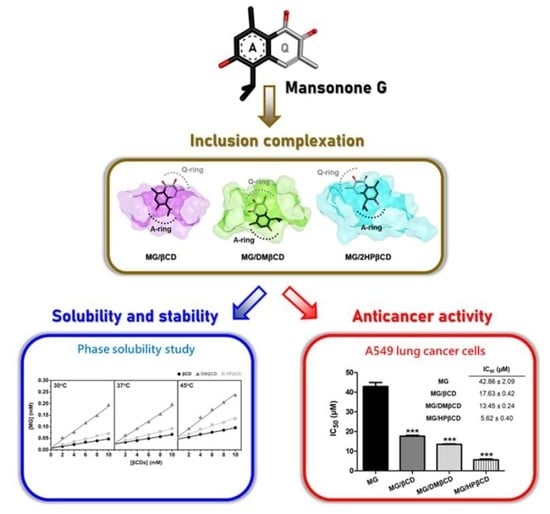
Enhanced Solubility and Anticancer Potential of Mansonone G By β-Cyclodextrin-Based Host-Guest Complexation: A Computational and Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Details
2.2. Experimental Part
2.2.1. Chemical Reagents
2.2.2. Cell lines and Culture
2.2.3. Phase Solubility Study and Evaluation of Thermodynamic Parameters
2.2.4. Inclusion Complex Preparation
2.2.5. Determination of Solubility
2.2.6. Inclusion Complex Characterization
Differential Scanning Calorimetry (DSC)
Scanning Electron Microscope (SEM)
2.2.7. Cytotoxicity of MG toward Lung Cancer Cells
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Ligand Mobility Inside βCD’s Hydrophobic Cavity
3.2. βCDs Conformations Upon MG Binding
3.3. Solvent Accessibility Toward Inclusion Complexes
3.4. Binding Free Energy of Inclusion Complexes
3.5. Phase Solubility Study and Thermodynamic Parameters
3.6. Inclusion Complex Characterization
3.6.1. Thermal Behavior of MG and Its Inclusion Complexes
3.6.2. Surface Morphological Changes upon Inclusion Complexation
3.7. Cytotoxicity of MG/βCDs Inclusion Complexes toward Lung Cancer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Pardee, A.B.; Li, Y.Z.; Li, C.J. Cancer therapy with beta-lapachone. Curr. Cancer Drug Targets 2002, 2, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Dreckschmidt, N.E.; Verma, A.K. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008, 68, 9024–9032. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Xu, J.; Liu, X.; Xia, X.; Li, N.; Bi, X. Shikonin induces apoptosis in the human gastric cancer cells HGC-27 through mitochondria-mediated pathway. Pharmacogn. Mag. 2015, 11, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghdadi, M.A.; Al-Abbasi, F.A.; El-Halawany, A.M.; Aseeri, A.H.; Al-Abd, A.M. Anticancer Profiling for Coumarins and Related O-Naphthoquinones from Mansonia gagei against Solid Tumor Cells In Vitro. Molecules 2018, 23, 1020. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xia, M.; Cui, Z.; Tashiro, S.-I.; Onodera, S.; Ikejima, T. Cytotoxic effects of mansonone E and F isolated from Ulmus pumila. Boil. Pharm. Bull. 2004, 27, 1025–1030. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, S.-L.; Li, M.-M.; Huang, Z.-S.; Lee, K.S.; Gu, L.-Q. Inhibition of thioredoxin reductase by mansonone F analogues: Implications for anticancer activity. Chem. Interactions 2009, 177, 48–57. [Google Scholar] [CrossRef]
- Johnson, J.; Gandhidasan, I.; Murugesan, R. Cytotoxicity and superoxide anion generation by some naturally occurring quinones. Free. Radic. Boil. Med. 1999, 26, 1072–1078. [Google Scholar] [CrossRef]
- Wu, W.-B.; Ou, J.-B.; Huang, Z.-H.; Chen, S.-B.; Ou, T.-M.; Tan, J.-H.; Li, D.; Shen, L.-L.; Huang, S.-L.; Gu, L.-Q.; et al. Synthesis and evaluation of mansonone F derivatives as topoisomerase inhibitors. Eur. J. Med. Chem. 2011, 46, 3339–3347. [Google Scholar] [CrossRef]
- Tanaka, N.; Yasue, M.; Imamura, H. The quinonoid pigments of mansonia altissima wood. Tetrahedron Lett. 1966, 7, 2767–2773. [Google Scholar] [CrossRef]
- Hairani, R.; Mongkol, R.; Chavasiri, W. Allyl and prenyl ethers of mansonone G, new potential semisynthetic antibacterial agents. Bioorganic Med. Chem. Lett. 2016, 26, 5300–5303. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, H.G.; Hegazy, G.A.; AlAmoudi, A.A.; Agabnoor, G.M.; El-Halawany, A.M.; Al-Abd, A.M. Abstract LB-073: Mansonone-G is more potent antiproliferative against liver cancer cells than its coumarin derivative (mansorin-A) despite their antagonistic interaction with 5-fluorouracil. Cancer Chem. 2018, 78. [Google Scholar] [CrossRef]
