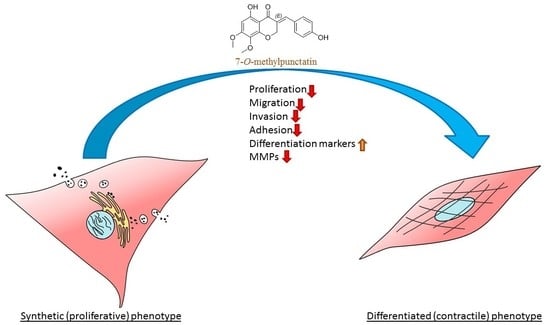
7-O-Methylpunctatin, a Novel Homoisoflavonoid, Inhibits Phenotypic Switch of Human Arteriolar Smooth Muscle Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Preparation of 7-O-methylpunctatin
2.4. MTT Assay
2.5. BrdU Incorporation Assay
2.6. Cell Cycle Analysis
2.7. RT-PCR
- Cyclin D1F: TCCTGTGCTGCGAAGTGGAAAC;
- Cyclin D1R: AAATCGTGCGGGGTCATTGC;
- cdk4F: AAGAGTGTGAGAGTCCCCAATGG;
- cdk4R: GATTTTGCCCAACTGGTCGG;
- Myocardin F: GAGAGGTCCATTCCAACTGC;
- Myocardin R: GGGCTGTGAGGCTGAGTC;
- SM-22α F: TCCAGGTCTGGCTGAAGAATGG;
- SM-22α R: CTGCTCCATCTGCTTGAAGACC;
- SM-α F: ACTGAGCGTGGCTATTCCTCCGTT
- SM-α R: GCAGTGGCCATCTCATTTTCA;
- GAPDH F: CGCTCTCTGCTCCTCCTGTTC;
- GAPDH R: TTGACTCCGACCTTCACCTTCC.
2.8. Phase Contrast Microscopy
2.9. Scanning Electron Microscopy
2.10. Wound Healing (Scratch) assay
2.11. Invasion Assay
2.12. Cell Adhesion Assay
2.13. Monocyte Adhesion Assay
2.14. Measurement of MMP-2 and MMP-9
2.15. Actin Staining
2.16. Luciferase Reporter Assay
2.17. Western Blotting
2.18. Statistical Analysis
3. Results
3.1. MP Inhibits FBS-Induced VSMC Proliferation
3.2. MP Induces Cell Cycle Arrest of FBS-Induced VSMC
3.3. MP Downregulates the Expression of Cyclin D1 and CDK4 and Upregulates the Expression of CDK Inhibitors, p21 and p27, in VSMCs
3.4. MP Induces VSMC Apoptosis
3.5. MP Attenuates FBS-Induced VSMC Migration, Invasion, and Adhesion
3.6. MP Inhibits MMP-2 and MMP-9 Secretion in VSMCs
3.7. MP Decreases the Phosphorylation of ERK1/2 and FAK
3.8. MP Increases the Expression of Early and Mid-Term Differentiation Markers and Decreases the Expression of a De-differentiation Marker
3.9. MP Inhibits Actin Polymerization
3.10. MP Inhibits PMA-induced Adhesion of THP-1 Monocytes on VSMCs
3.11. MP Inhibits FBS-Induced Expression of NF-κB in a Concentration-Dependent Manner
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pober, J.S.; Sessa, W.C. Inflammation and the blood microvascular system. Cold Spring Harb. Perspect. Biol. 2014, 7, a016345. [Google Scholar] [CrossRef]
- Rensen, S.S.; Doevendans, P.A.; van Eys, G.J. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth. Heart J. 2007, 15, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steucke, K.E.; Tracy, P.V.; Hald, E.S.; Hall, J.L.; Alford, P.W. Vascular smooth muscle cell functional contractility depends on extracellular mechanical properties. J. Biomech. 2015, 48, 3044–3051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fardoun, M.M.; Nassif, J.; Issa, K.; Baydoun, E.; Eid, A.H. Raynaud’s phenomenon: A brief review of the underlying mechanisms. Front. Pharmacol. 2016, 7, 438. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Owens, G.K. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc. Res. 2012, 95, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tachjian, A.; Maria, V.; Jahangir, A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J. Am. Coll. Cardiol. 2010, 55, 515–525. [Google Scholar] [CrossRef]
- Li, L.; Zhou, X.; Li, N.; Sun, M.; Lv, J.; Xu, Z. Herbal drugs against cardiovascular disease: Traditional medicine and modern development. Drug Discov. Today 2015, 20, 1074–1086. [Google Scholar] [CrossRef]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012, 95, 454–464. [Google Scholar] [CrossRef]
- van Dam, R.M.; Naidoo, N.; Landberg, R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: Review of recent findings. Curr. Opin. Lipidol. 2013, 24, 25–33. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, dairy foods, and cardiovascular and metabolic health: A review of emerging biologic pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Croft, K.D. Tea flavonoids and cardiovascular health. Mol. Asp. Med. 2010, 31, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cohn, J.S.; Harvey, I.; Le Cornu, K.A.; Ryder, J.J.; Hall, W.L.; Cassidy, A. Flavonoids, flavonoid-rich foods, and cardiovascular risk: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2008, 88, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Mehendale, S.R.; Calway, T.; Yuan, C.S. Botanical flavonoids on coronary heart disease. Am. J. Chin. Med. 2011, 39, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhuang, Y.; Tian, Y.; Thomas, G.N.; Ying, M.; Tomlinson, B. Study of the effects of total flavonoids of Astragalus on atherosclerosis formation and potential mechanisms. Oxidative Med. Cell. Longev. 2012, 2012, 282383. [Google Scholar] [CrossRef]
