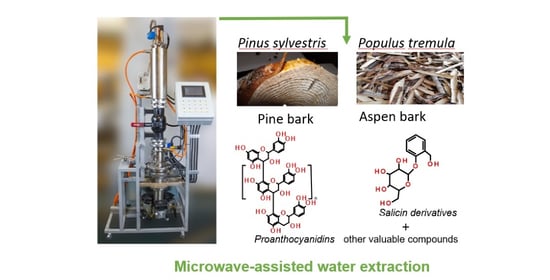
Microwave-Assisted Water Extraction of Aspen (Populus tremula) and Pine (Pinus sylvestris L.) Barks as a Tool for Their Valorization
Abstract
:1. Introduction
- The evaluation of the effectivity of the one-step water MAE of aspen (Populus tremula) and pine (Pinus sylvestris L.) barks in terms of yields of extractives;
- The characterization of the composition of bark water extracts obtained by microwave-assisted water extraction and reference-accelerated solvent extraction;
- The characterization of the advantages and effectiveness of aspen and pine barks MAE in terms of the isolation of salicin derivatives, flavonoids, proanthocyanidins and quinic acid, depending on the extraction conditions;
- The characterization of the biological activity of the obtained extracts in terms of their ability to inhibit xanthine oxidase enzyme, which is a validated target for the therapeutic treatment of hyperuricemia caused by uric acid overproduction and which has also been associated with a variety of conditions such as diabetes, hypertension and other cardiovascular diseases [29].
2. Results and Discussion
2.1. Composition of Aspen (Populus tremula) and Pine (Pinus sylvestris L.) Barks and the Efficiency of Their Water Extraction
2.2. Composition of Aspen Bark Water Extracts
2.3. Effectivity of Water MAE in Terms of the Isolation of Salicin, Flavonoids and Proanthocyanidins from Aspen Bark
2.4. Composition of Pine Bark Water Extracts
2.5. Effectivity of MAE in Terms of the Isolation of Proanthocyanidins, Flavonoids and Quinic Acid from Pine Bark
2.6. The Biological Activity of the Aspen Bark and Pine Bark Water Extracts Obtained by Microwave-Assisted Extraction
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Composition Characterization of Bark Biomass
3.3.1. Wet Chemistry Analysis
3.3.2. Element Analysis
3.3.3. Total Carbohydrates Content
3.3.4. FTIR Spectroscopy
3.4. Microwave-Assisted Water Extraction
3.5. Analytical Pyrolysis
3.6. Analysis of Extracts by Liquid Chromatography-Mass Spectrometry
3.6.1. Total Salicin Derivatives and Free Salicin Content
3.6.2. Alkaline Hydrolysis of Extracts
3.6.3. Free Salicin Analysis
3.6.4. Quantification of Taxifolin and Procyanidin B2
3.6.5. Quantification of Proanthocyanidin Dimers to Tetramers
3.6.6. Detection of Catechin Content
3.6.7. Quantification of Quinic Acid
3.7. Total Phenolics Content
3.8. Total Flavonoids Content
3.9. Total Proanthocyanidins Content
3.10. Xanthine Oxidase Inhibition Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, C.; Hofmann, T.; Visi-Rajczi, E.; Pásztory, Z. Low-Frequency, Green Sonoextraction of Antioxidants from Tree Barks of Hungarian Woodlands for Potential Food Applications. Chem. Eng. Processing-Process Intensif. 2021, 159, 108221. [Google Scholar] [CrossRef]