- Dai, Y.; Harinantenaina, L.; Brodie, P.J.; Callmander, M.W.; Randrianasolo, S.; Rakotobe, E.; Rasamison, V.E.; Kingston, D.G.I. Isolation and synthesis of two antiproliferative calamenene-type sesquiterpenoids from Sterculia tavia from the Madagascar rain forest. Bioorganic Med. Chem. 2012, 20, 6940–6944. [Google Scholar] [CrossRef] [PubMed]
- Mahalapbutr, P.; Wonganan, P.; Chavasiri, W.; Rungrotmongkol, T. Butoxy Mansonone G Inhibits STAT3 and Akt Signaling Pathways in Non-Small Cell Lung Cancers: Combined Experimental and Theoretical Investigations. Cancers 2019, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, L.B.; Burusco, K.K.; Jaime, C.; Venâncio, T.; De Carvalho, A.F.S.; Murgas, L.D.S.; Pinto, L.D.M.A.; Burusco-Goni, K.K. Complexes between methyltestosterone and β-cyclodextrin for application in aquaculture production. Carbohydr. Polym. 2018, 179, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, S.; Raneri, D.; Ficarra, R.; Calabrò, M.L.; Stancanelli, R.; Ficarra, P. Improvement in solubility and dissolution rate of flavonoids by complexation with β-cyclodextrin. J. Pharm. Biomed. Anal. 2004, 35, 379–387. [Google Scholar] [CrossRef]
- Veiga, M.D.; Díaz, P.J.; Ahsan, F. Interactions of Griseofulvin with Cyclodextrins in Solid Binary Systems. J. Pharm. Sci. 1998, 87, 891–900. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Vaidya, B.; Shukla, S.K.; Kolluru, S.; Huen, M.; Mulla, N.; Mehra, N.; Kanabar, D.; Palakurthi, S.; Ayehunie, S.; Muth, A.; et al. Nintedanib-cyclodextrin complex to improve bio-activity and intestinal permeability. Carbohydr. Polym. 2019, 204, 68–77. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, C.; Shi, F.; Firempong, C.K.; Yu, J.; Xu, X.; Zhang, W. Preparation, characterization, and pharmacokinetics study of capsaicin via hydroxypropyl-beta-cyclodextrin encapsulation. Pharm. Biol. 2016, 54, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Másson, M. Cyclodextrins in topical drug formulations: theory and practice. Int. J. Pharm. 2001, 225, 15–30. [Google Scholar] [CrossRef]
- de Araujo, D.R.; Tsuneda, S.S.; Cereda, C.M.S.; Del, G.F.; Carvalho, F.; Preté, P.S.C.; Fernandes, S.A.; Yokaichiya, F.; Franco, M.K.K.D.; Mazzaro, I.; et al. Development and pharmacological evaluation of ropivacaine-2-hydroxypropyl-beta-cyclodextrin inclusion complex. Eur. J. Pharm. Sci. 2008, 33, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Kai-Hang, L.; Mengying, S.; Guping, T.; Xiurong, H.U. [Preparation, characterization and antitumor of cyclodextrin inclusion of an anti-cancer drug regorafenib]. Zhejiang da xue xue bao. Yi xue ban = Journal of Zhejiang University. Med Sci. 2017, 46, 151–159. [Google Scholar]
