014, 289327. [Google Scholar] [CrossRef]
- Xian, Y.; Gao, Y.; Lv, W.; Ma, X.; Hu, J.; Chi, J.; Wang, W.; Wang, Y. Resveratrol Prevents Diabetic Nephropathy by Reducing Chronic Inflammation and Improving the Blood Glucose Memory Effect in Non-Obese Diabetic Mice. Naunyn-Schmiedeberg’s Arch. Pharm. 2020, 393, 2009–2017. [Google Scholar] [CrossRef]
- Yuan, D.; Liu, X.-M.; Fang, Z.; Du, L.-L.; Chang, J.; Lin, S.-H. Protective Effect of Resveratrol on Kidney in Rats with Diabetic Nephropathy and Its Effect on Endoplasmic Reticulum Stress. Eur. Rev. Med. Pharm. Sci. 2018, 22, 1485–1493. [Google Scholar] [CrossRef]
- Hu, H.-C.; Lei, Y.-H.; Zhang, W.-H.; Luo, X.-Q. Antioxidant and Anti-Inflammatory Properties of Resveratrol in Diabetic Nephropathy: A Systematic Review and Meta-Analysis of Animal Studies. Front. Pharmacol. 2022, 13, 841818. [Google Scholar] [CrossRef]
- Sattarinezhad, A.; Roozbeh, J.; Shirazi Yeganeh, B.; Omrani, G.R.; Shams, M. Resveratrol Reduces Albuminuria in Diabetic Nephropathy: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Diabetes Metab. 2019, 45, 53–59. [Google Scholar] [CrossRef]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol Supplementation Improves Glycemic Control in Type 2 Diabetes Mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef]
- García Trejo, E.; Arellano Buendía, A.; Sánchez Reyes, O.; García Arroyo, F.; Arguello García, R.; Loredo Mendoza, M.; Tapia, E.; Sánchez Lozada, L.; Osorio Alonso, H. The Beneficial Effects of Allicin in Chronic Kidney Disease Are Comparable to Losartan. Int. J. Mol. Sci. 2017, 18, 1980. [Google Scholar] [CrossRef] [Green Version]
- Toygar, I.; Tureyen, A.; Demir, D.; Cetinkalp, S. Effect of Allicin on Wound Healing: An Experimental Diabetes Model. J. Wound Care 2020, 29, 388–392. [Google Scholar] [CrossRef]
- Li, C.-L.; Liu, X.-H.; Qiao, Y.; Ning, L.-N.; Li, W.-J.; Sun, Y.-S.; Liu, D.-S.; Gao, W.; Ma, C.-M. Allicin Alleviates Inflammation of Diabetic Macroangiopathy via the Nrf2 and NF-KB Pathway. Eur. J. Pharm. 2020, 876, 173052. [Google Scholar] [CrossRef]
- Moon, S.W.; Shin, Y.U.; Cho, H.; Bae, S.H.; Kim, H.K. Effect of Grape Seed Proanthocyanidin Extract on Hard Exudates in Patients with Non-Proliferative Diabetic Retinopathy. Medicine 2019, 98, e15515. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, G.; Hu, Z.; Shi, W.; Chen, B.; Zou, P.; Li, X. Grape Seed Proanthocyanidins Protect against Streptozotocin-induced Diabetic Nephropathy by Attenuating Endoplasmic Reticulum Stress-induced Apoptosis. Mol. Med. Rep. 2018. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, Y.; Liu, Z.; Gao, Z.; Li, B.; Zhang, D.; Zhang, Z.; Jiang, X.; Liu, Z.; Meng, L.; et al. Grape Seed Proanthocyanidin Extract Ameliorates Diabetic Bladder Dysfunction via the Activation of the Nrf2 Pathway. PLoS ONE 2015, 10, e0126457. [Google Scholar] [CrossRef]
- Cui, X.; Li, B.; Gao, H.; Wei, N.; Wang, W.; Lu, M. Effects of Grape Seed Proanthocyanidin Extracts on Peripheral Nerves in Streptozocin-Induced Diabetic Rats. J. Nutr. Sci. Vitam. 2008, 54, 321–328. [Google Scholar] [CrossRef]
- Li, X.; Gao, Z.; Gao, H.; Li, B.; Peng, T.; Jiang, B.; Yang, X.; Hu, Z. Nephrin Loss Is Reduced by Grape Seed Proanthocyanidins in the Experimental Diabetic Nephropathy Rat Model. Mol. Med. Rep. 2017, 16, 9393–9400. [Google Scholar] [CrossRef]
