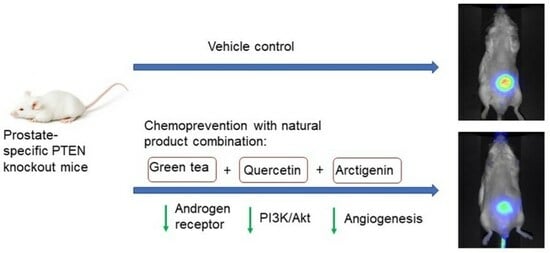
Enhanced Chemoprevention of Prostate Cancer by Combining Arctigenin with Green Tea and Quercetin in Prostate-Specific Phosphatase and Tensin Homolog Knockout Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line and Cell Culture
2.2. Cell Proliferation Assay
2.3. Preparation of GT, Q Diet, and Arc Solution for Animal Study
2.4. Animal Study
2.5. Pathological Evaluation of Tumor Stage
2.6. Western Blot Analysis
2.7. Tissue Microarray and Immunohistochemical Analysis of Ki67 and Microvessel Density
2.8. Statistical Analysis
3. Results
3.1. Synergistic Anti-Proliferative Effects In Vitro of the Combination of Arc with EGCG and Q
3.2. Increased Inhibition of Prostate Tumorigenesis by Arc in Combination with GT and Q
3.3. Amelioration of the Prostate Pathology by GT, Q, and Arc
3.4. Modulation of Signaling Molecules Involved in Proliferation and Angiogenesis
4. Discussion
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | androgen receptor |
| Arc | arctigenin |
| ATP | adenosine triphosphate |
| CI | combination index |
| COMT | catechol-O-methyltransferase |
| EC | epicatechin |
| ECG | epicatechin-3-gallate |
| EGC | epigallocatechin |
| EGCG | epigallocatechin-3-gallate |
| GT | green tea |
| GTPs | green tea polyphenols |
| KO | knockout |
| MRP | multidrug resistance-associated protein |
| mTOR | mammalian target of rapamycin |
| NT | non-treatment |
| PI3K | phosphatidylinositol 3-kinases |
| PIN | prostatic intraepithelial neoplasia |
| PSMA | prostate-specific membrane antigen |
| PTEN | phosphatase and tensin homolog |
| Q | quercetin |
| SCID | severe combined immunodeficiency |
References
- American Cancer Society. Cancer Facts & Figures 2023; American Cancer Society: Atlanta, GA, USA, 2023. [Google Scholar]
- Griffiths, K.; Prezioso, D.; Turkes, A.; Denis, L.J. The prevention of prostate cancer. In Recent Results Cancer Research; Springer: Berlin/Heidelberg, Germany, 2007; Volume 175, pp. 33–63. [Google Scholar]
- Neal, D.E.; Donovan, J.L. Prostate cancer: To screen or not to screen? Lancet Oncol. 2000, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Delpierre, C.; Lamy, S.; Kelly-Irving, M.; Molinie, F.; Velten, M.; Tretarre, B.; Woronoff, A.S.; Buemi, A.; Lapotre-Ledoux, B.; Bara, S.; et al. Life expectancy estimates as a key factor in over-treatment: The case of prostate cancer. Cancer Epidemiol. 2013, 37, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Diamond, T.H.; Higano, C.S.; Smith, M.R.; Guise, T.A.; Singer, F.R. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: Recommendations for diagnosis and therapies. Cancer 2004, 100, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Adhami, V.M.; Siddiqui, I.A.; Ahmad, N.; Gupta, S.; Mukhtar, H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004, 64, 8715–8722. [Google Scholar] [CrossRef] [PubMed]
- Sporn, M.B. Approaches to prevention of epithelial cancer during the preneoplastic period. Cancer Res. 1976, 36, 2699–2702. [Google Scholar] [PubMed]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar]
- Henning, S.M.; Wang, P.; Heber, D. Chemopreventive effects of tea in prostate cancer: Green tea versus black tea. Mol. Nutr. Food Res. 2011, 55, 905–920. [Google Scholar] [CrossRef]