- Pásztory, Z.; Mohácsiné, I.R.; Gorbacheva, G.; Börcsök, Z. The Utilization of Tree Bark. BioRes 2016, 11, 7859–7888. [Google Scholar] [CrossRef]
- Warmiński, K.; Stolarski, M.J.; Gil, Ł.; Krzyżaniak, M. Willow Bark and Wood as a Source of Bioactive Compounds and Bioenergy Feedstock. Ind. Crops Prod. 2021, 171, 113976. [Google Scholar] [CrossRef]
- Jablonsky, M.; Nosalova, J.; Sladkova, A.; Haz, A.; Kreps, F.; Valka, J.; Miertus, S.; Frecer, V.; Ondrejovic, M.; Sima, J.; et al. Valorisation of Softwood Bark through Extraction of Utilizable Chemicals. A Review. Biotechnol. Adv. 2017, 35, 726–750. [Google Scholar] [CrossRef]
- Neiva, D.M.; Luís, Â.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark Residues Valorization Potential Regarding Antioxidant and Antimicrobial Extracts. Wood Sci. Technol. 2020, 54, 559–585. [Google Scholar] [CrossRef]
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Abedini, A.; Chollet, S.; Angelis, A.; Borie, N.; Nuzillard, J.-M.; Skaltsounis, A.-L.; Reynaud, R.; Gangloff, S.C.; Renault, J.-H.; Hubert, J. Bioactivity-Guided Identification of Antimicrobial Metabolites in Alnus glutinosa Bark and Optimization of Oregonin Purification by Centrifugal Partition Chromatography. J. Chromatogr. B 2016, 1029–1030, 121–127. [Google Scholar] [CrossRef]
- Soquetta, M.B.; de Marsillac Terra, L.; Bastos, C.P. Green Technologies for the Extraction of Bioactive Compounds in Fruits and Vegetables. CyTA-J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Lea, C.S.; Simhadri, C.; Bradbury, S.G.; Wulff, J.E.; Constabel, C.P. Efficient Purification of the Diarylheptanoid Oregonin from Red Alder (Alnus rubra) Leaves and Bark Combining Aqueous Extraction, Spray Drying and Flash-chromatography. Phytochem. Anal. 2021, 32, 554–561. [Google Scholar] [CrossRef]
- Alberti, Á.; Riethmüller, E.; Béni, S. Characterization of Diarylheptanoids: An Emerging Class of Bioactive Natural Products. J. Pharm. Biomed. Anal. 2018, 147, 13–34. [Google Scholar] [CrossRef] [Green Version]
- Amalinei, R.L.M.; Trifan, A.; Cioanca, O.; Miron, S.D.; Mihai, C.T.; Rotinberg, P.; Miron, A. Polyphenol-Rich Extract from Pinus sylvestris L. Bark--Chemical and Antitumor Studies. Rev. Med. -Chir. A Soc. De Med. Si Nat. Din Iasi 2014, 118, 551–557. [Google Scholar]
- Adamiak, K.; Lewandowska, K.; Sionkowska, A. The Infuence of Salicin on Rheological and Film-Forming Properties of Collagen. Molecules 2021, 26, 1661. [Google Scholar] [CrossRef]
- Dumitraşcu, R.; Lunguleasa, A.; Spîrchez, C. Renewable Pellets Obtained from Aspen and Birch Bark. BioResources 2019, 13, 6985–7001. [Google Scholar] [CrossRef]
- Gauthier, G. Pellet Market Overview; Report from Bioenergy Europe; Bioenergy Europe: Brussels, Belgium, 2019; Volume 31. [Google Scholar]
- PhytoCide Aspen Bark Extract|Lotioncrafter. Available online: https://lotioncrafter.com/products/phytocide-aspen-bark-extract (accessed on 2 May 2022).