- Li, X.; Xiao, Y.; Gao, H.; Li, B.; Xu, L.; Cheng, M.; Jiang, B.; Ma, Y. Grape Seed Proanthocyanidins Ameliorate Diabetic Nephropathy via Modulation of Levels of AGE, RAGE and CTGF. Nephron Exp. Nephrol. 2009, 111, e31–e41. [Google Scholar] [CrossRef]
- Han, H.; Wang, H.; Du, Y.; Gao, L. Grape Seed Procyanidins Attenuates Cisplatin-Induced Human Embryonic Renal Cell Cytotoxicity by Modulating Heme Oxygenase-1 in Vitro. Cell Biochem. Biophys. 2019, 77, 367–377. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, H.-J.; Kang, K.S. Procyanidin C1 Activates the Nrf2/HO-1 Signaling Pathway to Prevent Glutamate-Induced Apoptotic HT22 Cell Death. Int. J. Mol. Sci. 2019, 20, 142. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Li, H.; Li, Y.; Liu, D.; Zhang, L.; Wang, T.; Liu, T.; Ma, L.; De la Puerta, R. Protective Effects of Grape Seed Proanthocyanidins on the Kidneys of Diabetic Rats through the Nrf2 Signalling Pathway. Evid. Based Complementary Altern. Med. 2020, 2020, 5205903. [Google Scholar] [CrossRef] [PubMed]
- Do, M.; Hur, J.; Choi, J.; Kim, M.; Kim, M.; Kim, Y.; Ha, S. Eucommia Ulmoides Ameliorates Glucotoxicity by Suppressing Advanced Glycation End-Products in Diabetic Mice Kidney. Nutrients 2018, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Feng, T.; Jin, G.; Li, Z. Rutin Prevents High Glucose-Induced Renal Glomerular Endothelial Hyperpermeability by Inhibiting the ROS/Rhoa/ROCK Signaling Pathway. Planta Med. 2016, 82, 1252–1257. [Google Scholar] [CrossRef]
- Kamalakkannan, N.; Prince, P.S.M. The Influence of Rutin on the Extracellular Matrix in Streptozotocin-Induced Diabetic Rat Kidney. J. Pharm. Pharmacol. 2010, 58, 1091–1098. [Google Scholar] [CrossRef]
- Han, C.-S.; Liu, K.; Zhang, N.; Li, S.-W.; Gao, H.-C. Rutin Suppresses High Glucose-Induced ACTA2 and P38 Protein Expression in Diabetic Nephropathy. Exp. Ther. Med. 2017, 14, 181–186. [Google Scholar] [CrossRef]
- Ganesan, D.; Albert, A.; Paul, E.; Ananthapadmanabhan, K.; Andiappan, R.; Sadasivam, S.G. Rutin Ameliorates Metabolic Acidosis and Fibrosis in Alloxan Induced Diabetic Nephropathy and Cardiomyopathy in Experimental Rats. Mol. Cell. Biochem. 2020, 471, 41–50. [Google Scholar] [CrossRef]
- Kim, D.; Cheon, J.; Yoon, H.; Jun, H.-S. Cudrania Tricuspidata Root Extract Prevents Methylglyoxal-Induced Inflammation and Oxidative Stress via Regulation of the PKC-NOX4 Pathway in Human Kidney Cells. Oxidative Med. Cell. Longev. 2021, 2021, 5511881. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, K.; Zhang, Y.; Duan, Y.; Xiao, K.; Liu, S.; Ding, X. Icariin Ameliorates Diabetic Renal Tubulointerstitial Fibrosis by Restoring Autophagy via Regulation of the MiR-192-5p/GLP-1R Pathway. Front. Pharmacol. 2021, 12, 720387. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, X.; Pan, Z.; Yao, W.; Gao, X.; Wang, X.; Ding, X. Icariin Prevents Extracellular Matrix Accumulation and Ameliorates Experimental Diabetic Kidney Disease by Inhibiting Oxidative Stress via GPER Mediated P62-Dependent Keap1 Degradation and Nrf2 Activation. Front. Cell Dev. Biol. 2020, 8, 559. [Google Scholar] [CrossRef]