- Du, G.J.; Zhang, Z.; Wen, X.D.; Yu, C.; Calway, T.; Yuan, C.S.; Wang, C.Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Wang, P.; Vadgama, J.V.; Said, J.W.; Magyar, C.E.; Doan, N.; Heber, D.; Henning, S.M. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J. Nutr. Biochem. 2014, 25, 73–80. [Google Scholar] [CrossRef]
- van Zanden, J.J.; Wortelboer, H.M.; Bijlsma, S.; Punt, A.; Usta, M.; Bladeren, P.J.; Rietjens, I.M.; Cnubben, N.H. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem. Pharmacol. 2005, 69, 699–708. [Google Scholar] [CrossRef]
- Nagai, M.; Conney, A.H.; Zhu, B.T. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab. Dispos. 2004, 32, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Naidu, P.S.; Kulkarni, S.K. Quercetin potentiates L-Dopa reversal of drug-induced catalepsy in rats: Possible COMT/MAO inhibition. Pharmacology 2003, 68, 81–88. [Google Scholar] [CrossRef]
- Kim, K.A.; Park, P.W.; Park, J.Y. Short-term effect of quercetin on the pharmacokinetics of fexofenadine, a substrate of P-glycoprotein, in healthy volunteers. Eur. J. Clin. Pharmacol. 2009, 65, 609–614. [Google Scholar] [CrossRef]
- Cen, J.; Zhang, R.; Zhao, T.; Zhang, X.; Zhang, C.; Cui, J.; Zhao, K.; Duan, S.; Guo, Y. A Water-Soluble Quercetin Conjugate with Triple Targeting Exerts Neuron-Protective Effect on Cerebral Ischemia by Mitophagy Activation. Adv. Health Mater. 2022, 11, e2200817. [Google Scholar] [CrossRef]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; El-Aleem, S.A.A.; El-Tahawy, N.F.G. Neuroprotective effect of quercetin nanoparticles: A possible prophylactic and therapeutic role in alzheimer’s disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Phan, T.; Gordon, D.; Chung, S.; Henning, S.M.; Vadgama, J.V. Arctigenin in combination with quercetin synergistically enhances the antiproliferative effect in prostate cancer cells. Mol. Nutr. Food Res. 2015, 59, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, R.; Mochizuki, N.; Ikeda, M.; Sato, A.; Nomura, S.; Owada, S.; Yomoda, S.; Tsuchihara, K.; Kishino, S.; Esumi, H. Change in plasma lactate concentration during arctigenin administration in a phase I clinical trial in patients with gemcitabine-refractory pancreatic cancer. PLoS ONE 2018, 13, e0198219. [Google Scholar] [CrossRef]
- Awale, S.; Lu, J.; Kalauni, S.K.; Kurashima, Y.; Tezuka, Y.; Kadota, S.; Esumi, H. Identification of arctigenin as an antitumor agent having the ability to eliminate the tolerance of cancer cells to nutrient starvation. Cancer Res. 2006, 66, 1751–1757. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, X.B.; Yuan, P.C.; Shao, T.L.; Wang, G.D.; Liu, X.P. Arctigenin inhibits proliferation of ER-positive breast cancer cells through cell cycle arrest mediated by GSK3-dependent cyclin D1 degradation. Life Sci. 2020, 256, 117983. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, L.; Guo, X.; Fang, R.; Zhang, H.; Dong, G.; Fu, J.; Yan, F.; Zhang, J.; Ning, Z.; et al. Arctigenin Attenuates Breast Cancer Progression through Decreasing GM-CSF/TSLP/STAT3/beta-Catenin Signaling. Int. J. Mol. Sci. 2020, 21, 6357. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lou, Z.; Lee, S.H. Arctigenin represses TGF-beta-induced epithelial mesenchymal transition in human lung cancer cells. Biochem. Biophys. Res. Commun. 