- Chen, J.; Thilakarathna, W.P.D.W.; Astatkie, T.; Rupasinghe, H.P.V. Optimization of Catechin and Proanthocyanidin Recovery from Grape Seeds Using Microwave-Assisted Extraction. Biomolecules 2020, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Ajao, O.; Benali, M.; Faye, A.; Li, H.; Maillard, D.; Ton-That, M.T. Multi-Product Biorefinery System for Wood-Barks Valorization into Tannins Extracts, Lignin-Based Polyurethane Foam and Cellulose-Based Composites: Techno-Economic Evaluation. Ind. Crops Prod. 2021, 167, 113435. [Google Scholar] [CrossRef]
- Jerez, M.; Sineiro, J.; Guitián, E.; Núñez, M.J. Identification of Polymeric Procyanidins from Pine Bark by Mass Spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 4013–4018. [Google Scholar] [CrossRef]
- Jerez, M.; Touriño, S.; Sineiro, J.; Torres, J.L.; Núñez, M.J. Procyanidins from Pine Bark: Relationships between Structure, Composition and Antiradical Activity. Food Chem. 2007, 104, 518–527. [Google Scholar] [CrossRef]
- Zhu, H.; Li, P.; Ren, S.; Tan, W.; Fang, G. Low-Cost Ru/C-Catalyzed Depolymerization of the Polymeric Proanthocyanidin-Rich Fraction from Bark to Produce Oligomeric Proanthocyanidins with Antioxidant Activity. ACS Omega 2019, 4, 16471–16480. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Pereira, C.; Gomes, A.P.; Neto, R.T.; Almeida, A.; Santos, S.A.O.; Silva, A.M.S.; Silvestre, A.J.D. Chemical Characterisation, Antioxidant and Antibacterial Activities of Pinus pinaster Ait. and Pinus pinea L. Bark Polar Extracts: Prospecting Forestry By-Products as Renewable Sources of Bioactive Compounds. Appl. Sci. 2022, 12, 784. [Google Scholar] [CrossRef]
- Rytter, L.; Ingerslev, M.; Kilpeläinen, A.; Torssonen, P.; Lazdina, D.; Löf, M.; Madsen, P.; Muiste, P.; Stener, L.-G. Increased Forest Biomass Production in the Nordic and Baltic Countries–a Review on Current and Future Opportunities. Silva Fenn. 2016, 50, 1660. [Google Scholar] [CrossRef] [Green Version]
- Karonen, M.; Loponen, J.; Ossipov, V.; Pihlaja, K. Analysis of Procyanidins in Pine Bark with Reversed-Phase and Normal-Phase High-Performance Liquid Chromatography–Electrospray Ionization Mass Spectrometry. Anal. Chim. Acta 2004, 522, 105–112. [Google Scholar] [CrossRef]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical Solvent Extraction of Procyanidins from Dried Red Grape Pomace. J. Agric. Food Chem. 2010, 58, 4014–4021. [Google Scholar] [CrossRef]
- García-Marino, M.; Rivas-Gonzalo, J.C.; Ibáñez, E.; García-Moreno, C. Recovery of Catechins and Proanthocyanidins from Winery By-Products Using Subcritical Water Extraction. Anal. Chim. Acta 2006, 563, 44–50. [Google Scholar] [CrossRef]
- Akyshin, Y.; Semenischev, A.; Arshanitsa, A.; Telysheva, G. Chemical Engineering for Improvement of the Efficiency of Microwave Energy Use in Processing of Plant Biomass. Key Eng. Mater. 2021, 903, 87–92. [Google Scholar] [CrossRef]