- Cha, S.-H.; Hwang, Y.; Heo, S.-J.; Jun, H.-S. Diphlorethohydroxycarmalol Attenuates Methylglyoxal-Induced Oxidative Stress and Advanced Glycation End Product Formation in Human Kidney Cells. Oxidative Med. Cell. Longev. 2018, 2018, 3654095. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Kong, L.; Tang, Z.-Z.; Zhang, Y.-M.; Liu, Y.; Wang, T.-Y.; Liu, Y.-W. Hesperetin Ameliorates Diabetic Nephropathy in Rats by Activating Nrf2/ARE/Glyoxalase 1 Pathway. Biomed. Pharmacother. 2019, 111, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-J.; Wang, H.-R.; Wang, Y.-I.; Zhai, Z.-H.; Wang, L.-W.; Li, L.; Zhang, C.; Tang, L. Myricetin Attenuated Diabetes-Associated Kidney Injuries and Dysfunction via Regulating Nuclear Factor (Erythroid Derived 2)-Like 2 and Nuclear Factor-ΚB Signaling. Front. Pharmacol. 2019, 10, 647. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, G.; Cheng, X.; Teng, Z.; Cai, X.; Yang, J.; Sun, X.; Lu, W.; Wang, X.; Yao, Y.; et al. Therapeutic Potential of Digitoflavone on Diabetic Nephropathy: Nuclear Factor Erythroid 2-Related Factor 2-Dependent Anti-Oxidant and Anti-Inflammatory Effect. Sci. Rep. 2015, 5, 12377. [Google Scholar] [CrossRef]
- Hu, Q.; Qu, C.; Xiao, X.; Zhang, W.; Jiang, Y.; Wu, Z.; Song, D.; Peng, X.; Ma, X.; Zhao, Y. Flavonoids on Diabetic Nephropathy: Advances and Therapeutic Opportunities. Chin. Med. 2021, 16, 74. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, X.; Teng, S.; Zhang, Y.; Liu, Y.; Li, X.; Li, Y. The Antidiabetic and Antinephritic Activities of Auricularia Cornea (An Albino Mutant Strain) via Modulation of Oxidative Stress in the Db/Db Mice. Front. Immunol. 2019, 10, 1039. [Google Scholar] [CrossRef]
- Samimi, F.; Baazm, M.; Eftekhar, E.; Rajabi, S.; Goodarzi, M.; Mashayekhi, F. Possible Antioxidant Mechanism of Coenzyme Q10 in Diabetes: Impact on Sirt1/Nrf2 Signaling Pathways. Res. Pharm. Sci. 2019, 14, 524. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The Mitochondria-Targeted Antioxidant MitoQ Ameliorated Tubular Injury Mediated by Mitophagy in Diabetic Kidney Disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Su, J.; Gao, C.; Xie, L.; Fan, Y.; Shen, Y.; Huang, Q.; Wang, N.; Xu, Y.; Yang, N.; Gui, D. Astragaloside II Ameliorated Podocyte Injury and Mitochondrial Dysfunction in Streptozotocin-Induced Diabetic Rats. Front. Pharmacol. 2021, 12, 638422. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and Its Effects in Inflammatory Responses and Inflammation-Associated Diseases: Recent Advances and Future Directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Kohandel, Z.; Farkhondeh, T.; Aschner, M.; Samarghandian, S. Nrf2 a Molecular Therapeutic Target for Astaxanthin. Biomed. Pharmacother. 2021, 137, 111374. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, Y.; Chen, Q.; Yang, H.; Xie, X. Astaxanthin Promotes Nrf2/ARE Signaling to Alleviate Renal Fibronectin and Collagen IV Accumulation in Diabetic Rats. J. Diabetes Res. 2018, 2018, 6730315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tao, J.; Xie, X. Astaxanthin Promotes Nrf2/ARE Signaling to Inhibit HG-Induced Renal Fibrosis in GMCs. Mar. Drugs 2018, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, J.; Guo, W.; Li, F.; Sun, W.; Chen, J.; Zhang, C.; Lu, X.; Tan, Y.; Feng, W.; et al. Up-Regulation of Nrf2 Is Involved in FGF21-Mediated Fenofibrate Protection against Type 1 Diabetic Nephropathy. Free Radic. Biol. Med. 2016, 93, 94–109. [Google Scholar] [CrossRef]
- Yun, J.S.W.; Yap, T.; Martyres, R.; Kern, J.S.; Varigos, G.; Scardamaglia, L. The Association of Mycophenolate Mofetil and Human Herpes Virus Infection. J. Dermatol. Treat. 2020, 31, 46–55. [Google Scholar] [CrossRef]
- Shahzad, K.; Bock, F.; Al-Dabet, M.M.; Gadi, I.; Nazir, S.; Wang, H.; Kohli, S.; Ranjan, S.; Mertens, P.R.; Nawroth, P.P.; et al. Stabilization of Endogenous Nrf2 by Minocycline Protects against Nlrp3-Inflammasome Induced Diabetic Nephropathy. Sci. Rep. 2016, 6, 34228. [Google Scholar] [CrossRef]
