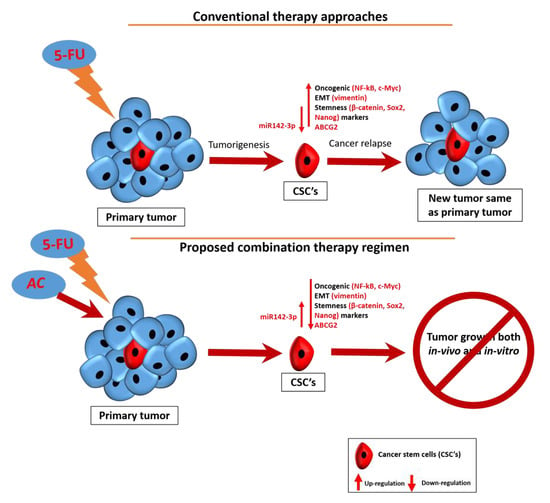
Antrodia cinnamomea Enhances Chemo-Sensitivity of 5-FU and Suppresses Colon Tumorigenesis and Cancer Stemness via Up-Regulation of Tumor Suppressor miR-142-3p
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, Chemicals and Reagents
2.2. Cell Viability Test
2.3. Apoptosis Assay
2.4. RNA Isolation, RT-PCR and Quantitative RT-PCR
2.5. Colony Formation Assays
2.6. SDS-Page and Western Blotting
2.7. ALDEFLUOR Assay and ALDH1+ Population Cell Sorting by FACS
2.8. In Vivo Studies
2.9. Statistical Analysis
3. Results
3.1. AC Inhibits the CRC Tumorigenesis and Colon Sphere Formation
3.2. AC Suppresses the Tumorsphere Formation and Reduces the ALDH1+ and Side Population of Stem Likes Cells
3.3. AC Treatment Increased 5-FU Sensitivity In Vitro
3.4. AC Treatment Effect and Colon Cancer Signaling Pathway
3.5. AC treatment Inhibits Tumor-Initiating Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, S.S.; Poolla, A.; Majumdar, A.P. Regulation of colon cancer recurrence and development of therapeutic strategies. World J. Gastrointest. Pathophysiol. 2012, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abulafi, A.M.; Williams, N.S. Local recurrence of colorectal cancer: The problem, mechanisms, management and adjuvant therapy. BJS 1994, 81, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Ryuk, J.P.; Choi, G.-S.; Park, J.S.; Kim, H.J.; Park, S.Y.; Yoon, G.S.; Jun, S.H.; Kwon, Y.C. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann. Surg. Treat. Res. 2014, 86, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Moharil, R.B.; Dive, A.; Khandekar, S.; Bodhade, A. Cancer Stem Cells: An Insight. J. Oral Maxillofac. Pathol. JOMFP 2017, 21, 463. (In English) [Google Scholar] [CrossRef]
- Prieto-Vila, M.; Takahashi, R.-U.; Usuba, W.; Kohama, I.; Ochiya, T. Drug Resistance Driven by Cancer Stem Cells and Their Niche. Int. J. Mol. Sci. 2017, 18, 2574. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Iodice, C.; Bogdanović, G.; Kojić, V.; Tešević, V. Stictic acid inhibits cell growth of human colon adenocarcinoma HT-29 cells. Arab. J. Chem. 2017, 10, S1240–S1242. [Google Scholar] [CrossRef] [Green Version]
- Pejin, B.; Kojic, V.; Bogdanovic, G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014, 28, 2053–2056. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Thang, M.W.C.; Chan, Y.-T.; Huang, Y.-F.; Ma, N.; Yu, A.L.; Wu, C.-Y.; Hu, M.-L.; Chiu, K.P. Global assessment of Antrodia cinnamomea-induced microRNA alterations in hepatocarcinoma cells. PLoS ONE 2013, 8, e82751. [Google Scholar] [CrossRef]
- Yue, P.Y.; Wong, Y.Y.; Chan, T.Y.; Law, C.K.; Tsoi, Y.K.; Leung, K.S. Review of biological and pharmacological activities of the endemic Taiwanese bitter medicinal mushroom, Antrodia camphorata (M. Zang et C. H. Su) Sh. H. Wu et al. (higher Basidiomycetes). Int. J. Med. Mushrooms 2012, 14, 241–256. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Rao, Y.K.; Yao, C.-J.; Yeh, C.-F.; Li, C.-H.; Chuang, S.-E.; Luong, J.H.T.; Lai, G.-M.; Tzeng, Y.-M. Cytotoxic triterpenes from Antrodia camphorata and their mode of action in HT-29 human colon cancer cells. Cancer Lett. 2009, 285, 73–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.C.; Rao, Y.K.; Wu, C.C.; Huang, C.Y.; Geethangili, M.; Hsu, S.L.; Tzeng, Y.M. Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin- and Bax-triggered mitochondrial pathway. Chem. Res. Toxicol. 2010, 23, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Rao, Y.K.; Lin, S.S.; Chou, M.Y.; Shen, Y.T.; Wu, C.H.; Geethangili, M.; Yang, C.C.; Tzeng, Y.M. Methylantcinate A induces tumor specific growth inhibition in oral cancer cells via Bax-mediated mitochondrial apoptotic pathway. Bioorg. Med. Chem. Lett. 2010, 20, 6145–6148. [Google Scholar] [CrossRef]