- Maaliki, D.; Shaito, A.A.; Pintus, G.; El-Yazbi, A.; Eid, A.H. Flavonoids in hypertension: A brief review of the underlying mechanisms. Curr. Opin. Pharmacol. 2019, 45, 57–65. [Google Scholar] [CrossRef]
- Alali, F.; El-Elimat, T.; Albataineh, H.; Al-Balas, Q.; Al-Gharaibeh, M.; Falkinham, J.O., 3rd; Chen, W.L.; Swanson, S.M.; Oberlies, N.H. Cytotoxic homoisoflavones from the bulbs of Bellevalia eigii. J. Nat. Prod. 2015, 78, 1708–1715. [Google Scholar] [CrossRef]
- Paudel, K.R.; Karki, R.; Kim, D.W. Cepharanthine inhibits in vitro VSMC proliferation and migration and vascular inflammatory responses mediated by RAW264.7. Toxicol. Vitr. 2016, 34, 16–25. [Google Scholar] [CrossRef]
- Aoki, C.; Hattori, Y.; Tomizawa, A.; Jojima, T.; Kasai, K. Anti-inflammatory role of cilostazol in vascular smooth muscle cells in vitro and in vivo. J. Atheroscler. Thromb. 2010, 17, 503–509. [Google Scholar] [CrossRef]
- Heiss, E.H.; Liu, R.; Waltenberger, B.; Khan, S.; Schachner, D.; Kollmann, P.; Zimmermann, K.; Cabaravdic, M.; Uhrin, P.; Stuppner, H.; et al. Plumericin inhibits proliferation of vascular smooth muscle cells by blocking STAT3 signaling via S-glutathionylation. Sci. Rep. 2016, 6, 20771. [Google Scholar] [CrossRef]
- Lin, L.G.; Liu, Q.Y.; Ye, Y. Naturally occurring homoisoflavonoids and their pharmacological activities. Planta Med. 2014, 80, 1053–1066. [Google Scholar] [CrossRef]
- El-Elimat, T.; Rivera-Chavez, J.; Burdette, J.E.; Czarnecki, A.; Alhawarri, M.B.; Al-Gharaibeh, M.; Alali, F.; Oberlies, N.H. Cytotoxic homoisoflavonoids from the bulbs of Bellevalia flexuosa. Fitoterapia 2018, 127, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.M.; Thu, C.V.; Dat, N.T.; Ryoo, S.W.; Lee, J.H.; Kim, J.C.; Na, M.; Jung, H.J.; Bae, K.; Min, B.S. Homoisoflavonoid derivatives from the roots of Ophiopogon japonicus and their in vitro anti-inflammation activity. Bioorg. Med. Chem. Lett. 2010, 20, 2412–2416. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.K.; Lee, S.H.; Lee, M.O.; Kim, S.G. Effects of Brazilin on glucose oxidation, lipogenesis and therein involved enzymes in adipose tissues from diabetic KK-mice. Life Sci. 1993, 53, 1291–1297. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C.; Manikumar, G.; Taylor, H.; McGivney, R. Plant antimutagens, 6. Intricatin and intricatinol, new antimutagenic homoisoflavonoids from Hoffmanosseggia intricata. J. Nat. Prod. 1989, 52, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Niranjan Reddy, V.L.; Ravikanth, V.; Jansi Lakshmi, V.V.; Suryanarayan Murty, U.; Venkateswarlu, Y. Inhibitory activity of homoisoflavonoids from Caesalpinia sappan against Beauveria bassiana. Fitoterapia 2003, 74, 600–602. [Google Scholar] [CrossRef]
- Tait, S.; Salvati, A.L.; Desideri, N.; Fiore, L. Antiviral activity of substituted homoisoflavonoids on enteroviruses. Antivir. Res. 2006, 72, 252–255. [Google Scholar] [CrossRef]
- Calvo, M.I. Three new homoisoflavanones from the bulbs of Ledebouria floribunda. Fitoterapia 2009, 80, 394–398. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, L.; Li, D.; Pu, W. Antioxidant activities of different extracts and homoisoflavanones isolated from the Polygonatum odoratum. Nat. Prod. Res. 2013, 27, 1111–1114. [Google Scholar] [CrossRef]
- Tribble, D.L. AHA Science Advisory. Antioxidant consumption and risk of coronary heart disease: Emphasison vitamin C, vitamin E, and β-carotene: A statement for healthcare professionals from the American Heart Association. Circulation 1999, 99, 591–595. [Google Scholar] [CrossRef]
- Mulholland, D.A.; Schwikkard, S.L.; Crouch, N.R. The chemistry and biological activity of the Hyacinthaceae. Nat. Prod. Rep. 2013, 30, 1165–1210. [Google Scholar] [CrossRef] [Green Version]
- Al-Eisawi, D.M. Field Guide to Wild Flowers of Jordan and Neighbouring Countries; Jordan Press Foundation: Amman, Jordan, 1998. [Google Scholar]
- Jeyaraj, S.C.; Unger, N.T.; Eid, A.H.; Mitra, S.; Paul El-Dahdah, N.; Quilliam, L.A.; Flavahan, N.A.; Chotani, M.A. Cyclic AMP-Rap1A signaling activates RhoA to induce α(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2012, 303, C499–C511. [Google Scholar] [CrossRef] [PubMed]