- Kikuchi, M.; Uekama, K. Effect of Dimethyl β-Cyclodextrin on Oral or Rectal Absorption of 1-Hexylcarbamoyl-5-fluorouracil (HCFU). Yakugaku Zasshi 1988, 108, 156–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kicuntod, J.; Sangpheak, K.; Mueller, M.; Wolschann, P.; Viernstein, H.; Yanaka, S.; Kato, K.; Chavasiri, W.; Pongsawasdi, P.; Kungwan, N.; et al. Theoretical and Experimental Studies on Inclusion Complexes of Pinostrobin and beta-Cyclodextrins. Sci. Pharm. 2018, 86, 5. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.I.; Vaz, P.D.; Braga, S.S.; Silva, A.M.S. Association of aescin with β- and γ-cyclodextrins studied by DFT calculations and spectroscopic methods. Beilstein J. Nanotechnol. 2017, 8, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Aree, T.; Jongrungruangchok, S. Structure-antioxidant activity relationship of beta-cyclodextrin inclusion complexes with olive tyrosol, hydroxytyrosol and oleuropein: Deep insights from X-ray analysis, DFT calculation and DPPH assay. Carbohydr. Polym. 2018, 199, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Mahalapbutr, P.; Chusuth, P.; Kungwan, N.; Chavasiri, W.; Wolschann, P.; Rungrotmongkol, T. Molecular recognition of naphthoquinone-containing compounds against human DNA topoisomerase IIα ATPase domain: A molecular modeling study. J. Mol. Liq. 2017, 247, 374–385. [Google Scholar] [CrossRef]
- Kicuntod, J.; Khuntawee, W.; Wolschann, P.; Pongsawasdi, P.; Chavasiri, W.; Kungwan, N.; Rungrotmongkol, T. Inclusion complexation of pinostrobin with various cyclodextrin derivatives. J. Mol. Graph. Model. 2016, 63, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sangpheak, W.; Khuntawee, W.; Wolschann, P.; Pongsawasdi, P.; Rungrotmongkol, T. Enhanced stability of a naringenin/2,6-dimethyl beta-cyclodextrin inclusion complex: Molecular dynamics and free energy calculations based on MM- and QM-PBSA/GBSA. J. Mol. Graph. Model. 2014, 50, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Hairani, R.; Jeong, H.; Jeong, M.G.; Chavasiri, W.; Hwang, E.S. CBMG, a novel derivative of mansonone G suppresses adipocyte differentiation via suppression of PPARgamma activity. Chemico-Biol. Interact. 2017, 273, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Hanpaibool, C.; Chakcharoensap, T.; Arifin; Hijikata, Y.; Irle, S.; Wolschann, P.; Kungwan, N.; Pongsawasdi, P.; Ounjai, P.; Rungrotmongkol, T. Theoretical analysis of orientations and tautomerization of genistein in β-cyclodextrin. J. Mol. Liq. 2018, 265, 16–23. [Google Scholar] [CrossRef]
- Mahalapbutr, P.; Thitinanthavet, K.; Kedkham, T.; Nguyen, H.; Theu, L.T.H.; Dokmaisrijan, S.; Huynh, L.; Kungwan, N.; Rungrotmongkol, T. A theoretical study on the molecular encapsulation of luteolin and pinocembrin with various derivatized beta-cyclodextrins. J. Mol. Struct. 2019, 1180, 480–490. [Google Scholar] [CrossRef]
- Mahalapbutr, P.; Darai, N.; Panman, W.; Opasmahakul, A.; Kungwan, N.; Hannongbua, S.; Rungrotmongkol, T. Atomistic mechanisms underlying the activation of the G protein-coupled sweet receptor heterodimer by sugar alcohol recognition. Sci. Rep. 2019, 9, 10205. [Google Scholar] [CrossRef]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; Gonzalez-Outeirino, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Luty, B.A.; Van Gunsteren, W.F. Calculating Electrostatic Interactions Using the Particle−Particle Particle−Mesh Method with Nonperiodic Long-Range Interactions. J. Phys. Chem. 1996, 100, 2581–2587. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Higuchi, T. A phase solubility technique. Adv. Anal. Chem. Instrum. 1965, 4, 117–211. [Google Scholar]