- Arshanitsa, A.; Ponomarenko, J.; Lauberte, L.; Jurkjane, V.; Pals, M.; Akishin, Y.; Lauberts, M.; Jashina, L.; Bikovens, O.; Telysheva, G. Advantages of MW-Assisted Water Extraction, Combined with Steam Explosion, of Black Alder Bark in Terms of Isolating Valuable Compounds and Energy Efficiency. Ind. Crops Prod. 2022, 181, 114832. [Google Scholar] [CrossRef]
- Arshanitsa, A.; Ponomarenko, J.; Lauberts, M.; Jurkjane, V.; Jashina, L.; Semenischev, A.; Akishin, J.; Telysheva, G. Composition of Extracts Isolated from Black Alder Bark by Microwave Assisted Water Extraction. In Proceedings of the Research for Rural Development, Jelgava, Latvia, 6 October 2020; pp. 87–94. [Google Scholar]
- Luna, G.; Dolzhenko, A.V.; Mancera, R.L. Inhibitors of Xanthine Oxidase: Scaffold Diversity and Structure-Based Drug Design. ChemMedChem 2019, 14, 714–743. [Google Scholar] [CrossRef] [Green Version]
- Technical Association of Pulp and Paper Industry. T222 Om-02 Lignin in Wood and Pulp. In TAPPI Test Methods; TAPPI: Peachtree Corners, GA, USA, 2011; pp. 1–7. [Google Scholar] [CrossRef]
- Wang, Z.; Winestrand, S.; Gillgren, T.; Jönsson, L.J. Chemical and Structural Factors Influencing Enzymatic Saccharification of Wood from Aspen, Birch and Spruce. Biomass Bioenergy 2018, 109, 125–134. [Google Scholar] [CrossRef]
- Korkalo, P.; Korpinen, R.; Beuker, E.; Sarjala, T.; Hellström, J.; Kaseva, J.; Lassi, U.; Jyske, T. Clonal Variation in the Bark Chemical Properties of Hybrid Aspen: Potential for Added Value Chemicals. Molecules 2020, 25, 4403. [Google Scholar] [CrossRef] [PubMed]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of Heating Values of Biomass Fuel from Elemental Composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Sillero, L.; Prado, R.; Welton, T.; Labidi, J. Energy and Environmental Analysis of Flavonoids Extraction from Bark Using Alternative Solvents. J. Clean. Prod. 2021, 308, 127286. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araújo, S.; Gominho, J.; de Cássia Carneiro, A.; Pereira, H. Potential of Eucalyptus globulus Industrial Bark as a Biorefinery Feedstock: Chemical and Fuel Characterization. Ind. Crops Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Leite, C.; Pereira, H. Cork-Containing Barks—A Review. Front. Mater. 2017, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- St-Pierre, A.; Blondeau, D.; Lajeunesse, A.; Bley, J.; Bourdeau, N.; Desgagné-Penix, I. Phytochemical Screening of Quaking Aspen (Populus tremuloides) Extracts by UPLC-QTOF-MS and Evaluation of Their Antimicrobial Activity. Molecules 2018, 23, 1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentín, L.; Kluczek-Turpeinen, B.; Willför, S.; Hemming, J.; Hatakka, A.; Steffen, K.; Tuomela, M. Scots Pine (Pinus sylvestris) Bark Composition and Degradation by Fungi: Potential Substrate for Bioremediation. Bioresour. Technol. 2010, 101, 2203–2209. [Google Scholar] [CrossRef]