- Fardoun, M.; Al-Shehabi, T.; El-Yazbi, A.; Issa, K.; Zouein, F.; Maaliki, D.; Iratni, R.; Eid, A.H. Ziziphus nummularia inhibits inflammation-induced atherogenic phenotype of human aortic smooth muscle cells. Oxidative Med. Cell. Longev. 2017, 2017, 4134093. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.H. cAMP induces adhesion of microvascular smooth muscle cells to fibronectin via an Epac-mediated but PKA-independent mechanism. Cell. Physiol. Biochem. 2012, 30, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.H.; Chotani, M.A.; Mitra, S.; Miller, T.J.; Flavahan, N.A. Cyclic AMP acts through Rap1 and JNK signaling to increase expression of cutaneous smooth muscle alpha(2C)-adrenoceptors. Am. J. Physiol. Heart C 2008, 295, H266–H272. [Google Scholar] [CrossRef] [PubMed]
- Häcker, G. The morphology of apoptosis. Cell Tissue Res. 2000, 301, 5–17. [Google Scholar] [CrossRef]
- Kamada, R.; Tano, F.; Kudoh, F.; Kimura, N.; Chuman, Y.; Osawa, A.; Namba, K.; Tanino, K.; Sakaguchi, K. Effective cellular morphology analysis for differentiation processes by a fluorescent 1,3a,6a-triazapentalene derivative probe in live cells. PLoS ONE 2016, 11, e0160625. [Google Scholar] [CrossRef]
- Shi, P.; Laude, A.; Yeong, W.Y. Investigation of cell viability and morphology in 3D bio-printed alginate constructs with tunable stiffness. J. Biomed. Mater. Res. A 2017, 105, 1009–1018. [Google Scholar] [CrossRef]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Tixeira, R.; Paone, S.; Mathivanan, S.; Collins, C.; Liem, M.; Goodall, K.J.; Ravichandran, K.S.; Hulett, M.D.; Poon, I.K. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat. Commun. 2015, 6, 7439. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, M.; Yang, F.; Zhu, J.; Xiao, Q.; Zhang, L. Matrix metalloproteinases: Inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediat. Inflamm. 2013, 2013, 928315. [Google Scholar] [CrossRef] [PubMed]
- Uzui, H.; Lee, J.D.; Shimizu, H.; Tsutani, H.; Ueda, T. The role of protein-tyrosine phosphorylation and gelatinase production in the migration and proliferation of smooth muscle cells. Atherosclerosis 2000, 149, 51–59. [Google Scholar] [CrossRef]
- Mason, D.P.; Kenagy, R.D.; Hasenstab, D.; Bowen-Pope, D.F.; Seifert, R.A.; Coats, S.; Hawkins, S.M.; Clowes, A.W. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ. Res. 1999, 85, 1179–1185. [Google Scholar] [CrossRef]
- Yu, L.; Huang, X.; Huang, K.; Gui, C.; Huang, Q.; Wei, B. Ligustrazine attenuates the platelet-derived growth factor-BB-induced proliferation and migration of vascular smooth muscle cells by interrupting extracellular signal-regulated kinase and P38 mitogen-activated protein kinase pathways. Mol. Med. Rep. 2015, 12, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Gerthoffer, W.T. Mechanisms of vascular smooth muscle cell migration. Circ. Res. 2007, 100, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Kim, S.; Namba, M.; Yasumoto, H.; Miyazaki, H.; Hoshiga, M.; Kaneda, Y.; Morishita, R.; Zhan, Y.; Iwao, H. Gene transfer of dominant-negative mutants of extracellular signal-regulated kinase and c-Jun NH2-terminal kinase prevents neointimal formation in balloon-injured rat artery. Circ. Res. 2001, 88, 1120–1126. [Google Scholar] [CrossRef]
- Guan, J.L.; Trevithick, J.E.; Hynes, R.O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991, 2, 951–964. [Google Scholar] [CrossRef]
- Han, Y.L.; Xiao, Y.P.; Qi, Y.M.; Kang, J.; Yan, C.H. Expression of vascular smooth muscle cell markers during early stage of embryonic stem cell-derived embryoid bodies differentiation. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2008, 24, 385–390. [Google Scholar]
- Giachelli, C.M.; Bae, N.; Almeida, M.; Denhardt, D.T.; Alpers, C.E.; Schwartz, S.M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J. Clin. Investig. 1993, 92, 1686–1696. [Google Scholar] [CrossRef]
- Trion, A.; van der Laarse, A. Vascular smooth muscle cells and calcification in atherosclerosis. Am. Heart J. 2004, 147, 808–814. [Google Scholar] [CrossRef]
- Kim, H.R.; Gallant, C.; Leavis, P.C.; Gunst, S.J.; Morgan, K.G. Cytoskeletal remodeling in differentiated vascular smooth muscle is actin isoform dependent and stimulus dependent. Am. J. Physiol. Cell Physiol. 2008, 295, C768–C778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.; Lincoff, A.M.; Plow, E.F.; Topol, E.J. Cell adhesion molecules in coronary artery disease. J. Am. Coll. Cardiol. 1994, 24, 1591–1601. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Sun, C.; Zhang, J.; Lin, S.; Zhao, L.; Wang, L.; Lin, R.; Lv, J.; Xin, S. Proliferation of vascular smooth muscle cells under inflammation is regulated by NF-κB p65/microRNA-17/RB pathway activation. Int. J. Mol. Med. 2018, 41, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sumagin, R.; Sarelius, I.H. Emerging understanding of roles for arterioles in inflammation. Microcirculation 2013, 20, 679–692. [Google Scholar] [CrossRef]