- Mahalapbutr, P.; Nutho, B.; Wolschann, P.; Chavasiri, W.; Kungwan, N.; Rungrotmongkol, T. Molecular insights into inclusion complexes of mansonone E and H enantiomers with various beta-cyclodextrins. J. Mol. Graph. Model. 2018, 79, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Ma, S.X.; Zhou, S.Y.; Chen, W.; Yuan, M.W.; Yin, Y.Q.; Yang, X.D. Preparation and characterization of inclusion complexes of naringenin with beta-cyclodextrin or its derivative. Carbohydr. Polym. 2013, 98, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Wongpituk, P.; Nutho, B.; Panman, W.; Kungwan, N.; Wolschann, P.; Rungrotmongkol, T.; Nunthaboot, N. Structural dynamics and binding free energy of neral-cyclodextrins inclusion complexes: molecular dynamics simulation. Mol. Simul. 2017, 43, 1356–1363. [Google Scholar] [CrossRef]
- Ren, B.; Gao, H.; Cao, Y.; Jia, L. In Silico understanding of the cyclodextrin–phenanthrene hybrid assemblies in both aqueous medium and bacterial membranes. J. Hazard. Mater. 2015, 285, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Mongan, J.; Simmerling, C.; McCammon, J.A.; Case, D.A.; Onufriev, A. Generalized Born model with a simple, robust molecular volume correction. J. Chem. Theory Comput. 2007, 3, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chang, C.-E.; Gilson, M.K. Calculation of Cyclodextrin Binding Affinities: Energy, Entropy, and Implications for Drug Design. Biophys. J. 2004, 87, 3035–3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fermeglia, M.; Ferrone, M.; Lodi, A.; Pricl, S. Host–guest inclusion complexes between anticancer drugs and β-cyclodextrin: computational studies. Carbohydr. Polym. 2003, 53, 15–44. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.-X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Jullian, C.; Cifuentes, C.; Alfaro, M.; Miranda, S.; Barriga, G.; Olea-Azar, C. Spectroscopic characterization of the inclusion complexes of luteolin with native and derivatized β-cyclodextrin. Bioorganic Med. Chem. 2010, 18, 5025–5031. [Google Scholar] [CrossRef]
- Wang, D.W.; Ouyang, C.B.; Liu, Q.; Yuan, H.L.; Liu, X.H. Inclusion of quinestrol and 2,6-di-O-methyl-beta-cyclodextrin: Preparation, characterization, and inclusion mode. Carbohydr. Polym. 2013, 93, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Jullian, C.; Morales-Montecinos, J.; Zapata-Torres, G.; Aguilera, B.; Rodriguez, J.; Arán, V.; Olea-Azar, C.; Aguilera-Venegas, B.; Rodríguez-Becerra, J. Characterization, phase-solubility, and molecular modeling of inclusion complex of 5-nitroindazole derivative with cyclodextrins. Bioorganic Med. Chem. 2008, 16, 5078–5084. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Wang, L.; Ma, X.; Xu, K.; Xiong, X.; Liao, X.; Li, H. Characterization and In Vitro Evaluation of the Complexes of Posaconazole with β- and 2,6-di-O-methyl-β-cyclodextrin. AAPS PharmSciTech 2017, 18, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, S.; Tang, P.; Yan, J.; Xu, K.; Li, H. Characterization and evaluation of synthetic riluzole with β-cyclodextrin and 2,6-di-O-methyl-β-cyclodextrin inclusion complexes. Carbohydr. Polym. 2015, 129, 9–16. [Google Scholar] [CrossRef] [PubMed]
- I Saleh, S.; A Rahman, A.; E Aboutaleb, A.; Nakai, Y.; O Ahmed, M. Effect of dimethyl-beta-cyclodextrin on nitrazepam stability. J. de Pharm. de Belg. 1993, 48, 383–388. [Google Scholar]
- Sangpheak, W.; Kicuntod, J.; Schuster, R.; Rungrotmongkol, T.; Wolschann, P.; Kungwan, N.; Viernstein, H.; Mueller, M.; Pongsawasdi, P. Physical properties and biological activities of hesperetin and naringenin in complex with methylated beta-cyclodextrin. Beilstein J. Org. Chem. 2015, 11, 2763–2773. [Google Scholar] [CrossRef]