- Hamad, A.M.A.; Ates, S.; Olgun, Ç.; Gur, M. Chemical Composition and Antioxidant Properties of Some Industrial Tree Bark Extracts. BioResources 2019, 14, 5657–5671. [Google Scholar]
- Snyder, D.T.; Schilling, M.C.; Hochwender, C.G.; Kaufman, A.D. Profiling Phenolic Glycosides in Populus deltoides and Populus grandidentata by Leaf Spray Ionization Tandem Mass Spectrometry. Anal. Methods 2015, 7, 870–876. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; He, Y.; Li, L.; Xu, Y.; Zhang, Q.; Zheng, Q.; Winterhalter, P.; Ito, Y. Semi-Industrial Isolation of Salicin and Amygdalin from Plant Extracts Using Slow Rotary Counter-Current Chromatography. J. Chromatogr. A 2005, 1074, 43–46. [Google Scholar] [CrossRef]
- Kenstavičienė, P.; Nenortienė, P.; Kiliuvienė, G.; Ževžikovas, A.; Lukošius, A.; Kazlauskienė, D. Application of High-Performance Liquid Chromatography for Research of Salicin in Bark of Different Varieties of Salix. Medicina 2009, 45, 644. [Google Scholar] [CrossRef] [Green Version]
- Pobłocka-Olech, L.; van Nederkassel, A.-M.; Heyden, Y.V.; Krauze-Baranowska, M.; Glód, D.; Baczek, T. Chromatographic Analysis of Salicylic Compounds in Different Species of the GenusSalix. J. Sep. Sci. 2007, 30, 2958–2966. [Google Scholar] [CrossRef]
- Engström, M.T.; Pälijärvi, M.; Fryganas, C.; Grabber, J.H.; Mueller-Harvey, I.; Salminen, J.-P. Rapid Qualitative and Quantitative Analyses of Proanthocyanidin Oligomers and Polymers by UPLC-MS/MS. J. Agric. Food Chem. 2014, 62, 3390–3399. [Google Scholar] [CrossRef]
- Imran, I.B.; Karonen, M.; Salminen, J.-P.; Engström, M.T. Modification of Natural Proanthocyanidin Oligomers and Polymers Via Chemical Oxidation under Alkaline Conditions. ACS Omega 2021, 6, 4726–4739. [Google Scholar] [CrossRef]
- Havlik, J.; de la Huebra, R.G.; Hejtmankova, K.; Fernandez, J.; Simonova, J.; Melich, M.; Rada, V. Xanthine Oxidase Inhibitory Properties of Czech Medicinal Plants. J. Ethnopharmacol. 2010, 132, 461–465. [Google Scholar] [CrossRef]
- Li, Y.; Wan, Y.; Li, R.; Xu, L.; Xie, M.; Fu, G. Solvent Extraction of Caffeoylquinic Acids from Artemisia selengensis Turcz Leaves and Their In Vitro Inhibitory Activities on Xanthine Oxidase. Ind. Crops Prod. 2018, 118, 296–301. [Google Scholar] [CrossRef]
- Li, Y.; Lai, P.; Chen, J.; Shen, H.; Tang, B.; Wu, L.; Weng, M. Extraction Optimization of Polyphenols, Antioxidant and Xanthine Oxidase Inhibitory Activities from Prunus salicina Lindl. Food Sci. Technol. 2016, 36, 520–525. [Google Scholar] [CrossRef] [Green Version]
- Moini, H.; Guo, Q.; Packer, L. Enzyme Inhibition and Protein-Binding Action of the Procyanidin-Rich French Maritime Pine Bark Extract, Pycnogenol: Effect on Xanthine Oxidase. J. Agric. Food Chem. 2000, 48, 5630–5639. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A Simple and Rapid Preparation of Alditol Acetates for Monosaccharide Analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Lauberts, M.; Pals, M. Antioxidant Activity of Different Extracts from Black Alder (Alnus glutinosa) Bark with Greener Extraction Alternative. Plants 2021, 10, 2531. [Google Scholar] [CrossRef]