- Granger, D.N.; Rodrigues, S.F.; Yildirim, A.; Senchenkova, E.Y. Microvascular responses to cardiovascular risk factors. Microcirculation 2010, 17, 192–205. [Google Scholar] [CrossRef]
- Stokes, K.Y.; Granger, D.N. The microcirculation: A motor for the systemic inflammatory response and large vessel disease induced by hypercholesterolaemia? J. Physiol. 2005, 562, 647–653. [Google Scholar] [CrossRef]
- Nellore, K.; Harris, N.R. l-arginine and antineutrophil serum enable venular control of capillary perfusion in hypercholesterolemic rats. Microcirculation 2002, 9, 477–485. [Google Scholar] [CrossRef]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Seo, K.; Yamashita, H.; Hosoya, Y.; Ohsugi, M.; Tobe, K.; Kadowaki, T.; Nagai, R.; et al. In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J. Clin. Investig. 2008, 118, 710–721. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Hosoya, Y.; Yamashita, H.; Fujita, H.; Ohsugi, M.; Tobe, K.; Kadowaki, T.; Nagai, R.; et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 2007, 56, 1517–1526. [Google Scholar] [CrossRef]
- Manlove, F.R. Retinal and choroidal arterioles in malignant hypertension; a clinical and pathologic study of 15 cases. Arch. Intern. Med. 1946, 78, 419–440. [Google Scholar] [CrossRef]
- Feihl, F.; Liaudet, L.; Waeber, B.; Levy, B.I. Hypertension: A disease of the microcirculation? Hypertension 2006, 48, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Dehaini, H.; Fardoun, M.; Abou-Saleh, H.; El-Yazbi, A.; Eid, A.A.; Eid, A.H. Estrogen in vascular smooth muscle cells: A friend or a foe? Vasc. Pharmacol. 2018, 111, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.R.; Sinha, S.; Owens, G.K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 2016, 118, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Rudijanto, A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med. Indones 2007, 39, 86–93. [Google Scholar]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef]
- Fu, M.; Wang, C.; Li, Z.; Sakamaki, T.; Pestell, R.G. Minireview: Cyclin D1: Normal and abnormal functions. Endocrinology 2004, 145, 5439–5447. [Google Scholar] [CrossRef]
- Sherr, C.J. G1 phase progression: Cycling on cue. Cell 1994, 79, 551–555. [Google Scholar] [CrossRef]
- Guo, J.; Li, L.; Wu, Y.J.; Yan, Y.; Xu, X.N.; Wang, S.B.; Yuan, T.Y.; Fang, L.H.; Du, G.H. Inhibitory effects of Brazilin on the vascular smooth muscle cell proliferation and migration induced by PDGF-BB. Am. J. Chin. Med. 2013, 41, 1283–1296. [Google Scholar] [CrossRef]
- Song, J.H.; Jeong, G.H.; Park, S.L.; Won, S.Y.; Paek, N.S.; Lee, B.H.; Moon, S.K. Inhibitory effects of fermented extract of Ophiopogon japonicas on thrombin-induced vascular smooth muscle cells. Mol. Med. Rep. 2016, 13, 426–432. [Google Scholar] [CrossRef]
- Marra, D.E.; Simoncini, T.; Liao, J.K. Inhibition of vascular smooth muscle cell proliferation by sodium salicylate mediated by upregulation of p21(Waf1) and p27(Kip1). Circulation 2000, 102, 2124–2130. [Google Scholar] [CrossRef]
- Mebratu, Y.; Tesfaigzi, Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle 2009, 8, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Pyles, J.M.; March, K.L.; Franklin, M.; Mehdi, K.; Wilensky, R.L.; Adam, L.P. Activation of MAP kinase in vivo follows balloon overstretch injury of porcine coronary and carotid arteries. Circ. Res. 1997, 81, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Roy, A.; Overbeck, H.W. Culture of renal arteriolar smooth muscle cells. Mitogenic responses to angiotensin II. Circ. Res. 1992, 71, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Abberton, K.M.; Taylor, N.H.; Healy, D.L.; Rogers, P.A. Vascular smooth muscle cell proliferation in arterioles of the human endometrium. Hum. Reprod. 1999, 14, 1072–1079. [Google Scholar] [CrossRef]
- El-Beblawy, N.M.; Andrawes, N.G.; Ismail, E.A.; Enany, B.E.; El-Seoud, H.S.; Erfan, M.A. Serum and urinary orosomucoid in young patients with type 1 diabetes: A link between inflammation, microvascular complications, and subclinical atherosclerosis. Clin. Appl. Thromb. Hemost. 2016, 22, 718–726. [Google Scholar] [CrossRef]
- Gibbons, G.H.; Dzau, V.J. The emerging concept of vascular remodeling. N. Engl. J. Med. 1994, 330, 1431–1438. [Google Scholar] [CrossRef]
