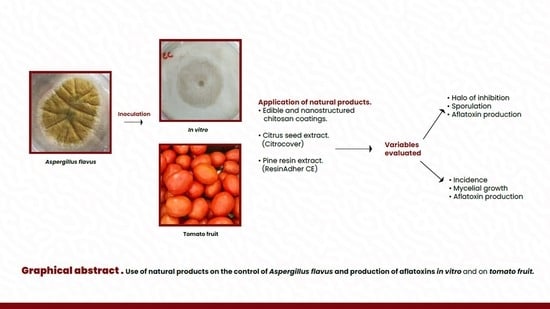
Use of Natural Products on the Control of Aspergillus flavus and Production of Aflatoxins In Vitro and on Tomato Fruit
Abstract
:1. Introduction
2. Results
2.1. In Vitro
2.2. In Situ
3. Discussion
3.1. In Vitro
3.2. In Situ
4. Materials and Methods
4.1. Fungal Strain
4.2. Materials
4.3. Treatments Preparation
4.4. Application of the Treatments In Vitro and on Tomatoe
4.5. Aflatoxin Production by A. flavus
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arrúa, A.A.; Moura, J.; Fernandez, D.; Cazal, C. Aspergillus y micotoxinas. Rev. UN Med. 2013, 2, 141–169. [Google Scholar]
- Guzman, D. La exposición a la aflatoxina B1 en animales de laboratorio y su significado en la salud pública. Salud Pública Méx. 2007, 49, 227–235. [Google Scholar]
- Urrego, J.R.; Días, G.J. Aflatoxinas: Mecanismos de toxicidad en la etiología de cáncer hepático celular. Rev. Fac. Med. 2006, 54, 108–116. [Google Scholar]
- Orozco, N.; Montes de Oca, J.H.; Parada, S.; Tlazola, R.Y.; Jiménez, S.A.; Reynoso, J.; Lopez, L.; Hernandez, I.; Ruvalcaba, J.C. The ingestion of aflatoxigenic foods and their possible implications with cervical cancer. J. Negat. No Posit. Results 2019, 4, 130–140. [Google Scholar]
- Buchanan, J.R.; Sommer, N.F.; Fortlage, R.J. Aspergillus flavus infection and aflatoxin production in fig fruits. Appl. Microbiol. 1975, 30, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Baiyewu, R.A.; Amusa, N.A.; Ayoola, O.A.; Babalola, O.O. Survey of the post harvest diseases and aflatoxin contamination of marketed pawpaw fruit (Carica papaya L.) in South Western Nigeria. Afr. J. Agric. Res. 2007, 24, 178–181. [Google Scholar]
- Mariutti, L.R.; Valente, L.M. Survey of aflatoxins in tomato products. Ciênc. Tecnol. Aliment. 2009, 29, 431–434. [Google Scholar] [CrossRef] [Green Version]
- Ortega, E.G.; Carrera, M.A.; Delgadillo, D.; Intriago, M.P.; Lares, E.F.; Escorza, Q. Asociación de la exposición ocupacional a plaguicidas organofosforados con el daño oxidativo y actividad de acetilcolinesterasa. Rev. Toxicol. 2016, 33, 39–43. [Google Scholar]
- Singh, A.; Singla, A.; Kamboj, P.; Singh, J. Pesticide residues in food grains, vegetables and fruits: A hazard to human health. J. Med. Chem. Toxicol. 2017, 2, 1–7. [Google Scholar]
- De Ancos, B.; González-Peña, D.; Colina-Coca, C.; Sánchez-Moreno, C. Uso de películas/recubrimientos comestibles en los productos de IV y V gama. Rev. Iberoam. De Tecnol. Postcosech 2015, 16, 8–17. [Google Scholar]
- Do Nascimento, F.J.; Diniz, E.T.; de Mesquita, L.X.; de Oliveira, A.M.; Costa, T.F. Extractos vegetales en el control de plagas. Rev. Verde Agroecol. Desenvolv. Sustentável 2008, 3, 1–5. [Google Scholar]
- Guédez, C.; Cañizalaez, L.; Avendaño, L.; Scorza, J.; Castillo, C.; Olivar, R.; Méndez, Y.; Sánchez, L. Actividad antifúngica del aceite esencial de naranja (Citrus sinensis L.) sobre hongos postcosecha en frutos de lechosa (Carica papaya L.). Rev. Soc. Ven. Microbiol. 2014, 34, 81–85. [Google Scholar]
- Cortes-Higareda, M.; Ramos-García, M.L.; Correa-Pacheco, Z.; Del Rio, J.C.; Bautista-Baños, S. Nanostructured chitosan/propolis formulations: Characterization and effect on the growth of Aspergillus flavus and production of aflatoxins. Heliyon 2019, 5, e01776. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-García, P.F.; Ventura-Aguilar, R.I.; Del Rio, J.C.; Hernandez-Lopez, M.; Guillen-Sanchez, D.; Salazar-Piña, D.A.; Ramos-García, M.L.; Bautista-Baños, S. Edible chitosan/propolis coatings and their effect on ripening, development of Aspergillus flavus, and sensory quality in fig fruit, during controlled storage. Plants 2021, 10, 112. [Google Scholar] [CrossRef]
- Gonzalez-Andrade, C.; Ramos-García, M.L. Efecto de Productos Naturales Nanoestructurados Sobre el Desarrollo de Aspergillus flavus In Vitro en Maíz (zea mays L.). XXII Congreso Internacional & XLVII Congreso Nacional de la Sociedad Mexicana de Fitopatología. 2020. Suplemento de la Revista Mexicana de Fitopatología. 100p. Available online: https://www.smf.org.mx/rmf/suplemento/suplemento382020/Resumenes_Posters.pdf (accessed on 10 October 2021).