- Dong, W.; Jia, Y.; Liu, X.; Zhang, H.; Li, T.; Huang, W.; Chen, X.; Wang, F.; Sun, W.; Wu, H. Sodium Butyrate Activates NRF2 to Ameliorate Diabetic Nephropathy Possibly via Inhibition of HDAC. J. Endocrinol. 2017, 232, 71–83. [Google Scholar] [CrossRef]
- Du, L.; Wang, L.; Wang, B.; Wang, J.; Hao, M.; Chen, Y.; Li, X.; Li, Y.; Jiang, Y.; Li, C.; et al. A Novel Compound AB38b Attenuates Oxidative Stress and ECM Protein Accumulation in Kidneys of Diabetic Mice through Modulation of Keap1/Nrf2 Signaling. Acta Pharm. Sin. 2020, 41, 358–372. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zhou, M.; Xie, Y.; Dong, X.; Bai, F.; Zhang, J. DPHC From Alpinia Officinarum Ameliorates Oxidative Stress and Insulin Resistance via Activation of Nrf2/ARE Pathway in Db/Db Mice and High Glucose-Treated HepG2 Cells. Front. Pharmacol. 2022, 12, 792977. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Liang, D.; Jiang, Y.; Liang, W.; Chi, Z.-H.; Ma, J. Protective Effects of Berberine on Renal Injury in Streptozotocin (STZ)-Induced Diabetic Mice. Int. J. Mol. Sci. 2016, 17, 1327. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Kim, H.-J.; Cha, S.-H.; Jun, H.-S. Protective Effects of Broussonetia Kazinoki Siebold Fruit Extract against Palmitate-Induced Lipotoxicity in Mesangial Cells. Evid. Based Complementary Altern. Med. 2019, 2019, 4509403. [Google Scholar] [CrossRef] [PubMed]
- Adelusi, T.I.; Du, L.; Hao, M.; Zhou, X.; Xuan, Q.; Apu, C.; Sun, Y.; Lu, Q.; Yin, X. Keap1/Nrf2/ARE Signaling Unfolds Therapeutic Targets for Redox Imbalanced-Mediated Diseases and Diabetic Nephropathy. Biomed. Pharmacother. 2020, 123, 109732. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Behl, T.; Sehgal, A.; Bhatia, S.; Jaglan, D.; Bungau, S. Therapeutic Potential of Nrf-2 Pathway in the Treatment of Diabetic Neuropathy and Nephropathy. Mol. Biol. Rep. 2021, 48, 2761–2774. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Li, S.; Zhang, C.; Chen, H.; Wang, N.; Feng, Y. Clinical Efficacies, Underlying Mechanisms and Molecular Targets of Chinese Medicines for Diabetic Nephropathy Treatment and Management. Acta Pharm. Sin. B 2021, 11, 2749–2767. [Google Scholar] [CrossRef]

| NRF2 Activators and Ref. | Strengths | Weaknesses | Future Perspectives |
|---|---|---|---|
| Bardoxolone methyl [69,73,77,78,80,82] | GFR ↑ | Albuminuria ↑ | Its strengths could be present without its weaknesses in subjects who: - have not previously been hospitalized for heart failure and with BNP levels less than 200 pg/mL -other etiologies of CKD (ADPKD, Alport syndrome) |
| Delayed progression to ESRD | Morality↑ and serious side effects in some groups | ||
| Curcumin [84,94,98] | Serum creatinine levels ↓ | No significant impact on proteinuria, blood urea nitrogen | Curcumin derivatives could be a better option from a pharmacokinetic point of view while maintaining the beneficial effects of the original compound |
| Fasting blood glucose ↓ | Unstable molecular structure | ||
| Systolic blood pressure ↓ | High dosages to obtain the known effects | ||
| Total cholesterol ↓ | Absorption ↓ | ||
| Degradation and elimination ↑ | |||
| Sulforaphane [65,105,106,108] | Good bioavailability | Unstable molecular structure | A clinical trial could bring a better understanding of its future potential |