- Ruiz, E.; Gordillo-Moscoso, A.; Padilla, E.; Redondo, S.; Rodriguez, E.; Reguillo, F.; Briones, A.M.; van Breemen, C.; Okon, E.; Tejerina, T. Human vascular smooth muscle cells from diabetic patients are resistant to induced apoptosis due to high Bcl-2 expression. Diabetes 2006, 55, 1243–1251. [Google Scholar] [CrossRef]
- Ruiz, E.; Redondo, S.; Gordillo-Moscoso, A.; Tejerina, T. Pioglitazone induces apoptosis in human vascular smooth muscle cells from diabetic patients involving the transforming growth factor-β/activin receptor-like kinase-4/5/7/Smad2 signaling pathway. J. Pharmacol. Exp. Ther. 2007, 321, 431–438. [Google Scholar] [CrossRef]
- Bottone, M.G.; Santin, G.; Aredia, F.; Bernocchi, G.; Pellicciari, C.; Scovassi, A.I. Morphological features of organelles during apoptosis: An overview. Cells 2013, 2, 294–305. [Google Scholar] [CrossRef]
- Sheikh, A.Q.; Lighthouse, J.K.; Greif, D.M. Recapitulation of developing artery muscularization in pulmonary hypertension. Cell Rep. 2014, 6, 809–817. [Google Scholar] [CrossRef]
- Voelkel, N.F.; Gomez-Arroyo, J.; Mizuno, S. COPD/emphysema: The vascular story. Pulm. Circ. 2011, 1, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Louis, S.F.; Zahradka, P. Vascular smooth muscle cell motility: From migration to invasion. Exp. Clin. Cardiol. 2010, 15, e75–e85. [Google Scholar] [PubMed]
- Chang, S.; Song, S.; Lee, J.; Yoon, J.; Park, J.; Choi, S.; Park, J.K.; Choi, K.; Choi, C. Phenotypic modulation of primary vascular smooth muscle cells by short-term culture on micropatterned substrate. PLoS One 2014, 9, e88089. [Google Scholar] [CrossRef] [PubMed]
- Motawea, H.K.; Jeyaraj, S.C.; Eid, A.H.; Mitra, S.; Unger, N.T.; Ahmed, A.A.; Flavahan, N.A.; Chotani, M.A. Cyclic AMP-Rap1A signaling mediates cell surface translocation of microvascular smooth muscle α2C-adrenoceptors through the actin-binding protein filamin-2. Am. J. Physiol. Cell Physiol. 2013, 305, C829–C845. [Google Scholar] [CrossRef] [PubMed]
- DeMali, K.A.; Wennerberg, K.; Burridge, K. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 2003, 15, 572–582. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.E.; Horwitz, A.F.; Lauffenburger, D.A.; Sheetz, M.P. Integrin-cytoskeletal interactions in migrating fibroblasts are dynamic, asymmetric, and regulated. J. Cell Biol. 1993, 123, 977–991. [Google Scholar] [CrossRef]
- Sokolova, A.; Hill, M.D.; Rahimi, F.; Warden, L.A.; Halliday, G.M.; Shepherd, C.E. Monocyte chemoattractant protein-1 plays a dominant role in the chronic inflammation observed in Alzheimer’s disease. Brain Pathol. 2009, 19, 392–398. [Google Scholar] [CrossRef]
- Herbin, O.; Regelmann, A.G.; Ramkhelawon, B.; Weinstein, E.G.; Moore, K.J.; Alexandropoulos, K. Monocyte adhesion and plaque recruitment during atherosclerosis development is regulated by the adapter protein Chat-H/SHEP1. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1791–1801. [Google Scholar] [CrossRef]
- Gwon, W.G.; Joung, E.J.; Kwon, M.S.; Lim, S.J.; Utsuki, T.; Kim, H.R. Sargachromenol protects against vascular inflammation by preventing TNF-α-induced monocyte adhesion to primary endothelial cells via inhibition of NF-κB activation. Int. Immunopharmacol. 2017, 42, 81–89. [Google Scholar] [CrossRef]
- Granger, D.N.; Kubes, P. The microcirculation and inflammation: Modulation of leukocyte-endothelial cell adhesion. J. Leukoc. Biol. 1994, 55, 662–675. [Google Scholar] [CrossRef]
- Bruce, A.C.; Kelly-Goss, M.R.; Heuslein, J.L.; Meisner, J.K.; Price, R.J.; Peirce, S.M. Monocytes are recruited from venules during arteriogenesis in the murine spinotrapezius ligation model. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Cerda-Nicolas, M.; Naim Abu Nabah, Y.; Mata, M.; Issekutz, A.C.; Panes, J.; Lobb, R.R.; Sanz, M.J. Direct evidence of leukocyte adhesion in arterioles by angiotensin II. Blood 2004, 104, 402–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boneh, A.; Mandla, S.; Tenenhouse, H.S. Phorbol myristate acetate activates protein kinase C, stimulates the phosphorylation of endogenous proteins and inhibits phosphate transport in mouse renal tubules. Biochim. Biophys. Acta 1989, 1012, 308–316. [Google Scholar] [CrossRef]
- Gamet-Payrastre, L.; Manenti, S.; Gratacap, M.P.; Tulliez, J.; Chap, H.; Payrastre, B. Flavonoids and the inhibition of PKC and PI 3-kinase. Gen. Pharmacol. 1999, 32, 279–286. [Google Scholar] [CrossRef]