2017, 493, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Solorzano, W.; Diaz, T.; Magyar, C.E.; Henning, S.M.; Vadgama, J.V. Arctigenin inhibits prostate tumor cell growth in vitro and in vivo. Clin. Nutr. Exp. 2017, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Diaz, T.; Verduzco, A.D.R.; Magyar, C.E.; Zhong, J.; Elshimali, Y.; Rettig, M.B.; Henning, S.M.; Vadgama, J.V.; Wang, P. Arctigenin inhibits prostate tumor growth in high-fat diet fed mice through dual actions on adipose tissue and tumor. Sci. Rep. 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- McCall, P.; Witton, C.J.; Grimsley, S.; Nielsen, K.V.; Edwards, J. Is PTEN loss associated with clinical outcome measures in human prostate cancer? Br. J. Cancer 2008, 99, 1296–1301. [Google Scholar] [CrossRef]
- Sarker, D.; Reid, A.H.; Yap, T.A.; de Bono, J.S. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin. Cancer Res. 2009, 15, 4799–4805. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, J.; Lei, Q.; Rozengurt, N.; Pritchard, C.; Jiao, J.; Thomas, G.V.; Li, G.; Roy-Burman, P.; Nelson, P.S.; et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003, 4, 209–221. [Google Scholar] [CrossRef]
- Webber, M.M.; Quader, S.T.; Kleinman, H.K.; Bello-DeOcampo, D.; Storto, P.D.; Bice, G.; DeMendonca-Calaca, W.; Williams, D.E. Human cell lines as an in vitro/in vivo model for prostate carcinogenesis and progression. Prostate 2001, 47, 1–13. [Google Scholar] [CrossRef]
- Yang, G.Y.; Liao, J.; Li, C.; Chung, J.; Yurkow, E.J.; Ho, C.T.; Yang, C.S. Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: Possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis 2000, 21, 2035–2039. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Wu, X.; Wu, J.; Huang, J.; Powell, W.C.; Zhang, J.; Matusik, R.J.; Sangiorgi, F.O.; Maxson, R.E.; Sucov, H.M.; Roy-Burman, P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech. Dev. 2001, 101, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Wang, S.; Qiao, R.; Vivanco, I.; Watson, P.A.; Sawyers, C.L.; Wu, H. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007, 67, 6083–6091. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Fujiwara, Y.; Orkin, S.H. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Bouchelouche, K.; Choyke, P.L.; Capala, J. Prostate specific membrane antigen- a target for imaging and therapy with radionuclides. Discov. Med. 2010, 9, 55–61. [Google Scholar]
- Shappell, S.B.; Thomas, G.V.; Roberts, R.L.; Herbert, R.; Ittmann, M.M.; Rubin, M.A.; Humphrey, P.A.; Sundberg, J.P.; Rozengurt, N.; Barrios, R.; et al. Prostate pathology of genetically engineered mice: Definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004, 64, 2270–2305. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Heber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Ronchi, M.; Petrangolini, G.; Bosisio, S.; Allegrini, P. Improved Oral Absorption of Quercetin from Quercetin Phytosome(R), a New Delivery System Based on Food Grade Lecithin. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 169–177. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Khabatova, V.V.; Gudkov, S.V.; Plotnikov, E.Y.; Turovsky, E.A. Cytoprotective Properties of a New Nanocomplex of Selenium with Taxifolin in the Cells of the Cerebral Cortex Exposed to Ischemia/Reoxygenation. Pharmaceutics 2022, 14, 2477. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Uspalenko, N.I.; Khmil, N.V.; Shigaeva, M.I.; Stepanov, M.R.; Ananyan, M.A.; Timchenko, M.A.; Molchanov, M.V.; Mironova, G.D.; Turovsky, E.A. A Comparative Analysis of Neuroprotective Properties of Taxifolin and Its Water-Soluble Form in Ischemia of Cerebral Cortical Cells of the Mouse. Int. J. Mol. Sci. 2023, 24, 11436. [Google Scholar] [CrossRef]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin Gallate-Gold Nanoparticles Exhibit Superior Antitumor Activity Compared to Conventional Gold Nanoparticles: Potential Synergistic Interactions. Nanomaterials 2019, 9, 396. [Google Scholar] [CrossRef]