- Iglesias, D. Actividad Antifúngica In Vitro de Extractos Hidroalcohólicos de Hojas de Citrus spp. Frente a Hongos Fitopatogenos de Lycopersicum esculentum Mill. Licenciatura en Agronomía. Universidad Central “Marta Abreu” de Las Villas. Facultad de Ciencias Agropecuarias. Departamento de Biología. Santa Clara, Cuba. 2012. Available online: https://dspace.uclv.edu.cu/bitstream/handle/123456789/1859/Dianella%20Iglesias%20HOJAS%20DE%20CITRUS.pdf?sequence=1&isAllowed=y (accessed on 10 October 2021).
- Ramírez, Y. Actividad Antifúngica In Vivo de Extractos de Citrus reticulata Blanco y Citrus aurantiifolia (Chrisrm.) Swingle Frente a Passarola fulva (Cooke) U. Braun&Crous. Licenciatura en Agronomía, Universidad Central “Marta Abreu” de las Villas. Santa Clara, Cuba. 2013. Available online: https://dspace.uclv.edu.cu/bitstream/handle/123456789/1817/A0019.pdf?sequence=1&isAllowed=y (accessed on 5 October 2021).
- Amini, M.N.; Safaie, M.J. Antifungal activity of three medicinal plant essential oils against some phytopathogenic fungi. Trakia J. Sci. 2012, 10, 1–8. [Google Scholar]
- Juarez-Morales, L.A.; Hernandez-Cocoletzi, H.; Chigo-Anona, E.; Aguila-Almanza, E.; Tenorio-Arvide, G. Chitosan-aflatoxins B1, M1 interaction: A computational approach. Curr. Org. Chem. 2017, 21, 2877–2883. [Google Scholar] [CrossRef]
- Barkai-Golan, R. Aspergillus Mycotoxins. In Mycotoxins in Fruits and Vegetables; Barkai-Golan, R., Paster, N., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2008; pp. 115–151. [Google Scholar]
- Klich, M. Environmental and developmental factors influencing aflatoxin production by Aspergillus flavus and Aspergillus parasiticus. Mycosciencie 2007, 48, 71–80. [Google Scholar] [CrossRef]
- Montero-Álvarez, J.A.; Paredes-Bautista, M.J.; Rivera-Morales, M.C. Utilización de quitosana para la remoción de arsénico (As) del agua. Superf. Vacío 2010, 23, 136–139. [Google Scholar]
- Gavira-Hernández, V.; Patiño-Hoyos, L.F.; Saldarriaga-Cardona, A. Evaluación in vitro de fungicidas comerciales para el control de Colletotrichum spp., in blackberry. Cienc. Tecnol. Agropecu. 2013, 14, 67–75. [Google Scholar] [CrossRef]
- Silva, M.E.; Schwan-Estrada, K.R.F.; Balbi-Peña, M.I.; Terumi, A.; Clemente, E.; Stangarlin, J.R. Control del moho azul en poscosecha de manzana con productos naturales. Idesia 2015, 33, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Quintana-Obregón, E.A.; Sánchez-Mariñez, R.I.; Cortez-Rocha, M.O.; González-Aguilar, G.A. Actividad antifúngica in vitro de mezcla de terpenos de naranja contra Alternaria tenuissima. Rev. Mex. Mic. 2017, 45, 7–12. [Google Scholar] [CrossRef]
- Barroso, A.K.; Ochoa, F.Y.; Cerna, E.; Toruch, M.; Olalde, P.V.; Robles, Y.L. Manejo un vitro de antracnosis (Colletotrichium acutatum Simmonds) en aguacate mediante el uso de principios activos botánicos. Ecosistemas Recur. Agropecu. 2021, 8, e3038. [Google Scholar]
- Perera, S.D.; Pérez, E.; Hernandez, J.; Lobo, G.; López- Cepero, J.; Puerta, M.; Torres, J.M.; Lopez, C. Evaluación de la Eficacia de Fungicidas Naturales y Químicos en el Control de Enfermedades Postcosecha Sobre Distintas Variedades de Papaya (II). Cabildo Insular de Tenerife, COAGISORA, COPLACA, Instituto Canario de Investigaciones Agrarias ICIA. 2010. Available online: https://www.agrocabildo.org/publica/Publicaciones/subt_336_papaya.pdf (accessed on 10 October 2021).