- Lauberts, M.; Telysheva, G.; Venskutonis, P.R.; Lauberte, L.; Dizhbite, T.; Kazernavičiūte, R.; Pukalskas, A. Diarylheptanoid-Rich Extract of Grey and Black Alder Barks: An Effective Dietary Antioxidant in Mayonnaise. Chem. Pap. 2017, 71, 1007–1012. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 6th ed.; Council of Europe: Strasbourg, France, 2007. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; pp. 152–178. [Google Scholar]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The Conversion of Procyanidins and Prodelphinidins to Cyanidin and Delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]










| Feedstock | Content, % on DM | |||
|---|---|---|---|---|
| Ash 1 | Carbohydrates Total 1 | Klason Lignin Acid Insoluble 2 | Extractives 3 | |
| Aspen bark | 3.77 ± 0.10 | 43.6 ± 1.2 | 26.1 ± 1.1 | 26.5 ± 0.7 |
| Pine bark | 1.79 ± 0.05 | 42.3 ± 0.8 | 41.8 ± 1.8 | 14.1 ± 0.3 |
| Feedstock | Content, % on Ash Free DM 1 | |||
|---|---|---|---|---|
| C | H | O 2 | N | |
| Aspen bark | 51.53 ± 1.34 | 5.40 ± 0.16 | 42.36 ± 0.81 | 0.71 ± 0.01 |
| Pine bark | 52.94 ± 1.32 | 5.07 ± 0.11 | 41.53 ± 1.21 | 0.46 ± 0.01 |
| Oil products [6] | 83–87 | 10–14 | 0.1–1.5 | 0.1–2 |
| Bark Source | Solvent, Extraction Conditions | Procedure | Extractives Yield, % on Bark D.M |
|---|---|---|---|
| Hybrid aspen (Populus tremula L. × tremuloides Michx.) | Acetone (95%, aq.) | Accelerated solvent extraction, 100 °C, 3 cycles of 15 min each | 16.2–23.9% [32] |
| Quaking aspen (Populus tremuloides) | Acetone (99.8%) | Soxhlet system for 7 h over 6 cycles of extraction | ≈14.5% [37] |
| Quaking aspen (Populus tremuloides) | Distilled water | Accelerated solvent extraction, 100 °C, 6 cycles of 10 min each, 1500 psi | ≈15% [37] |
| Quaking aspen (Populus tremuloides) | Methanol (99.9%) | Accelerated solvent extraction, 100 °C, 6 cycles of 10 min each, 1500 psi | ≈19% [37] |
| Quaking aspen (Populus tremuloides) | Denaturised ethanol | Soxhlet system for 7 h over 6 cycles of extraction | ≈18% [37] |
| Pinus sylvestris | Acetone:water (95:5; v/v) → water (100%) | Sequential accelerated solvent extraction, 150 °C, 3 static cycles of 5 min each, 2000 psi | 19.4% (acetone 5.5% + water 13.9%) [38] |
| Pinus sylvestris | Hot water | TAPPI 1993a | 18.72% [39] |
| Pinus sylvestris | Alcohol | TAPPI 1988a | 18.33% [39] |
| Pinus sylvestris L. | Methanol (80%) | Extraction using a magnetic stirrer, 3 cycles of 3 h each | 17.35% [11] |
| Peak | [M-H]− | Fragments | Compounds | Compound Class | Ret. Time, min |
|---|---|---|---|---|---|
| 1 | 331 | 285, 123 | Salicin | Phenolic Glycosides | 1.62 |
| 2 | 373 | 327 | 2O-acetyl-salicin | 2.37 | |
| 3 | 423 | 469; 317 | Salicortin | 2.86 | |
| 4 | 405 | 451; 283 | Salicyloyl-salicin | 3.62 | |
