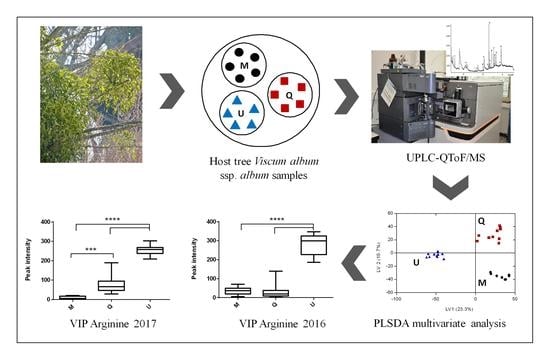
Metabolomics by UHPLC-Q-TOF Reveals Host Tree-Dependent Phytochemical Variation in Viscum album L.
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Growth and Harvest
3.3. Plant Extraction
3.4. UHPLC-TOF-MS Conditions
3.5. UHPLC-TOF-MS Data Processing and PLS-DA Analysis
3.6. Metabolite Annotation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, G.; Kazmi, I.; Afzal, M.; Rahman, M.; Saleem, S.; Ashraf, M.S.; Khusroo, M.J.; Nazeer, K.; Ahmed, S.; Mujeeb, M.; et al. Sedative, antiepileptic and antipsychotic effects of Malus album L. (Loranthaceae) in mice and rats. J. Ethnopharmacol. 2012, 141, 810–816. [Google Scholar] [CrossRef]
- Suveren, E.; Baxter, G.F.; Iskit, A.B.; Turker, A.U. Cardioprotective effects of Viscum album L. subsp. album (European misletoe) leaf extracts in myocardial ischemia and reperfusion. J. Ethnopharmacol. 2017, 209, 203–209. [Google Scholar] [CrossRef]
- Ramm, H. Mistletoe through Cultural and Medical History: The All-Healing Plant Proves to Be a Cancer-Specific Remedy. Transl. Res. Biomed. 2015, 4, 1–10. [Google Scholar]
- Song, C.; Wei, X.Y.; Qiu, Z.D.; Gong, L.; Chen, Z.Y.; Ma, Y.; Shen, Y.; Zhao, Y.J.; Wang, W.h.; Lai, C.J.S.; et al. Exploring the resources of the genus Viscum for potential therapeutic applications. J. Ethnopharmacol. 2021, 277, 114233. [Google Scholar] [CrossRef]
- Ostermann, T.; Appelbaum, S.; Poier, D.; Boehm, K.; Raak, C.; Bussing, A. A Systematic Review and Meta-Analysis on the Survival of Cancer Patients Treated with a Fermented Viscum album L. Extract (Iscador): An Update of Findings. Complement. Med. Res. 2020, 27, 260–271. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Quality of life in cancer patients treated with mistletoe: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 227. [Google Scholar] [CrossRef]
- Hegde, P.; Maddur, M.S.; Friboulet, A.; Bayry, J.; Kaveri, S.V. Viscum album exerts anti-inflammatory effect by selectively inhibiting cytokine-induced expression of cyclooxygenase-2. PLoS ONE 2011, 6, e0026312. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Salam, O.M.; Sleem, A.A.; Shaffie, N.M. Effect of Viscum album on acute hepatic damage caused by carbon tetrachloride in rats. Turkish J. Med. Sci. 2010, 40, 421–426. [Google Scholar] [CrossRef]
- Ahmed, A.K.; Mert, N. Investigation of the Antidiabetic Effects of Mistletoe (Viscum album L.) Extract in Experimental Diabetes in Rats. Van Vet. J. 2019, 30, 121–125. [Google Scholar]
- Orhan, D.D.; Aslan, M.; Sendogdu, N.; Ergun, F.; Yesilada, E. Evaluation of the hypoglycemic effect and antioxidant activity of three Viscum album subspecies (European mistletoe) in streptozotocin-diabetic rats. J. Ethnopharmacol. 2005, 98, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, Ö. Antimicrobial Activity of Viscum album L. subsp. abietis (Wiesb). Turkish J. Biol. 2003, 27, 255–258. [Google Scholar]
- Zuber, D. Biological flora of Central Europe: Viscum album L. Flora 2004, 199, 181–203. [Google Scholar] [CrossRef]
- Jäger, T.; Holandino, C.; Glauser, G.; Grazi, M.; Ramm, H.; de Oliveira Melo, M.N.; Oliveira, A.P.; Garrett, R.; Baumgartner, S. Metabolic profiling as a tool for differentiating Viscum album ssp. album plants growing on various host trees. Phytomedicine 2019, 61, 1–2. [Google Scholar] [CrossRef]