- Rungrotmongkol, T.; Chakcharoensap, T.; Pongsawasdi, P.; Kungwan, N.; Wolschann, P. The inclusion complexation of daidzein with β-cyclodextrin and 2,6-dimethyl-β-cyclodextrin: a theoretical and experimental study. Mon. für Chem. - Chem. Mon. 2018, 149, 1739–1747. [Google Scholar] [CrossRef]
- Suarez, D.; Diaz, N. Affinity Calculations of Cyclodextrin Host-Guest Complexes: Assessment of Strengths and Weaknesses of End-Point Free Energy Methods. J. Chem. Inf. Modeling 2019, 59, 421–440. [Google Scholar] [CrossRef]
- Genheden, S. MM/GBSA and LIE estimates of host–guest affinities: Dependence on charges and solvation model. J. Comput. Mol. Des. 2011, 25, 1085–1093. [Google Scholar] [CrossRef]
- Suwandecha, T.; Rungnim, C.; Namuangruk, S.; Ruktanonchai, U.; Sawatdee, S.; Dechraksa, J.; Srichana, T. Host-guest interactions between sildenafil and cyclodextrins: Spectrofluorometric study and molecular dynamic modeling. J. Mol. Graph. Model. 2017, 77, 115–120. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Paunescu, V.; Tatu, C.; Ordodi, V.L.; Bandur, G.; Lupea, A.X. Thermal stability of the linoleic acid/α- and β-cyclodextrin complexes. Food Chem. 2006, 99, 500–508. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodriguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Rajendiran, N.; Siva, S. Inclusion complex of sulfadimethoxine with cyclodextrins: Preparation and characterization. Carbohydr. Polym. 2014, 101, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.A.; Giannone, I.; Paolino, D.; Pistara, V.; Corsaro, A.; Puglisi, G. Preparation of celecoxib-dimethyl-beta-cyclodextrin inclusion complex: Characterization and in vitro permeation study. Eur. J. Med. Chem. 2005, 40, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Simal-Gándara, J.; Torrado-Agrasar, A. Antioxidant and antimicrobial properties of encapsulated guava leaf oil in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2018, 111, 219–225. [Google Scholar] [CrossRef]
- Zarif, M.S.; Afidah, A.R.; Abdullah, J.M.; Shariza, A.R. Physicochemical characterization of vancomycin and its complexes with β-cyclodextrin. Biomed. Res. (In India) 2012, 23, 513–520. [Google Scholar]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B: Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Rajbanshi, B.; Saha, S.; Das, K.; Barman, B.K.; Sengupta, S.; Bhattacharjee, A.; Roy, M.N. Study to Probe Subsistence of Host-Guest Inclusion Complexes of α and β-Cyclodextrins with Biologically Potent Drugs for Safety Regulatory Dischargement. Sci. Rep. 2018, 8, 13031. [Google Scholar] [CrossRef]
- Gil Choi, S.; Lee, S.-E.; Kang, B.-S.; Ng, C.L.; Davaa, E.; Park, J.-S. Thermosensitive and Mucoadhesive Sol-Gel Composites of Paclitaxel/Dimethyl-β-Cyclodextrin for Buccal Delivery. PLOS ONE 2014, 9, e109090. [Google Scholar]
- Wong, M.C.S.; Lao, X.Q.; Ho, K.-F.; Goggins, W.B.; Tse, S.L.A. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci. Rep. 2017, 7, 14300. [Google Scholar] [CrossRef]
- Wang, F.; Yang, B.; Zhao, Y.; Liao, X.; Gao, C.; Jiang, R.; Han, B.; Yang, J.; Liu, M.; Zhou, R. Host-guest inclusion system of scutellarein with 2-hydroxypropyl-beta-cyclodextrin: preparation, characterization, and anticancer activity. J. Biomater. Sci. Polym. Ed. 2014, 25, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Drug permeation through biomembranes: cyclodextrins and the unstirred water layer. Die Pharm. 2012, 67, 363–370. [Google Scholar]
