Effect of Antifreeze Glycoproteins on Organoid Survival during and after Hypothermic Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. AFGP Purification
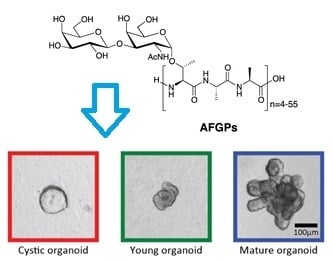
2.2. Organoid Culture
2.3. Cold Storage Experiements
2.4. Fluorescein Diacetate Test
2.5. Imaging of FITC-AFGP
2.6. Statistical Analysis
3. Results
Antifreeze Glycoproteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rubinsky, B. Principles of Low Temperature Cell Preservation. Heart Failure Rev. 2003, 8, 277–284. [Google Scholar] [CrossRef]
- Mathew, A.J.; Baust, J.M.; Van Buskirk, R.G.; Baust, J.G. Cell preservation in reparative and regenerative medicine: Evolution of individualized solution composition. Tissue Eng. 2004, 10, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.L.; Wohlschlag, D.E. Freezing resistance in some Antarctic fishes. Science 1969, 163, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Duman, J.G. Antifreeze and Ice Nucleator Proteins in Terrestrial Arthropods. Annu. Rev. Physiol. 2001, 63, 327–357. [Google Scholar] [CrossRef] [PubMed]
- Olijve, L.L.C.; Meister, K.; DeVries, A.L.; Duman, J.G.; Guo, S.; Bakker, H.J.; Voets, I.K. Blocking rapid ice crystal growth through nonbasal plane adsorption of antifreeze proteins. Proc. Natl. Acad. Sci. USA 2016, 113, 3740–3745. [Google Scholar] [CrossRef]
- Raymond, J.A.; DeVries, A.L. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc. Natl. Acad. Sci. USA 1977, 74, 2589–2593. [Google Scholar] [CrossRef] [PubMed]
- Amir, G.; Horowitz, L.; Rubinsky, B.; Yousif, B.S.; Lavee, J.; Smolinsky, A.K. Subzero nonfreezing cryopresevation of rat hearts using antifreeze protein I and antifreeze protein III. Cryobiology 2004, 48, 273–282. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, H.J.; Kim, H.J.; Lee, J.H.; Ko, Y.; Kim, S.M.; Lee, J.R.; Suh, C.S.; Kim, S.H. Effects of antifreeze proteins on the vitrification of mouse oocytes: Comparison of three different antifreeze proteins. Hum. Reprod. 2015, 30, 2110–2119. [Google Scholar] [CrossRef]
- Kamijima, T.; Sakashita, M.; Miura, A.; Nishimiya, Y.; Tsuda, S. Antifreeze protein prolongs the life-time of insulinoma cells during hypothermic preservation. PLoS ONE 2013, 8, e73643. [Google Scholar] [CrossRef]
- Tablin, F.; Oliver, A.E.; Walker, N.J.; Crowe, L.M.; Crowe, J.H. Membrane phase transition of intact human platelets: Correlation with cold-induced activation. J. Cell. Physiol. 1996, 168, 305–313. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Fletcher, G.L. Hypothermic protection--a fundamental property of “antifreeze” proteins. Biochem. Biophys. Res. Commun. 1991, 180, 566–571. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Hong, J.S.; Lee, C.Y. Freezing of mammalian livers with glycerol and antifreeze proteins. Biochem. Biophys. Res. Commun. 1994, 200, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, Q.; Yang, X.; Layne, J.R., Jr.; Devries, A.L. Antifreeze glycoproteins from antarctic notothenioid fishes fail to protect the rat cardiac explant during hypothermic and freezing preservation. Cryobiology 1994, 31, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Paramo, S.; Barbosa, V.; Perez-Cerezales, S.; Robles, V.; Herraez, M.P. Cryoprotective effects of antifreeze proteins delivered into zebrafish embryos. Cryobiology 2009, 58, 128–133. [Google Scholar] [CrossRef] [PubMed]
- de Souza, N. Organoids. Nat. Methods 2018, 15, 23. [Google Scholar] [CrossRef]
- Fatehullah, A.; Tan, S.H.; Barker, N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016, 18, 246. [Google Scholar] [CrossRef] [PubMed]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Evans, C.W.; Gubala, V.; Nooney, R.; Williams, D.E.; Brimble, M.A.; Devries, A.L. How do Antarctic notothenioid fishes cope with internal ice? A novel function for antifreeze glycoproteins. Antarct. Sci. 2011, 23, 57–64. [Google Scholar] [CrossRef]