- Opitz, F.; Schenke-Layland, K.; Cohnert, T.U.; Stock, U.A. Phenotypical plasticity of vascular smooth muscle cells-effect of in vitro and in vivo shear stress for tissue engineering of blood vessels. Tissue Eng. 2007, 13, 2505–2514. [Google Scholar] [CrossRef]
- Bacakova, L.; Travnickova, M.; Filova, E.; Matějka, R.; Stepanovska, J.; Musilkova, J.; Zarubova, J.; Molitor, M. The role of vascular smooth muscle cells in the physiology and pathophysiology of blood vessels. Muscle Cell Tissue Curr. Status Res. Field 2018, 229. [Google Scholar] [CrossRef]
- Rzucidlo, E.M.; Martin, K.A.; Powell, R.J. Regulation of vascular smooth muscle cell differentiation. J. Vasc. Surg. 2007, 45 (Suppl. A), A25–A32. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Lun, Y.; Wu, X.; Xia, Q.; Zhang, X.; Xin, S.; Zhang, J. Association between the hypomethylation of osteopontin and integrin β3 promoters and vascular smooth muscle cell phenotype switching in great saphenous varicose veins. Int. J. Mol. Sci. 2014, 15, 18747–18761. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Vascular smooth muscle cell in atherosclerosis. Acta Physiol. (Oxf.) 2015, 214, 33–50. [Google Scholar] [CrossRef]
- Wang, D.; Chang, P.S.; Wang, Z.; Sutherland, L.; Richardson, J.A.; Small, E.; Krieg, P.A.; Olson, E.N. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell 2001, 105, 851–862. [Google Scholar] [CrossRef]
- Chen, J.; Kitchen, C.M.; Streb, J.W.; Miano, J.M. Myocardin: A component of a molecular switch for smooth muscle differentiation. J. Mol. Cell. Cardiol. 2002, 34, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Du, K.L.; Ip, H.S.; Li, J.; Chen, M.; Dandre, F.; Yu, W.; Lu, M.M.; Owens, G.K.; Parmacek, M.S. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol. Cell. Biol. 2003, 23, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.Z.; Pipes, G.C.; Olson, E.N. Myocardin is a master regulator of smooth muscle gene expression. Proc. Natl. Acad. Sci. USA 2003, 100, 7129–7134. [Google Scholar] [CrossRef] [Green Version]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Kawai-Kowase, K.; Owens, G.K. Forced expression of myocardin is not sufficient for induction of smooth muscle differentiation in multipotential embryonic cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.Z.; Hockemeyer, D.; McAnally, J.; Nordheim, A.; Olson, E.N. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 2004, 428, 185–189. [Google Scholar] [CrossRef]
- Taurin, S.; Sandbo, N.; Yau, D.M.; Sethakorn, N.; Kach, J.; Dulin, N.O. Phosphorylation of myocardin by extracellular signal-regulated kinase. J. Biol. Chem. 2009, 284, 33789–33794. [Google Scholar] [CrossRef]
- Liao, X.H.; Wang, N.; Zhao, D.W.; Zheng, D.L.; Zheng, L.; Xing, W.J.; Ma, W.J.; Bao, L.Y.; Dong, J.; Zhang, T.C. STAT3 protein regulates vascular smooth muscle cell phenotypic switch by interaction with myocardin. J. Biol. Chem. 2015, 290, 19641–19652. [Google Scholar] [CrossRef]
- Xie, C.; Guo, Y.; Zhu, T.; Zhang, J.; Ma, P.X.; Chen, Y.E. Yap1 protein regulates vascular smooth muscle cell phenotypic switch by interaction with myocardin. J. Biol. Chem. 2012, 287, 14598–14605. [Google Scholar] [CrossRef]
- Tang, R.H.; Zheng, X.L.; Callis, T.E.; Stansfield, W.E.; He, J.; Baldwin, A.S.; Wang, D.Z.; Selzman, C.H. Myocardin inhibits cellular proliferation by inhibiting NF-κB(p65)-dependent cell cycle progression. Proc. Natl. Acad. Sci. USA 2008, 105, 3362–3367. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Noda, M.; O’Regan, A.W.; Pavlin, D.; Berman, J.S. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Investig. 2001, 107, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.R.; Garvin, M.R.; Stewart, D.K.; Hinohara, T.; Simpson, J.B.; Schwartz, S.M.; Giachelli, C.M. Osteopontin is synthesized by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler. Thromb. 1994, 14, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xu, H.; Liang, F.; He, J.; Zhang, J. Association of osteopontin expression with the prognosis of glioma patient: A meta-analysis. Tumour. Biol. 2015, 36, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, A.H.; Abdolrahmani, L.; Mirzaei, H.; Sahebkar, A.; Moohebati, M.; Ghorbani, M.; Ferns, G.A.; Ghayour-Mobarhan, M. Serum osteopontin concentrations in relation to coronary artery disease. Arch. Med. Res. 2015, 46, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Parrish, A.R.; Ramos, K.S. Differential processing of osteopontin characterizes the proliferative vascular smooth muscle cell phenotype induced by allylamine. J. Cell. Biochem. 1997, 65, 267–275. [Google Scholar] [CrossRef]