- Gokila Vani, M.; Kumar, K.J.; Liao, J.W.; Chien, S.C.; Mau, J.L.; Chiang, S.S.; Lin, C.C.; Kuo, Y.H.; Wang, S.Y. Antcin C from Antrodia cinnamomea Protects Liver Cells against Free Radical-Induced Oxidative Stress and Apoptosis In Vitro and In Vivo through Nrf2-Dependent Mechanism. Evid.-Based Complement. Altern. Med. eCAM 2013, 296082. [Google Scholar] [CrossRef]
- Kumar, K.J.; Chu, F.H.; Hsieh, H.W.; Liao, J.W.; Li, W.H.; Lin, J.C.; Shaw, J.F.; Wang, S.Y. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J. Ethnopharmacol. 2011, 136, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Chen, C.C.; Wu, M.J.; Chen, Y.S.; Chen, C.C.; Sheu, S.J.; Lin, T.W.; Chou, S.H.; Lin, S.C.; Liu, C.J.; et al. Active Component of Antrodia cinnamomea Mycelia Targeting Head and Neck Cancer Initiating Cells through Exaggerated Autophagic Cell Death. Evid.-Based Complement. Altern. Med. eCAM 2013, 946451. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Chou, P.Y.; Chien, Y.C.; Wu, C.H.; Wu, T.S.; Sheu, M.J. Ethanol extracts of fruiting bodies of Antrodia cinnamomea exhibit anti-migration action in human adenocarcinoma CL1-0 cells through the MAPK and PI3K/AKT signaling pathways. Phytomed. Int. J. Phytother. Phytopharmacol. 2012, 19, 768–778. [Google Scholar] [CrossRef]
- Liu, F.C.; Lai, M.T.; Chen, Y.Y.; Lin, W.H.; Chang, S.J.; Sheu, M.J.; Wu, C.H. Elucidating the inhibitory mechanisms of the ethanolic extract of the fruiting body of the mushroom Antrodia cinnamomea on the proliferation and migration of murine leukemia WEHI-3 cells and their tumorigenicity in a BALB/c allograft tumor model. Phytomed. Int. J. Phytother. Phytopharm. 2013, 20, 874–882. [Google Scholar] [CrossRef]
- Liu, Y.M.; Liu, Y.K.; Huang, P.I.; Tsai, T.H.; Chen, Y.J. Antrodia cinnamomea mycelial fermentation broth inhibits the epithelial-mesenchymal transition of human esophageal adenocarcinoma cancer cells. Food Chem. Toxicol. 2018, 119, 380–386. [Google Scholar] [CrossRef]
- Dotse, E.; Bian, Y. Isolation of colorectal cancer stem-like cells. Cytotechnology 2016, 68, 609–619. [Google Scholar] [CrossRef]
- Yang, C.M.; Zhou, Y.J.; Wang, R.J.; Hu, M.L. Anti-angiogenic effects and mechanisms of polysaccharides from Antrodia cinnamomea with different molecular weights. J. Ethnopharmacol. 2009, 123, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Tsai, W.C.; Ko, C.J.; Rao, Y.K.; Yang, C.R.; Chen, D.R.; Yang, M.H.; Yang, C.C.; Tzeng, Y.M. Anticancer effects of eleven triterpenoids derived from Antrodia camphorata. Anticancer Res. 2012, 32, 2727–2734. [Google Scholar] [PubMed]
- Lin, Y.-S.; Lin, Y.-Y.; Yang, Y.-H.; Lin, C.-L.; Kuan, F.-C.; Lu, C.-N.; Chang, G.-H.; Tsai, M.-S.; Hsu, C.-M.; Yeh, R.-A.; et al. Antrodia cinnamomea extract inhibits the proliferation of tamoxifen-resistant breast cancer cells through apoptosis and skp2/microRNAs pathway. BMC Complement. Altern. Med. 2018, 18, 152. [Google Scholar] [CrossRef]
- Huang, T.-T.; Lan, Y.-W.; Chen, C.-M.; Ko, Y.-F.; Ojcius, D.M.; Martel, J.; Young, J.D.; Chong, K.-Y. Antrodia cinnamomea induces anti-tumor activity by inhibiting the STAT3 signaling pathway in lung cancer cells. Sci. Rep. 2019, 9, 5145. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Liu, F.-G.; Wei, C.-F.; Lu, C.-C.; Chen, C.-C.; Lin, H.-C.; Ojcius, D.M.; Lai, H.-C. Activation of Multiple Apoptotic Pathways in Human Nasopharyngeal Carcinoma Cells by the Prenylated Isoflavone, Osajin. PLoS ONE 2011, 6, e18308. [Google Scholar] [CrossRef]