- Mahe, M.M.; Aihara, E.; Schumacher, M.A.; Zavros, Y.; Montrose, M.H.; Helmrath, M.A.; Sato, T.; Shroyer, N.F. Establishment of Gastrointestinal Epithelial Organoids. Curr. Protocols Mouse Biol. 2013, 3, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Widholm, J.M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972, 47, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yui, S.; Azzolin, L.; Maimets, M.; Pedersen, M.T.; Fordham, R.P.; Hansen, S.L.; Larsen, H.L.; Guiu, J.; Alves, M.R.P.; Rundsten, C.F.; et al. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell 2018, 22, 35–49.e37. [Google Scholar] [CrossRef] [PubMed]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordonez-Moran, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.R.; Swain, J.R.; Davies, P.S.; Gallagher, A.C.; Parappilly, M.S.; Beach, C.Z.; Streeter, P.R.; Williamson, I.A.; Magness, S.T.; Wong, M.H. Monoclonal Antibodies Reveal Dynamic Plasticity Between Lgr5- and Bmi1-Expressing Intestinal Cell Populations. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, J.F.; Wiegerinck, C.L.; de Jonge, H.R.; Bronsveld, I.; Janssens, H.M.; de Winter-de Groot, K.M.; Brandsma, A.M.; de Jong, N.W.M.; Bijvelds, M.J.C.; Scholte, B.J.; et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 2013, 19, 939. [Google Scholar] [CrossRef] [PubMed]
- Zomer-van Ommen, D.D.; Pukin, A.V.; Fu, O.; Quarles van Ufford, L.H.C.; Janssens, H.M.; Beekman, J.M.; Pieters, R.J. Functional Characterization of Cholera Toxin Inhibitors Using Human Intestinal Organoids. J. Med. Chem. 2016, 59, 6968–6972. [Google Scholar] [CrossRef] [PubMed]
- Strubberg, A.M.; Liu, J.; Walker, N.M.; Stefanski, C.D.; MacLeod, R.J.; Magness, S.T.; Clarke, L.L. Cftr Modulates Wnt/beta-Catenin Signaling and Stem Cell Proliferation in Murine Intestine. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 253–271. [Google Scholar] [CrossRef]
- Rubinsky, B.; Mattioli, M.; Arav, A.; Barboni, B.; Fletcher, G.L. Inhibition of Ca2+ and K+ currents by antifreeze proteins. Am. J. Physiol. 1992, 262, R542–R545. [Google Scholar] [CrossRef]
- Rubinsky, B.; Arav, A.; Mattioli, M.; Devries, A.L. The effect of antifreeze glycopeptides on membrane potential changes at hypothermic temperatures. Biochem. Biophys. Res. Commun. 1990, 173, 1369–1374. [Google Scholar] [CrossRef]
- Hays, L.M.; Feeney, R.E.; Crowe, L.M.; Crowe, J.H.; Oliver, A.E. Antifreeze glycoproteins inhibit leakage from liposomes during thermotropic phase transitions. Proc. Natl. Acad. Sci. USA 1996, 93, 6835–6840. [Google Scholar] [CrossRef]
- Tomczak, M.M.; Hincha, D.K.; Estrada, S.D.; Wolkers, W.F.; Crowe, L.M.; Feeney, R.E.; Tablin, F.; Crowe, J.H. A mechanism for stabilization of membranes at low temperatures by an antifreeze protein. Biophys. J. 2002, 82, 874–881. [Google Scholar] [CrossRef]
- Garner, J.; Inglis, S.R.; Hook, J.; Separovic, F.; Harding, M.M. A solid-state NMR study of the interaction of fish antifreeze proteins with phospholipid membranes. Eur. Biophys. J. 2008, 37, 1031–1038. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huelsz-Prince, G.; DeVries, A.L.; Bakker, H.J.; van Zon, J.S.; Meister, K. Effect of Antifreeze Glycoproteins on Organoid Survival during and after Hypothermic Storage. Biomolecules 2019, 9, 110. https://doi.org/10.3390/biom9030110
Huelsz-Prince G, DeVries AL, Bakker HJ, van Zon JS, Meister K. Effect of Antifreeze Glycoproteins on Organoid Survival during and after Hypothermic Storage. Biomolecules. 2019; 9(3):110. https://doi.org/10.3390/biom9030110
Chicago/Turabian StyleHuelsz-Prince, Guizela, Arthur L. DeVries, Huib J. Bakker, Jeroen S. van Zon, and Konrad Meister. 2019. "Effect of Antifreeze Glycoproteins on Organoid Survival during and after Hypothermic Storage" Biomolecules 9, no. 3: 110. https://doi.org/10.3390/biom9030110
APA StyleHuelsz-Prince, G., DeVries, A. L., Bakker, H. J., van Zon, J. S., & Meister, K. (2019). Effect of Antifreeze Glycoproteins on Organoid Survival during and after Hypothermic Storage. Biomolecules, 9(3), 110. https://doi.org/10.3390/biom9030110