- Liaw, L.; Skinner, M.P.; Raines, E.W.; Ross, R.; Cheresh, D.A.; Schwartz, S.M.; Giachelli, C.M. The adhesive and migratory effects of osteopontin are mediated via distinct cell surface integrins. Role of alpha v beta 3 in smooth muscle cell migration to osteopontin in vitro. J. Clin. Investig. 1995, 95, 713–724. [Google Scholar] [CrossRef]
- Li, J.J.; Han, M.; Wen, J.K.; Li, A.Y. Osteopontin stimulates vascular smooth muscle cell migration by inducing FAK phosphorylation and ILK dephosphorylation. Biochem. Biophys. Res. Commun. 2007, 356, 13–19. [Google Scholar] [CrossRef]
- Gao, H.; Steffen, M.C.; Ramos, K.S. Osteopontin regulates α-smooth muscle actin and calponin in vascular smooth muscle cells. Cell Biol. Int. 2012, 36, 155–161. [Google Scholar] [CrossRef]
- Williams, E.S.; Wilson, E.; Ramos, K.S. NF-kappaB and matrix-dependent regulation of osteopontin promoter activity in allylamine-activated vascular smooth muscle cells. Oxid. Med. Cell. Longev. 2012, 2012, 496540. [Google Scholar] [CrossRef]
- Adams, C.C.; Workman, J.L. Binding of disparate transcriptional activators to nucleosomal DNA is inherently cooperative. Mol. Cell. Biol. 1995, 15, 1405–1421. [Google Scholar] [CrossRef] [Green Version]
- Shanahan, C.M.; Weissberg, P.L.; Metcalfe, J.C. Isolation of gene markers of differentiated and proliferating vascular smooth muscle cells. Circ. Res. 1993, 73, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.J.; Kim, G.R.; Cho, S.N.; Kwon, J.S.; Ahn, Y.; Kook, H.; Jeong, M.H. miR-18a-5p MicroRNA increases vascular smooth muscle cell differentiation by downregulating syndecan4. Korean Circ. J. 2014, 44, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Bockmeyer, C.L.; Kern, D.S.; Forstmeier, V.; Lovric, S.; Modde, F.; Agustian, P.A.; Steffens, S.; Birschmann, I.; Traeder, J.; Dammrich, M.E.; et al. Arteriolar vascular smooth muscle cell differentiation in benign nephrosclerosis. Nephrol. Dial. Transpl. 2012, 27, 3493–3501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.M.; Wang, B.W.; Pan, C.M.; Fang, W.J.; Chua, S.K.; Hou, S.W.; Chang, H.; Shyu, K.G. Effects of flavonoids on MicroRNA 145 regulation through Klf4 and myocardin in neointimal formation in vitro and in vivo. J. Nutr. Biochem. 2018, 52, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Mehrhof, F.B.; Schmidt-Ullrich, R.; Dietz, R.; Scheidereit, C. Regulation of vascular smooth muscle cell proliferation: Role of NF-κB revisited. Circ. Res. 2005, 96, 958–964. [Google Scholar] [CrossRef]
- Brasier, A.R. The nuclear factor-κB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc. Res. 2010, 86, 211–218. [Google Scholar] [CrossRef]
- Pamukcu, B.; Lip, G.Y.; Shantsila, E. The nuclear factor—Kappa B pathway in atherosclerosis: A potential therapeutic target for atherothrombotic vascular disease. Thromb. Res. 2011, 128, 117–123. [Google Scholar] [CrossRef]
- Pateras, I.; Giaginis, C.; Tsigris, C.; Patsouris, E.; Theocharis, S. NF-κB signaling at the crossroads of inflammation and atherogenesis: Searching for new therapeutic links. Expert Opin. Ther. Targets 2014, 18, 1089–1101. [Google Scholar] [CrossRef]
- Dickson, K.M.; Bhakar, A.L.; Barker, P.A. TRAF6-dependent NF-κB transcriptional activity during mouse development. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2004, 231, 122–127. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Ma, H.; Guo, R.; Kang, R.; Han, J.; Dong, Z. Coumestrol inhibits carotid sinus baroreceptor activity by cAMP/PKA dependent nitric oxide release in anesthetized male rats. Biochem. Pharmacol. 2015, 93, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Chotani, M.A.; Mitra, S.; Eid, A.H.; Han, S.A.; Flavahan, N.A. Distinct cAMP signaling pathways differentially regulate α2C-adrenoceptor expression: Role in serum induction in human arteriolar smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H69–H76. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, R.; Hansson, G.K. Treating inflammation in atherosclerotic cardiovascular disease: Emerging therapies. Eur. Heart J. 2009, 30, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.L.; Weaver, V.M.; Hammer, D.A. Integrin-mediated signalling through the MAP-kinase pathway. IET Syst. Biol. 2008, 2, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ketova, T.; Hardy, D.; Xu, X.; Gao, X.; Zijlstra, A.; Blobe, G.C. Endoglin mediates vascular maturation by promoting vascular smooth muscle cell migration and spreading. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.H.; Wen, J.K.; Liu, G.; McNutt, M.A.; Miao, S.B.; Gao, R.; Zheng, B.; Zhang, H.; Han, M. Blockade of the Ras-extracellular signal-regulated kinase 1/2 pathway is involved in smooth muscle 22 α-mediated suppression of vascular smooth muscle cell proliferation and neointima hyperplasia. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 683–691. [Google Scholar] [CrossRef]