- Butler, S.J.; Richardson, L.; Farias, N.; Morrison, J.; Coomber, B.L. Characterization of cancer stem cell drug resistance in the human colorectal cancer cell lines HCT116 and SW480. Biochem. Biophys. Res. Commun. 2017, 490, 29–35. [Google Scholar] [CrossRef]
- Yasgar, A.; Titus, S.A.; Wang, Y.; Danchik, C.; Yang, S.-M.; Vasiliou, V.; Jadhav, A.; Maloney, D.J.; Simeonov, A.; Martinez, N.J. A High-Content Assay Enables the Automated Screening and Identification of Small Molecules with Specific ALDH1A1-Inhibitory Activity. PLoS ONE 2017, 12, e0170937. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Liu, Y.-K.; Wang, L.-W.; Huang, Y.-C.; Huang, P.-I.; Tsai, T.-H.; Chen, Y.-J. The medicinal fungus Antrodia cinnamomea regulates DNA repair and enhances the radiosensitivity of human esophageal cancer cells. OncoTargets Ther. 2016, 9, 6651–6661. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Gao, W.; Zhang, Y.; Liu, Z.; Yan, L.; Huang, L.; Liu, C. Formosanin C-inhibited pulmonary metastasis through repression of matrix metalloproteinases on mouse lung adenocarcinoma. Cancer Biol. Ther. 2011, 11, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.T.; Lin, W.C.; Wang, S.Y.; Lin, L.J.; Yu, B.; Lee, T.T. Evaluation of potential antioxidant and anti-inflammatory effects of Antrodia cinnamomea powder and the underlying molecular mechanisms via Nrf2- and NF-kappaB-dominated pathways in broiler chickens. Poult. Sci. 2018, 97, 2419–2434. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Ohnuma, S.; Ambudkar, S.V. Discovering natural product modulators to overcome multidrug resistance in cancer chemotherapy. Curr. Pharm. Biotechnol. 2011, 12, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, H.L.; Yang, Y.J.; Wang, L.; Lee, S.C. Overcome Cancer Cell Drug Resistance Using Natural Products. Evid.-Based Complement. Altern. Med. eCAM 2015, 767136. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Huang, Z.N.; Yu, H.H.; Chang, L.H.; Li, S.L.; Chen, Y.P.; Lee, K.Y.; Chuu, J.J. The adjuvant effects of Antrodia Camphorata extracts combined with anti-tumor agents on multidrug resistant human hepatoma cells. J. Ethnopharmacol. 2008, 118, 387–395. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [Green Version]
- Favaloro, B.; Allocati, N.; Graziano, V.; Di Ilio, C.; De Laurenzi, V. Role of apoptosis in disease. Aging 2012, 4, 330–349. [Google Scholar] [CrossRef]
- Chiang, P.C.; Lin, S.C.; Pan, S.L.; Kuo, C.H.; Tsai, I.L.; Kuo, M.T.; Wen, W.C.; Chen, P.; Guh, J.H. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: A crucial role of AMPK and mTOR pathways. Biochem. Pharmacol. 2010, 79, 162–171. [Google Scholar] [CrossRef]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59 (Suppl. 2), 21–26. [Google Scholar] [CrossRef] [PubMed]
- Gerl, R.; Vaux, D.L. Apoptosis in the development and treatment of cancer. Carcinogenesis 2005, 26, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Hauke, R.; Mehta, P.P.; Batra, S.K. Recent advances in cancer stem/progenitor cell research: Therapeutic implications for overcoming resistance to the most aggressive cancers. J. Cell. Mol. Med. 2007, 11, 981–1011. [Google Scholar] [CrossRef] [PubMed]