| Liver first-pass effect↑ | |||
| Water solubility ↓ | |||
| Cinnamic aldehyde [130,131,132,133] | Improves DM symptoms | Bioavailability? | There is less data available on its effect on DKD, which highlights the direction future studies need to take. |
| Possible side effects | |||
| Resveratrol [137,139] | It can target at once several pathophysiological mechanisms | Bioavailability ↓ | There are some conflicting results, which need to be evaluated in further studies |
| Possible no significant side effects? | Metabolism ↑ | ||
| water solubility ↓ |
| Compound | Animal/Cells | General Effects | Doses and Time of Administration | Detection Site and Notable Findings | Ref. |
|---|---|---|---|---|---|
| Allicin | Male Wistar rats | antihypertensive, antidiabetic, antioxidant, antifibrotic effects | 16 mg/kg day/p.o. for 30 days |
| [4] |
| GSPE | STZ-induced diabetic rats | antioxidant effects, can decrease insulin resistance, delay DKD progression and improve DKD | I group (treated with 125-mg/kg/day GSPE for 8 weeks), and II group (treated with 250 mg/kg/day GSPE for 8 weeks) |
| [157] |
| EU | STZ-induced diabetic mice | antioxidant, anti-hypertensive, and anti-hyperglycemic effect | (200 mg/kg) orally for 6 weeks |
| [158] |
| Rutin | HRGECs | direct antioxidant effect on human renal glomerular endothelial cells | 12.5, 25, or 50 µM rutin and/or HG for 24 h |
| [160] |
| CTRE | HK-2 cells | antioxidant, antiinflammatory effects | (5–40 μg/mL), ranging between 3 and 24 h |
| [164] |
| Icariin | HMC and/or STZ-induced diabetic rats | antioxidant effect, can prevent the development of DKD, improve DKD-induced kidney injury | (20, 40, 80 mg/kg, i.g.) group for 9 weeks |
| [166] |
| Myricetin | STZ-induced diabetic rats | antioxidant, antiinflammatory effect | (100 mg/ kg/day) for 6 months |
| [169] |
| Coenzyme Q10 | Rats and/or mGECs | antioxidant effect | 0.1% in the food for 7 weeks |
| [18] |
| AST | HF diet and STZ-induced diabetic rats | antioxidant effect, can delay DKD progression | 25 mg/kg daily i.g. for 12 weeks |
| [178] |
| Sodium butyrate | wild-type and Nrf2-knockout mice | Antioxidant effect | 5 g/kg/day for 20 weeks |
| [183] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, D.M.; Gosav, E.M.; Anton, M.I.; Floria, M.; Seritean Isac, P.N.; Hurjui, L.L.; Tarniceriu, C.C.; Costea, C.F.; Ciocoiu, M.; Rezus, C. Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules 2022, 12, 1227. https://doi.org/10.3390/biom12091227
Tanase DM, Gosav EM, Anton MI, Floria M, Seritean Isac PN, Hurjui LL, Tarniceriu CC, Costea CF, Ciocoiu M, Rezus C. Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules. 2022; 12(9):1227. https://doi.org/10.3390/biom12091227
Chicago/Turabian StyleTanase, Daniela Maria, Evelina Maria Gosav, Madalina Ioana Anton, Mariana Floria, Petronela Nicoleta Seritean Isac, Loredana Liliana Hurjui, Claudia Cristina Tarniceriu, Claudia Florida Costea, Manuela Ciocoiu, and Ciprian Rezus. 2022. "Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives" Biomolecules 12, no. 9: 1227. https://doi.org/10.3390/biom12091227
APA StyleTanase, D. M., Gosav, E. M., Anton, M. I., Floria, M., Seritean Isac, P. N., Hurjui, L. L., Tarniceriu, C. C., Costea, C. F., Ciocoiu, M., & Rezus, C. (2022). Oxidative Stress and NRF2/KEAP1/ARE Pathway in Diabetic Kidney Disease (DKD): New Perspectives. Biomolecules, 12(9), 1227. https://doi.org/10.3390/biom12091227