- Wang, P.; Wang, B.; Chung, S.; Wu, Y.; Henning, S.M.; Vadgama, J.V. Increased chemopreventive effect by combining arctigenin, green tea polyphenol and curcumin in prostate and breast cancer cells. RSC Adv. 2014, 4, 35242–35250. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Gupta, S. Green tea and prostate cancer: From bench to clinic. Front. Biosci. Elite Ed. 2009, 1, 13–25. [Google Scholar] [PubMed]
- Sun, B.L.; Cai, E.B.; Zhao, Y.; Wang, Y.; Yang, L.M.; Wang, J.Y. Arctigenin Triggers Apoptosis and Autophagy via PI3K/Akt/mTOR Inhibition in PC-3M Cells. Chem. Pharm. Bull. 2021, 69, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.M.; Koreckij, T.D.; Corey, E. Targeted therapy for advanced prostate cancer: Inhibition of the PI3K/Akt/mTOR pathway. Curr. Cancer Drug Targets 2009, 9, 237–249. [Google Scholar] [CrossRef]
- Bhat, T.A.; Singh, R.P. Tumor angiogenesis--a potential target in cancer chemoprevention. Food Chem. Toxicol. 2008, 46, 1334–1345. [Google Scholar] [CrossRef]
- Sharma, R.A.; Harris, A.L.; Dalgleish, A.G.; Steward, W.P.; O’Byrne, K.J. Angiogenesis as a biomarker and target in cancer chemoprevention. Lancet Oncol. 2001, 2, 726–732. [Google Scholar] [CrossRef]
- Yip, H.Y.K.; Papa, A. Signaling Pathways in Cancer: Therapeutic Targets, Combinatorial Treatments, and New Developments. Cells 2021, 10, 659. [Google Scholar] [CrossRef]
- Georgi, B.; Korzeniewski, N.; Hadaschik, B.; Grullich, C.; Roth, W.; Sultmann, H.; Pahernik, S.; Hohenfellner, M.; Duensing, S. Evolving therapeutic concepts in prostate cancer based on genome-wide analyses (review). Int. J. Oncol. 2014, 45, 1337–1344. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Q.; Henning, S.M.; Magyar, C.E.; Said, J.; Zhong, J.; Rettig, M.B.; Vadgama, J.V.; Wang, P. Enhanced Chemoprevention of Prostate Cancer by Combining Arctigenin with Green Tea and Quercetin in Prostate-Specific Phosphatase and Tensin Homolog Knockout Mice. Biomolecules 2024, 14, 105. https://doi.org/10.3390/biom14010105
Hao Q, Henning SM, Magyar CE, Said J, Zhong J, Rettig MB, Vadgama JV, Wang P. Enhanced Chemoprevention of Prostate Cancer by Combining Arctigenin with Green Tea and Quercetin in Prostate-Specific Phosphatase and Tensin Homolog Knockout Mice. Biomolecules. 2024; 14(1):105. https://doi.org/10.3390/biom14010105
Chicago/Turabian StyleHao, Qiongyu, Susanne M. Henning, Clara E. Magyar, Jonathan Said, Jin Zhong, Matthew B. Rettig, Jaydutt V. Vadgama, and Piwen Wang. 2024. "Enhanced Chemoprevention of Prostate Cancer by Combining Arctigenin with Green Tea and Quercetin in Prostate-Specific Phosphatase and Tensin Homolog Knockout Mice" Biomolecules 14, no. 1: 105. https://doi.org/10.3390/biom14010105
APA StyleHao, Q., Henning, S. M., Magyar, C. E., Said, J., Zhong, J., Rettig, M. B., Vadgama, J. V., & Wang, P. (2024). Enhanced Chemoprevention of Prostate Cancer by Combining Arctigenin with Green Tea and Quercetin in Prostate-Specific Phosphatase and Tensin Homolog Knockout Mice. Biomolecules, 14(1), 105. https://doi.org/10.3390/biom14010105