| 5 | 527 | 573 | Tremulacin | 6.67 | |
| 6 | 435 | 389; 121 | Tremuloidin | 5.05 | |
| 7 | 477 | 285; 119 | Kaempferol-hexoside | 2.98 | |
| 9 | 447 | 285 | Luteolin-hexoside | 3.74 | |
| 10 | 487 | 307 | Acetylglycitin | 3.14 | |
| 11 | 423 | 307; 259; 145; 163 | Grandidentatin | 3.19 | |
| 12 | 435 | 273; 163 | Phloridzin | 4.07 | |
| 13 | 423 | 307; 259; 163; 145 | Grandidentatin | 4.62 | |
| 14 | 431 | 269 | Apigenin-7-O-glucoside | 5.97 | |
| 15 | 191 | 173, 111, 67 | Citric acid derivative | Phenolic acids | 0.47 |
| 16 | 325 | 163 | Coumaric acid glucoside | 2.05 | |
| 17 | 315 | 300/299 | Isorhamnetin | Flavonoids | 1.33 |
| 18 | 303 | 285, 285, 247, 111 | Ouercetin | 2.96 | |
| 19 | 521 | 423; 373 | Trilobolide | Terpenoid | 2.49 |
| 20 | 683 | 341; 281; 179, 161, 135 | Caffeic acid hexose and Hexose polymer | Phenolic acids/sugars | 0.40 |
| Extraction Conditions 1 | Content of Salicin, (%) on DM of Extract 2 | Content of Salicin, (%) on DM of Bark 3 | ||
|---|---|---|---|---|
| Free | Total | Free | Total | |
| 70 °C; 5 min. | 9.42 ± 0.21 | 16.52 ± 0.36 | 1.74 ± 0.05 | 2.92 ± 0.09 |
| 70 °C; 20 min. | 8.23 ± 0.18 | 12.07 ± 0.31 | 1.41 ± 0.05 | 2.11 ± 0.06 |
| 70 °C; 30 min. | 9.74 ± 0.17 | 19.33 ± 0.38 | 1.76 ± 0.06 | 3.53 ± 0.15 |
| 90 °C; 5 min. | 8.51 ± 0.19 | 18.71 ± 0.47 | 1.55 ± 0.03 | 3.92 ± 0.17 |
| 90 °C; 20 min. | 9.63 ± 0.22 | 18.12 ± 0.27 | 1.84 ± 0.05 | 3.37 ± 0.14 |
| 90 °C; 30 min. | 10.48 ± 0.28 | 19.21 ± 0.23 | 2.41 ± 0.06 | 3.63 ± 0.13 |
| 110 °C, 5 min. | 10.71 ± 0.17 | 19.04 ± 0.39 | 2.08 ± 0.05 | 3.68 ± 0.14 |
| 110 °C; 20 min. | 14.05 ± 0.33 | 17.64 ± 0.42 | 2.73 ± 0.11 | 3.42 ± 0.17 |
| 110 °C; 30 min. | 11.07 ± 0.21 | 16.13 ± 0.34 | 2.23 ± 0.08 | 3.24 ± 0.12 |
| 130 °C; 5 min. | 10.81 ± 0.25 | 17.32 ± 0.39 | 2.25 ± 0.05 | 3.57 ± 0.14 |
| 130 °C; 30 min. | 12.15 ± 0.27 | 15.38 ± 0.29 | 2.99 ± 0.08 | 3.72 ± 0.16 |
| 150 °C; 5 min. | 9.94 ± 0.14 | 13.71 ± 0.22 | 2.51 ± 0.09 | 3.52 ± 0.12 |
| 150 °C; 30 min. | 10.32 ± 0.29 | 13.62 ± 0.34 | 2.82 ± 0.07 | 3.77 ± 0.16 |
| Peak | [M-H]− | Fragments | Compounds | Compound Class | Ret.time, min |
|---|---|---|---|---|---|
| 1 | 191 | 173, 127, 111, 85 | Quinic acid | Phenolic acids | 0.47 |
| 2 | 299 | 137, 93 | P-Hydroxy benzoic acid hexoside | 0.86 | |
| 3 | 341 | 179, 161, 119, 101, | Sucrose | Carbohydrates | 0.41 |
| 4 | 465 | 447, 437, 259, 285, 125 | Taxifolin-O-hexoside | Flavonoids | 3.09 |
| 5 | 289 | 245 | Catechin or epicatechin | 2.33 | |
| 6 | 303 | 285, 177, 125 | Taxifolin | 3.23 | |
| 7 | 577 | 559, 461, 425, 289, 245, 203 | B-type proanthocyanidin dimer | Proanthocyanidins | 2.17 |
| 8 | 1153 | 865, 577, 425, | B-type proanthocyanidin tetramer | 2.20 | |
| 9 | 865 | 739, 577, 543, 425, 289, 245 | B-type proanthocyanidin trimer | 2.26 | |
| 10 | 865 | 822, 793, 713, 577 | B-type proanthocyanidin trimer isomer | 2.28 | |
| 11 | 1153 | 863, 575, 287 | B-type proanthocyanidin tetramer isomer | 2.39 | |