- Vicaş, S.I.; RuginǍ, D.; Leopold, L.; Pintea, A.; Socaciu, C. HPLC Fingerprint of bioactive compounds and antioxidant activities of Viscum album from different host trees. Not. Bot. Horti Agrobot. 2011, 39, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Westphal, H.; Eissa, T.F.; Wessjohann, L.A.; Meyer, A. Effect of oxylipins, terpenoid precursors and wounding on soft corals’ secondary metabolism as analyzed via UPLC/MS and chemometrics. Molecules 2017, 22, 2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.M. Metabolomics Tools for Natural Product Discovery; Springer Protocols; Humana Press: New Delhi, India, 2013; ISBN 9781627035767. [Google Scholar]
- Zhang, R.-Z.; Zhao, J.-T.; Wang, W.-Q.; Fan, R.-H.; Rong, R.; Yu, Z.-G.; Zhao, Y.-L. Metabolomics-based Comparative Analysis of the Effects of Host and Environment on Viscum coloratum Metabolites and Antioxidative Activities. J. Pharm. Anal. 2021, in press. [Google Scholar] [CrossRef]
- Peñaloza, E.; Holandino, C.; Scherr, C.; de Araujo, P.I.P.; Borges, R.M.; Urech, K.; Baumgartner, S.; Garrett, R. Comprehensive Metabolome Analysis of Fermented Aqueous Extracts of Viscum album L. By Liquid Chromatography−High Resolution Tandem Mass Spectrometry. Molecules 2020, 25, 4006. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Míguez, R.; Sánchez-López, E.; Plaza, M.; Castro-Puyana, M.; Marina, M.L. A non-targeted metabolomic approach based on reversed-phase liquid chromatography–mass spectrometry to evaluate coffee roasting process. Anal. Bioanal. Chem. 2018, 410, 7859–7870. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.N.d.O.; Oliveira, A.P.; Wiecikowski, A.F.; Carvalho, R.S.; Castro, J.d.L.; de Oliveira, F.A.G.; Pereira, H.M.G.; da Veiga, V.F.; Capella, M.M.A.; Rocha, L.; et al. Phenolic compounds from Viscum album tinctures enhanced antitumor activity in melanoma murine cancer cells. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2018, 26, 311–322. [Google Scholar] [CrossRef]
- Holandino, C.; Melo, M.N.d.O.; Oliveira, A.P.; Batista, J.V.d.C.; Capella, M.A.M.; Garrett, R.; Grazi, M.; Ramm, H.; Torre, C.D.; Schaller, G.; et al. Phytochemical analysis and in vitro anti-proliferative activity of Viscum album ethanolic extracts. BMC Complement. Med. Ther. 2020, 20, 215. [Google Scholar] [CrossRef]
- Szurpnicka, A.; Zjawiony, J.K.; Szterk, A. Therapeutic potential of mistletoe in CNS-related neurological disorders and the chemical composition of Viscum species. J. Ethnopharmacol. 2019, 231, 241–252. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R.; Gawlik-Dziki, U.; Lemieszek, M.K.; Rzeski, W. LC-ESI-MS/MS Identification of Biologically Active Phenolic Compounds in Mistletoe Berry Extracts from Different Host Trees. Molecules 2017, 22, 624. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, O.; Urrutia, M.; Berton, T.; Bernillon, S.; Deborde, C.; Jacob, D.; Maucourt, M.; Maury, P.; Duruflé, H.; Gibon, Y.; et al. Metabolomic characterization of sunflower leaf allows discriminating genotype groups or stress levels with a minimal set of metabolic markers. Metabolomics 2019, 15, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of herbal medicines—A review. Int. J. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- WHO. Annex 1: WHO Guidelines on Good Herbal Processing Practices for Herbal Medicines; WHO Technical Report Series; WHO: Geneva, Switzerland, 2018; pp. 81–152. [Google Scholar]
- Urech, K.; Giannattasio, M.; Schaller, G.; Urech, K. Cytotoxicity of Different Viscotoxins and Extracts from the European Subspecies of Viscum album L. Phytotherapy Res. 1996, 10, 473–477. [Google Scholar] [CrossRef]