- Hong, H.; McCullough, C.M.; Stegemann, J.P. The role of ERK signaling in protein hydrogel remodeling by vascular smooth muscle cells. Biomaterials 2007, 28, 3824–3833. [Google Scholar] [CrossRef] [Green Version]
- Li, R.C.; Cindrova-Davies, T.; Skepper, J.N.; Sellers, L.A. Prostacyclin induces apoptosis of vascular smooth muscle cells by a cAMP-mediated inhibition of extracellular signal-regulated kinase activity and can counteract the mitogenic activity of endothelin-1 or basic fibroblast growth factor. Circ. Res. 2004, 94, 759–767. [Google Scholar] [CrossRef]
- Pan, Q.; Liu, H.; Zheng, C.; Zhao, Y.; Liao, X.; Wang, Y.; Chen, Y.; Zhao, B.; Lazartigues, E.; Yang, Y.; et al. Microvesicles derived from inflammation-challenged endothelial cells modulate vascular smooth muscle cell functions. Front. Physiol. 2017, 7, 692. [Google Scholar] [CrossRef]
- Lee, S.J.; Baek, S.E.; Jang, M.A.; Kim, C.D. Osteopontin plays a key role in vascular smooth muscle cell proliferation via EGFR-mediated activation of AP-1 and C/EBPβ pathways. Pharmacol. Res. 2016, 108, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, H.; Hunter, T. p27, a novel inhibitor of G1 cyclin-CDK protein kinase activity, is related to p21. Cell 1994, 78, 67–74. [Google Scholar] [CrossRef]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yu, W.; Feng, J.; Fan, Z.; Li, J. Curcumin suppresses tumor necrosis factor-alpha-induced matrix metalloproteinase-2 expression and activity in rat vascular smooth muscle cells via the NF-κB pathway. Exp. Ther. Med. 2014, 7, 1653–1658. [Google Scholar] [CrossRef]
- Moon, S.K.; Cho, G.O.; Jung, S.Y.; Gal, S.W.; Kwon, T.K.; Lee, Y.C.; Madamanchi, N.R.; Kim, C.H. Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: Role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochem. Biophys. Res. Commun. 2003, 301, 1069–1078. [Google Scholar] [CrossRef]
- Moon, S.K.; Cha, B.Y.; Kim, C.H. ERK1/2 mediates TNF-α-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-κB and AP-1: Involvement of the ras dependent pathway. J. Cell. Physiol. 2004, 198, 417–427. [Google Scholar] [CrossRef]
- Chung, C.H.; Lin, K.T.; Chang, C.H.; Peng, H.C.; Huang, T.F. The integrin α2β1 agonist, aggretin, promotes proliferation and migration of VSMC through NF-κB translocation and PDGF production. Br. J. Pharmacol. 2009, 156, 846–856. [Google Scholar] [CrossRef]
- Guo, R.; Li, W.; Liu, B.; Li, S.; Zhang, B.; Xu, Y. Resveratrol protects vascular smooth muscle cells against high glucose-induced oxidative stress and cell proliferation in vitro. Med. Sci. Monit. Basic Res. 2014, 20, 82–92. [Google Scholar] [CrossRef]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fardoun, M.; Iratni, R.; Dehaini, H.; Eid, A.; Ghaddar, T.; El-Elimat, T.; Alali, F.; Badran, A.; Eid, A.H.; Baydoun, E. 7-O-Methylpunctatin, a Novel Homoisoflavonoid, Inhibits Phenotypic Switch of Human Arteriolar Smooth Muscle Cells. Biomolecules 2019, 9, 716. https://doi.org/10.3390/biom9110716
Fardoun M, Iratni R, Dehaini H, Eid A, Ghaddar T, El-Elimat T, Alali F, Badran A, Eid AH, Baydoun E. 7-O-Methylpunctatin, a Novel Homoisoflavonoid, Inhibits Phenotypic Switch of Human Arteriolar Smooth Muscle Cells. Biomolecules. 2019; 9(11):716. https://doi.org/10.3390/biom9110716
Chicago/Turabian StyleFardoun, Manal, Rabah Iratni, Hassan Dehaini, Assaad Eid, Tarek Ghaddar, Tamam El-Elimat, Feras Alali, Adnan Badran, Ali H. Eid, and Elias Baydoun. 2019. "7-O-Methylpunctatin, a Novel Homoisoflavonoid, Inhibits Phenotypic Switch of Human Arteriolar Smooth Muscle Cells" Biomolecules 9, no. 11: 716. https://doi.org/10.3390/biom9110716
APA StyleFardoun, M., Iratni, R., Dehaini, H., Eid, A., Ghaddar, T., El-Elimat, T., Alali, F., Badran, A., Eid, A. H., & Baydoun, E. (2019). 7-O-Methylpunctatin, a Novel Homoisoflavonoid, Inhibits Phenotypic Switch of Human Arteriolar Smooth Muscle Cells. Biomolecules, 9(11), 716. https://doi.org/10.3390/biom9110716