- Brewster, M.E.; Noppe, M.; Peeters, J.; Loftsson, T. Effect of the unstirred water layer on permeability enhancement by hydrophilic cyclodextrins. Int. J. Pharm. 2007, 342, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, J.; Shiota, S.; Mazima, K.; Inoue, Y.; Mizuno, H.; Yoshida, J. Interactions of Cyclodextrins with Dipalmitoyl, Distearoyl, and Dimyristoyl Phosphatidyl Choline Liposomes. A Study by Leakage of Carboxyfluorescein in Inner Aqueous Phase of Unilamellar Liposomes. Chem. Pharm. Bull. 2000, 48, 48–52. [Google Scholar] [CrossRef] [PubMed]








| Energy Component (kcal/mol) | MG/βCD | MG/DMβCD | MG/2HPβCD |
|---|---|---|---|
| ΔEvdW | −27.61 ± 1.19 | −29.19 ± 0.29 | −28.59 ± 0.64 |
| ΔEele | −11.79 ± 0.40 | −4.43 ± 0.19 | −6.40 ± 0.90 |
| ΔEMM | −39.40 ± 1.58 | −33.62 ± 0.26 | −34.99 ± 0.26 |
| ΔGsolv,polar | 22.14 ± 0.91 | 12.72 ± 0.13 | 17.17 ± 0.54 |
| ΔGsolv,non-polar | −2.83 ± 0.04 | −2.98 ± 0.01 | −3.10 ± 0.02 |
| ΔGsolv | 19.31 ± 0.86 | 9.73 ± 0.12 | 14.06 ± 0.57 |
| ΔGsolv,polar + ΔEele | 10.35 ± 0.53 | 8.29 ± 0.19 | 10.77 ± 0.47 |
| ΔGsolv,non-polar + ΔEvdW | −30.44 ± 1.24 | −32.18 ± 0.31 | −31.69 ± 0.66 |
| TΔS | −17.74 ± 0.36 | −18.16 ± 0.13 | −17.57 ± 0.42 |
| ΔGbind, MM/GBSA | −2.34 ± 0.35 | −5.73 ± 0.04 (***) | −3.35 ± 0.14 (*) |
| Temperature (°C) | Stability Constant (Kc, M−1) | ||
|---|---|---|---|
| MG/βCD | MG/DMβCD | MG/HPβCD | |
| 30 | 562 | 2245 | 684 |
| 37 | 165 | 643 | 245 |
| 45 | 109 | 358 | 173 |
| Thermodynamic Parameter (kcal/mol) | MG/βCD | MG/DMβCD | MG/HPβCD |
|---|---|---|---|
| ∆H | −20.77 | −23.27 | −17.39 |
| T∆S | −17.06 | −18.71 | −13.54 |
| ∆Gbind, exp (30 °C) | −3.71 | −4.56 | −3.85 |
| ∆Gbind, MM/GBSA (Table 1) | −2.34 ± 0.35 | −5.73 ± 0.04 | −3.35 ± 0.14 |
| Solubility of MG (mg/L) | |
|---|---|
| MG | 1.7 |
| MG/βCD | 11.5 |
| MG/DMβCD | 47.2 |
| MG/HPβCD | 17.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahalapbutr, P.; Wonganan, P.; Charoenwongpaiboon, T.; Prousoontorn, M.; Chavasiri, W.; Rungrotmongkol, T. Enhanced Solubility and Anticancer Potential of Mansonone G By β-Cyclodextrin-Based Host-Guest Complexation: A Computational and Experimental Study. Biomolecules 2019, 9, 545. https://doi.org/10.3390/biom9100545
Mahalapbutr P, Wonganan P, Charoenwongpaiboon T, Prousoontorn M, Chavasiri W, Rungrotmongkol T. Enhanced Solubility and Anticancer Potential of Mansonone G By β-Cyclodextrin-Based Host-Guest Complexation: A Computational and Experimental Study. Biomolecules. 2019; 9(10):545. https://doi.org/10.3390/biom9100545
Chicago/Turabian StyleMahalapbutr, Panupong, Piyanuch Wonganan, Thanapon Charoenwongpaiboon, Manchumas Prousoontorn, Warinthorn Chavasiri, and Thanyada Rungrotmongkol. 2019. "Enhanced Solubility and Anticancer Potential of Mansonone G By β-Cyclodextrin-Based Host-Guest Complexation: A Computational and Experimental Study" Biomolecules 9, no. 10: 545. https://doi.org/10.3390/biom9100545
APA StyleMahalapbutr, P., Wonganan, P., Charoenwongpaiboon, T., Prousoontorn, M., Chavasiri, W., & Rungrotmongkol, T. (2019). Enhanced Solubility and Anticancer Potential of Mansonone G By β-Cyclodextrin-Based Host-Guest Complexation: A Computational and Experimental Study. Biomolecules, 9(10), 545. https://doi.org/10.3390/biom9100545