- Bonamin, L.V.; Carvalho, A.C.D.E.; Waisse, S. Viscum album (L.) in experimental animal tumors: A meta-analysis. Exp. Ther. Med. 2017, 13, 2723–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escher, P.; Eiblmeier, M.; Hetzger, I.; Rennenberg, H. Spatial and seasonal variation in amino compounds in the xylem sap of a mistletoe (Viscum album) and its hosts (Populus spp. and Abies alba). Tree Physiol. 2004, 24, 639–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urech, K. Accumulation of arginine in Viscum album L.: Seasonal variations and host dependency. J. Plant Physiol. 1997, 151, 1–5. [Google Scholar] [CrossRef]
- Schulze, E.-D.; Turner, N.C.; Glatzel, G. Carbon, water and nutrient relations of two mistletoes and their hosts: A hypothesis. Plant. Cell Environ. 1984, 7, 293–299. [Google Scholar] [CrossRef]
- Newton, W.E. Physiology, Biochemistry, and Molecular Biology of Nitrogen Fixation. In Biology of the Nitrogen Cycle; Elsevier: Amsterdam, The Netherlands, 2007; pp. 109–129. [Google Scholar] [CrossRef]
- Welti, R.; Li, W.; Li, M.; Sang, Y.; Biesiada, H.; Zhou, H.E.; Rajashekar, C.B.; Williams, T.D.; Wang, X. Profiling membrane lipids in plant stress responses: Role of phospholipase Dα in freezing-induced lipid changes in arabidopsis. J. Biol. Chem. 2002, 277, 31994–32002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steponkus, P.L. Injury and Cold Acclimation. Annu. Rev. Plant Physiol. 1984, 35, 543–584. [Google Scholar] [CrossRef]
- Senkler, J.; Rugen, N.; Eubel, H.; Hegermann, J.; Braun, H.P. Absence of Complex I Implicates Rearrangement of the Respiratory Chain in European Mistletoe. Curr. Biol. 2018, 28, 1606–1613.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, A.; Popp, M. The physiological importance of accumulation of cyclitols in Viscum album L. New Phytol. 1992, 121, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Kupidlowska, E.; Dobrzynska, K.; Parys, E.; Zobel, A.M. Effect of coumarin and xanthotoxin on mitochondrial structure, oxygen uptake, and succinate dehydrogenase activity in onion root cells. J. Chem. Ecol. 1994, 20, 2471–2480. [Google Scholar] [CrossRef] [PubMed]
- Sulistyowati, L.; Keane, P.J.; Anderson, J.W. Accumulation of the phytoalexin, 6,7-dimethoxycoumarin, in roots and stems of citrus seedlings following inoculation with Phytophthora citrophthora. Physiol. Mol. Plant Pathol. 1990, 37, 451–461. [Google Scholar] [CrossRef]
- Higuchi, T. Lignin biochemistry: Biosynthesis and biodegradation. Wood Sci. Technol. 1990, 24, 23–63. [Google Scholar] [CrossRef]
- Wagner, H.; Jordan, E.; Feil, B. Studies on the standardization of mistletoe preparations. Oncology 1986, 43, 16–22. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Dhanaraj, A.L.; Arora, R.; Rowland, L.J.; Fu, Y.; Sun, L. Identification of cold acclimation-responsive Rhododendron genes for lipid metabolism, membrane transport and lignin biosynthesis: Importance of moderately abundant ESTs in genomic studies. Plant Cell Environ. 2006, 29, 558–570. [Google Scholar] [CrossRef] [Green Version]
- Climate-Data.org. Available online: https://en.climate-data.org/europe/switzerland/basel-city/basel-437/ (accessed on 16 May 2020).
- Gaillard, M.D.P.; Glauser, G.; Robert, C.A.M.; Turlings, T.C.J. Fine-tuning the ‘plant domestication-reduced defense’ hypothesis: Specialist vs. generalist herbivores. New Phytol. 2018, 217, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Vandergheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]



| VIP Score 2016 | VIP Score 2017 | tR (min) | m/z Theoretical [M + H]+ | Error (ppm) | Neutral Formula | Compounds | Deconvoluted MS/MS Ions |
|---|---|---|---|---|---|---|---|
| 15.68 | 15.68 | 0.24 | 175.11895 | 3.71 | C6H14N4O2 | Arginine | 175.12; 158.09; 116.07 |