- Benato, E.A.; Sigris, J.M.M.; Hanashiro, M.M.; Magalhães, M.J.M.; Binotti, C.S. Avaliação de fungicidas e produtos alternativos no controle de podridões pós-colheita em maracujá amarelo. Summa Phytopathol. 2002, 28, 299–304. [Google Scholar]
- Schultz, C.W. Potencial Antimicótico y Antibacterial In Vitro de la Colofonia y la Tremetina, Derivados de la Resina Oleosa de la Conífera Endémica Pinus Occidentalis, en el Municipio de Jarabacoa, Provincia La Vega. Licenciado en Farmacia, Universidad Nacional Pedro Henríquez Ureña Facultad de Ciencias de la Salud, Santo Domingo, Republica Dominicana. 2014. Available online: https://repositorio.unphu.edu.do/handle/123456789/1007 (accessed on 4 October 2021).
- Gallo, J.A.; Sarria, R.A. Obtención de colofonia y trementina a partir de resina de pino de la especia patula y posterior evaluación de los parámetros de calidad. Jou. Cie. Ing. 2013, 5, 88–91. [Google Scholar]
- Pavón, C.P. Material Para Impresión 2D Basado en Resinas Naturales de Colofonia. Master en Ingeniería, Procesado y Caracterización de Materiales., Universidad Politécnica de Valencia. Valencia, España. 2019. Available online: https://riunet.upv.es/bitstream/handle/10251/125974/Pav%C3%B3n%20-%20Material%20para%20impresion%203D%20basado%20en%20resinas%20naturales%20de%20colofonia.pdf?sequence=1 (accessed on 5 October 2021).
- Amri, I.; Gargouri, S.; Hamrouni, L.; Hanana, M.; Fezzani, T.; Jamoussi, B. Chemical composition, phytotoxic and antifungical activities of Pinus pinea essential oil. J. Pest Sci. 2012, 85, 199–207. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Valle-Marquina, M.A.; Hernandez-Lopez, M. The effect of nanostructured chitosan and chitosan-thyme essential oil coatings on Colletotrichum gloeosporioides growth in vitro and on cv Hass avocado and fruit quality. J. Phytopathol. 2017, 165, 297–305. [Google Scholar] [CrossRef]
- Cia, P.; Pascholati, S.; Benato, E.; Camili, E.; Santos, A. Effects of gamma and UV-C irradiation on the postharvest control of papaya anthracnose. Postharvest Biol. Technol. 2007, 43, 366–373. [Google Scholar] [CrossRef]

| Treatment | Halo of Inhibition (cm2) (x ± S.D.) | |||
|---|---|---|---|---|
| Days of Incubation | ||||
| 4 | 5 | 6 | 7 | |
| Citrocover | 10.4 ± 4.4 b* | 4.2 ± 5.1 b | 3.5 ± 4.7 b | 3.0 ± 4.5 a |
| Resinadher | 5.4 ± 5.0 ab | 2.6 ± 2.1 ab | 2.6 ± 2.4 ab | 2.6 ± 2.4 a |
| Chitosan | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| Chitosan coating | 0 ± 0 | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| Chitosan nanoparticles | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| Mancozeb | 2.6 ± 1.3 ab | 2.6 ± 1.5 ab | 1.1 ± 0.1 ab | 0.9 ± 0.15 a |
| Water | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a | 0 ± 0 a |
| Treatment | Sporulation (105 Spores mL−1) | Production of Aflatoxins (ppb) |