| 12 | 1153 | 865, 575, 289, 245 | B-type proanthocyanidin tetramer isomer | 2.43 | |
| 13 | 1153 | 1008, 865, 577, 289, 245 | B-type proanthocyanidin tetramer isomer | 2.48 | |
| 14 | 865 | 577, 289, 245 | B-type proanthocyanidin trimer isomer | 2.50 | |
| 15 | 1153 | 865, 577, 289, 245 | B-type proanthocyanidin tetramer isomer | 2.56 | |
| 16 | 1153 | 865, 577, 289, 245 | B-type proanthocyanidin tetramer isomer | 2.58 | |
| 18 | 1153 | 864, 491, 315, 289 | B-type proanthocyanidin tetramer isomer | 2.62 |
| Extraction Conditions | Dimers, % * | Trimers, % * | Tetramers, % * | Total Dimers-Tetramers, % * |
|---|---|---|---|---|
| ASE 90 °C (4 × 5 min.) | 0.98 ± 0.02 | 2.02 ± 0.05 | 0.54 ± 0.01 | 3.54 ± 0.07 |
| MAE 90 °C 0 min of isothermal heating | 1.19 ± 0.03 | 2.64 ± 0.07 | 0.57 ± 0.01 | 4.40 ± 0.09 |
| ASE 130 °C (4 × 5 min.) | 0.81 ± 0.02 | 1.12 ± 0.03 | 0.52 ± 0.02 | 2.44 ± 0.07 |
| MAE 130 °C 0 min of isothermal heating | 0.92 ± 0.02 | 2.93 ± 0.07 | 0.36 ± 0.01 | 4.21 ± 0.11 |
| Extraction Conditions | Quinic Acid, % * | Taxifolin, % * | B2 Dimer, % * |
|---|---|---|---|
| MAE 70 °C 0 min. of isothermal heating | 2.56 ± 0.07 | 0.10 ± 0.003 | 0.80 ± 0.02 |
| ASE 70 °C (4 × 5 min.) | 2.09 ± 0.05 | 0.13 ± 0.003 | 0.80 ± 0.02 |
| MAE 90 °C 0 min. of isothermal heating | 2.80 ± 0.08 | 0.21 ± 0.005 | 1.19 ± 0.03 |
| ASE 90 °C (4 × 5 min.) | 2.29 ± 0.07 | 0.54 ± 0.015 | 0.98 ± 0.03 |
| MAE 130 °C 0 min. of isothermal heating | 0.17 ± 0.004 | 0.12 ± 0.002 | 0.92 ± 0.02 |
| ASE 130 °C (4 × 5 min.) | 0.13 ± 0.003 | 0.24 ± 0.005 | 0.81 ± 0.02 |
| MAE 150 °C 0 min. of isothermal heating | 0.80 ± 0.02 | 0.01 ± 0.0003 | 1.18 ± 0.03 |
| ASE 150 °C (4 × 5 min.) | 0.23 ± 0.005 | 0.02 ± 0.0003 | 0.44 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pals, M.; Lauberte, L.; Ponomarenko, J.; Lauberts, M.; Arshanitsa, A. Microwave-Assisted Water Extraction of Aspen (Populus tremula) and Pine (Pinus sylvestris L.) Barks as a Tool for Their Valorization. Plants 2022, 11, 1544. https://doi.org/10.3390/plants11121544
Pals M, Lauberte L, Ponomarenko J, Lauberts M, Arshanitsa A. Microwave-Assisted Water Extraction of Aspen (Populus tremula) and Pine (Pinus sylvestris L.) Barks as a Tool for Their Valorization. Plants. 2022; 11(12):1544. https://doi.org/10.3390/plants11121544
Chicago/Turabian StylePals, Matiss, Liga Lauberte, Jevgenija Ponomarenko, Maris Lauberts, and Alexander Arshanitsa. 2022. "Microwave-Assisted Water Extraction of Aspen (Populus tremula) and Pine (Pinus sylvestris L.) Barks as a Tool for Their Valorization" Plants 11, no. 12: 1544. https://doi.org/10.3390/plants11121544
APA StylePals, M., Lauberte, L., Ponomarenko, J., Lauberts, M., & Arshanitsa, A. (2022). Microwave-Assisted Water Extraction of Aspen (Populus tremula) and Pine (Pinus sylvestris L.) Barks as a Tool for Their Valorization. Plants, 11(12), 1544. https://doi.org/10.3390/plants11121544