| 12.8 | 12.8 | 0.33 | 130.08626 | 1.84 | C6H11NO2 | Pipecolic acid or lysine | 130.09; 84.05; 56.05 |
| 9.03 | 9.03 | 1.31 | 207.06519 | 1.50 | C11H10O4 | Dimethoxycoumarin | 207.07; 175.04; 147.04; 119.05 |
| 7.97 | - * | 0.47 | 132.10191 | 2.95 | C6H13NO2 | Leucine or Isoleucine | 132.1; 86.1; 69.07 |
| 6.68 | - * | 4.01 | 353.26864 | 0.17 | C21H36O4 | Glyceryl linolenate | 353.27; 335.26; 317.25; 279.23; 261.22; 243.21 |
| 6.22 | - * | 3.98 | 520.33977 | −1.48 | C26H50NO7P | LysoPC (18:2) a | 520.34; 502.32; 184.07; 86.09 |
| 5.94 | 5.94 | 1.03 | 193.08592 | 4.56 | C11H14O4 | Sinapyl alcohol | 193.08; 161.06;133.06;105.07 |
| - * | 5.93 | 0.26 | 148.06043 | 5.88 | C5H9NO4 | Glutamic acid | 148.06; 130.05; 102.05 |
| - * | 5.90 | 0.25 | 195.08631 | 4.56 | C7H14O6 | Pinitol | 127.04; 109.03; 85.03; 81.03; 71.05 |
| - * | 5.88 | 3.96 | 478.29282 | −1.09 | C23H44NO7P | LysoPE (18:2) b | 337.27; 109.1; 95.08; 64.04 |
| tR (min) | m/z | m/z Theoretical [M + H]+ | Error (ppm) | Neutral Formula | Compounds | Deconvoluted MS/MS Ions |
|---|---|---|---|---|---|---|
| 0.30 | 127.0395 | 127.03897 | 4.17 | C6H6O3 | Phloroglucinol | 127.0394; 109.0117; 81.0085; 68.995; 53.0412 |
| 0.35 | 308.0915 | 308.09108 | 1.36 | C10H17N3O6S | Glutathione (reduced) | 308.0904; 179.0486; 162.0231; 84.0456; 76.022 |
| 0.40 | 182.0814 | 182.08117 | 1.26 | C9H11NO3 | Tyrosine | 182.0816; 165.056; 136.0571; 123.0463; 119.0497; 91.0548 |
| 0.85 | 355.1035 | 355.10236 | 3.21 | C16H18O9 | Chlorogenic acid | 355.1042; 163.0818; 145.0045; 135.0049; 117.0762; 107.0501 |
| 0.89 | 235.1452 | 235.1441 | 4.68 | C13H18N2O2 | Coumaroyl putrescin | 234.1451; 147.0448; 119.0503 |
| 1.04 | 390.176 | 390.17586 | 0.36 | C17H24O9 | Syringin | 193.087; 161.06505, 166.0655; 105.0706 |
| 1.50 | 465.1032 | 465.10275 | 0.97 | C21H20O12 | Quercetin-O-glucoside | 465.1021; 303.0875 |
| 1.69 | 435.128 | 435.12857 | −1.31 | C21H22O10 | Naringenin-O-glucoside | 435.1292; 273.0391 |
| 1.80 | 301.1084 | 301.10705 | 4.48 | C17H16O5 | Flavanone a | 301.1098; 181.0619 |
| 4.43 | 522.3555 | 522.35542 | 0.15 | C26H52NO7P | LysoPC (18:1) b | 522.3539; 184.0736; 104.1069 |
| 5.67 | 758.5683 | 758.56943 | 1.49 | C42H80NO8P | PC (16:0/18:2) c | 758.5747; 184.0746 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jäger, T.; Holandino, C.; Melo, M.N.d.O.; Peñaloza, E.M.C.; Oliveira, A.P.; Garrett, R.; Glauser, G.; Grazi, M.; Ramm, H.; Urech, K.; et al. Metabolomics by UHPLC-Q-TOF Reveals Host Tree-Dependent Phytochemical Variation in Viscum album L. Plants 2021, 10, 1726. https://doi.org/10.3390/plants10081726
Jäger T, Holandino C, Melo MNdO, Peñaloza EMC, Oliveira AP, Garrett R, Glauser G, Grazi M, Ramm H, Urech K, et al. Metabolomics by UHPLC-Q-TOF Reveals Host Tree-Dependent Phytochemical Variation in Viscum album L. Plants. 2021; 10(8):1726. https://doi.org/10.3390/plants10081726
Chicago/Turabian StyleJäger, Tim, Carla Holandino, Michelle Nonato de Oliveira Melo, Evelyn Maribel Condori Peñaloza, Adriana Passos Oliveira, Rafael Garrett, Gaétan Glauser, Mirio Grazi, Hartmut Ramm, Konrad Urech, and et al. 2021. "Metabolomics by UHPLC-Q-TOF Reveals Host Tree-Dependent Phytochemical Variation in Viscum album L." Plants 10, no. 8: 1726. https://doi.org/10.3390/plants10081726
APA StyleJäger, T., Holandino, C., Melo, M. N. d. O., Peñaloza, E. M. C., Oliveira, A. P., Garrett, R., Glauser, G., Grazi, M., Ramm, H., Urech, K., & Baumgartner, S. (2021). Metabolomics by UHPLC-Q-TOF Reveals Host Tree-Dependent Phytochemical Variation in Viscum album L. Plants, 10(8), 1726. https://doi.org/10.3390/plants10081726