|---|---|---|
| Citrocover | 0.2 ± 0 a* | 0.13 ± 0.16 a |
| Resinadher | 0.9± 0.02 a | 0.29 ± 0.25 ab |
| Chitosan | 4.7 ± 0.6 b | 0.10 ± 0.08 a |
| Chitosan coating | 14.5 ± 4.8 cd | 0.00 ± 0.00 a |
| Chitosan nanoparticles | 19.7 ± 4.1 d | 0.83 ± 2.20 ab |
| Mancozeb | 9.4 ± 3.1 bc | 0.09 ± 0.16 a |
| Water | 11.1 ± 1.14 c | 1.21 ± 1.50 b |
| Treatment | Mycelial Growth (cm2; x ± S.D.) Days of Storage | ||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |
| Citrocover | 0.0 ± 0.0 | 0.04 ± 0.1 ab* | 0.07 ± 0.2 a | 0.1 ± 0.5 a | 0.2 ± 0.8 ab |
| Resinadher | 0.0 ± 0.0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| Chitosan | 0.0 ± 0.0 | 0.2 ± 0.2 abc | 0.7 ± 1.0 bc | 0.9 ± 1.2 abc | 1.0 ± 1.3 abc |
| Chitosan coating | 0.0 ± 0.0 | 0.2 ± 0.2 bc | 0.3 ± 0.2 abc | 0.4 ± 0.2 ab | 1.2 ± 0.9 abc |
| Chitosan nanoparticles | 0.0 ± 0.0 | 0.2 ± 0.3 bc | 0.3 ± 0.3 ab | 0.4 ± 0.4 a | 1.5 ± 1.5 bc |
| Mancozeb | 0.0 ± 0.0 | 0.3 ± 0.2 c | 0.0 ± 0.5 c | 1.3 ± 0.9 bc | 1.3 ± 1.9 bc |
| Water | 0.0 ± 0.0 | 0.1 ± 0.2 abc | 0.6 ± 0.5 bc | 1.8 ± 1.8 c | 1.9 ± 1.8 c |
| Treatment | Aflatoxins (ppb) |
|---|---|
| Citrocover | 1.77 ± 0.21 a* |
| Resinadher | 0.95 ± 0.68 a |
| Chitosan | 2.12 ± 0.09 a |
| Chitosan coating | 2.50 ± 0.89 a |
| Chitosan nanoparticles | 1.87 ± 0.45 a |
| Mancozeb | 1.93 ± 0.65 a |
| Water | 5.00 ± 0.89 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segura-Palacios, M.A.; Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Martinez-Ramirez, O.C.; Salazar-Piña, D.A.; Ramos-García, M.d.L.; Bautista-Baños, S. Use of Natural Products on the Control of Aspergillus flavus and Production of Aflatoxins In Vitro and on Tomato Fruit. Plants 2021, 10, 2553. https://doi.org/10.3390/plants10122553
Segura-Palacios MA, Correa-Pacheco ZN, Corona-Rangel ML, Martinez-Ramirez OC, Salazar-Piña DA, Ramos-García MdL, Bautista-Baños S. Use of Natural Products on the Control of Aspergillus flavus and Production of Aflatoxins In Vitro and on Tomato Fruit. Plants. 2021; 10(12):2553. https://doi.org/10.3390/plants10122553
Chicago/Turabian StyleSegura-Palacios, Mario Alberto, Zormy Nacary Correa-Pacheco, Maria Luisa Corona-Rangel, Ollin Celeste Martinez-Ramirez, Dolores Azucena Salazar-Piña, Margarita de Lorena Ramos-García, and Silvia Bautista-Baños. 2021. "Use of Natural Products on the Control of Aspergillus flavus and Production of Aflatoxins In Vitro and on Tomato Fruit" Plants 10, no. 12: 2553. https://doi.org/10.3390/plants10122553
APA StyleSegura-Palacios, M. A., Correa-Pacheco, Z. N., Corona-Rangel, M. L., Martinez-Ramirez, O. C., Salazar-Piña, D. A., Ramos-García, M. d. L., & Bautista-Baños, S. (2021). Use of Natural Products on the Control of Aspergillus flavus and Production of Aflatoxins In Vitro and on Tomato Fruit. Plants, 10(12), 2553. https://doi.org/10.3390/plants10122553