- Goranova, T.E.; Ohue, M.; Shimoharu, Y.; Kato, K. Dynamics of cancer cell subpopulations in primary and metastatic colorectal tumors. Clin. Exp. Metastasis 2011, 28, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnenberg, V.S.; Donnenberg, A.D. Multiple drug resistance in cancer revisited: The cancer stem cell hypothesis. J. Clin. Pharmacol. 2005, 45, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, J.; Stantic, M.; Zobalova, R.; Chladova, J.; Wang, X.; Prochazka, L.; Dong, L.; Andera, L.; Ralph, S.J. Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: What’s in the name? Biochem. Biophys. Res. Commun. 2007, 355, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Scopelliti, A.; Cammareri, P.; Catalano, V.; Saladino, V.; Todaro, M.; Stassi, G. Therapeutic implications of Cancer Initiating Cells. Expert Opin. Biol. Ther. 2009, 9, 1005–1016. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Lugli, A.; Iezzi, G.; Hostettler, I.; Muraro, M.G.; Mele, V.; Tornillo, L.; Carafa, V.; Spagnoli, G.; Terracciano, L.; Zlobec, I. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br. J. Cancer 2010, 103, 382–390. [Google Scholar] [CrossRef]
- Meng, H.M.; Zheng, P.; Wang, X.Y.; Liu, C.; Sui, H.M.; Wu, S.J.; Zhou, J.; Ding, Y.Q.; Li, J. Over-expression of Nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol. Ther. 2010, 9, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Ioue, Y.; Miki, C.; Kusunoki, M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann. Surg. Oncol. 2009, 16, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Jeter, C.R.; Liu, B.; Liu, X.; Chen, X.; Liu, C.; Calhoun-Davis, T.; Repass, J.; Zaehres, H.; Shen, J.J.; Tang, D.G. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene 2011, 30, 3833–3845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, B.B.; Sengupta, R.; Qazi, S.; Vachhani, H.; Yu, Y.; Rishi, A.K.; Majumdar, A.P.N. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int. J. Cancer 2008, 122, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska, A.; Gornowicz, A.; Pawłowska, N.; Czarnomysy, R.; Nazaruk, J.; Szymanowski, W.; Bielawski, K. Anticancer Effect of a Novel Octahydropyrazino[2,1-a:5,4-a’]diisoquinoline Derivative and Its Synergistic Action with Nigella sativa in Human Gastric Cancer Cells. BioMed Res. Int. 2017, 9153403. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Kraehe, P.; Popper, B.; Goel, A.; Shakibaei, M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchymal transition and apoptosis in colorectal cancer. Biochem. Pharmacol. 2015, 98, 51–68. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzym. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Tallarida, R.J. The interaction index: A measure of drug synergism. Pain 2002, 98, 163–168. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-J.; Yadav, V.K.; Srivastava, P.; Wu, A.T.; Huynh, T.-T.; Wei, P.-L.; Huang, C.-Y.F.; Huang, T.-H. Antrodia cinnamomea Enhances Chemo-Sensitivity of 5-FU and Suppresses Colon Tumorigenesis and Cancer Stemness via Up-Regulation of Tumor Suppressor miR-142-3p. Biomolecules 2019, 9, 306. https://doi.org/10.3390/biom9080306
Huang Y-J, Yadav VK, Srivastava P, Wu AT, Huynh T-T, Wei P-L, Huang C-YF, Huang T-H. Antrodia cinnamomea Enhances Chemo-Sensitivity of 5-FU and Suppresses Colon Tumorigenesis and Cancer Stemness via Up-Regulation of Tumor Suppressor miR-142-3p. Biomolecules. 2019; 9(8):306. https://doi.org/10.3390/biom9080306
Chicago/Turabian StyleHuang, Yan-Jiun, Vijesh Kumar Yadav, Prateeti Srivastava, Alexander TH Wu, Thanh-Tuan Huynh, Po-Li Wei, Chi-Ying F. Huang, and Tse-Hung Huang. 2019. "Antrodia cinnamomea Enhances Chemo-Sensitivity of 5-FU and Suppresses Colon Tumorigenesis and Cancer Stemness via Up-Regulation of Tumor Suppressor miR-142-3p" Biomolecules 9, no. 8: 306. https://doi.org/10.3390/biom9080306
APA StyleHuang, Y. -J., Yadav, V. K., Srivastava, P., Wu, A. T., Huynh, T. -T., Wei, P. -L., Huang, C. -Y. F., & Huang, T. -H. (2019). Antrodia cinnamomea Enhances Chemo-Sensitivity of 5-FU and Suppresses Colon Tumorigenesis and Cancer Stemness via Up-Regulation of Tumor Suppressor miR-142-3p. Biomolecules, 9(8), 306. https://doi.org/10.3390/biom9080306