High-Dose Assessment of Transgenic Insect-Resistant Maize Events against Major Lepidopteran Pests in China
Abstract
:1. Introduction
2. Results
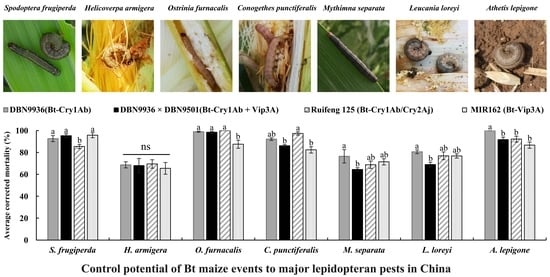
2.1. Corrected Mortalities of DBN9936 (Bt-Cry1Ab) to the Seven Pests
2.2. Corrected Mortalities of DBN9936 × DBN9501 (Bt-Cry1Ab + Vip3A) to the Seven Pests
2.3. Corrected Mortalities of Ruifeng 125 (Bt-Cry1Ab/Cry2Aj) to the Seven Pests
2.4. Corrected Mortalities of MIR162 (Bt-Vip3A) to the Seven Pests
2.5. Average Corrected Mortalities of Four Transformation Events to the Seven Pests
3. Discussion
4. Materials and Methods
4.1. The Transformation Events of Insect-Resistant Transgenic Maize
4.2. Collection and Culture of Insect Species
4.3. High-Dose Bioassays
4.4. Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koziel, M.G.; Beland, G.L.; Bowman, C.; Carozzi, N.B.; Crenshaw, R.; Crossland, L.; Dawson, J.; Desai, N.; Hill, M.; Kaell, S.; et al. Field performance of elite transgenic maize plants expressing an insecticidal protein derived from Bacillus thuringinesis. Nat. Biotechnol. 1993, 11, 194–200. [Google Scholar] [CrossRef]
- Mason, C.E.; Rice, M.E.; Calvin, D.D.; Van Duyn, J.W.; Showers, W.B.; Hutchinson, W.D.; Witkowski, J.F.; Higgins, R.A.; Onstad, D.W.; Dively, G.P. European Corn Borer Ecology and Management; North Central Regional Extension Publication, No.327; Iowa State University: Ames, IA, USA, 1996; p. 57. [Google Scholar]
- Hutchison, W.D.; Burkness, E.C.; Mitchell, P.D.; Moon, R.D.; Leslie, T.W.; Fleischer, S.J.; Abrahamson, M.; Hamilton, K.L.; Steffey, K.L.; Gray, M.E.; et al. Area wide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 2010, 330, 222–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dively, G.P.; Venugopal, P.D.; Bean, D.; Whalen, J.; Holmstrom, K.; Kuhar, T.P.; Doughty, H.B.; Patton, T.; Cissel, W.; Hutchison, W.D. Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. USA 2018, 115, 3320–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISAAA. Global Status of Commercialized Biotech/GM Crops in 2019: Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier; ISAAA: Ithaca, NY, USA, 2019. [Google Scholar]
- Gould, F. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 1998, 43, 701–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storer, N.P.; Babcock, J.M.; Schlenz, M.; Meade, T.; Thompson, G.D.; Bing, J.W.; Huckaba, R.M. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J. Econ. Entomol. 2010, 103, 1031–1038. [Google Scholar] [CrossRef]
- Chandrasena, D.I.; Signorini, A.M.; Abratti, G.; Storer, N.P.; Olaciregui, M.L.; Alves, A.P.; Pilcher, C.D. Characterization of field-evolved resistance to Bacillus thuringiensis-derived Cry1F δ-endotoxin in Spodoptera frugiperda populations from Argentina. Pest Manag. Sci. 2018, 74, 746–754. [Google Scholar] [CrossRef] [Green Version]
- Omoto, C.; Bernardi, O.; Salmeron, E.; Sorgatto, R.J.; Dourado, P.M.; Crivellari, A.; Carvalho, R.A.; Willse, A.; Martinelli, S.; Head, G.P. Field-evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag. Sci. 2016, 72, 1727–1736. [Google Scholar] [CrossRef]
- Van Rensburg, J.B.J. First report of field resistance by the stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. S. Afr. J. Plant Soil 2007, 24, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Dively, G.P.; Venugopal, P.D.; Finkenbinder, C. Field-evolved resistance in corn earworm to Cry proteins expressed by transgenic sweet corn. PLoS ONE 2016, 11, e0169115. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Guo, J.; Brown, S.; Head, G.P.; Price, P.A.; Paula-Moraes, S.; Ni, X.; Dimase, M.; Huang, F. Field-evolved resistance of Helicoverpa zea (Boddie) to transgenic maize expressing pyramided Cry1A.105/ Cry2Ab2 proteins in northeast Louisiana, the United States. J. Invertebr. Pathol. 2019, 163, 11–20. [Google Scholar] [CrossRef]
- Tabashnik, B.E.; Carrière, Y. Surge in insect resistance to transgenic crops and prospects for sustainability. Nat. Biotechnol. 2017, 35, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Carrière, Y. Global patterns of resistance to Bt crops highlighting pink bollworm in the United States, China and India. J. Econ. Entomol. 2019, 112, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Li, G.P.; Wu, K.M. Commercial strategy of transgenic insect-resistant maize in China. J. Plant Prot. 2022, 49, 17–32. (In Chinese) [Google Scholar]
- Carrière, Y.; Crickmore, N.; Tabashnik, B.E. Optimizing pyramided transgenic Bt crops for sustainable pest management. Nat. Biotechnol. 2015, 33, 161–168. [Google Scholar] [CrossRef] [PubMed]
- U.S. Environmental Protection Agency (EPA). The Environmental Protection Agency’s White Paper on Bt Plant-Pesticide Resistance Management; EPA/739/S-98/001; Biopesticides and Pollution Prevention Division: Washington, DC, USA, 1998. Available online: http://www.epa.gov/EPA-PEST/1998/January/Day-14/paper.pdf (accessed on 9 September 2021).
- FIFRA Scientific Advisory Panel. Transmittal of the Final Report of the FIFRA Scientific Advisory Panel Subpanel on Bacillus thuringiensis (Bt) Plant-Pesticides and Resistance Management, Meeting Held on February 9 and 10, 1998; US Environmental Protection Agency: Washington, DC, USA, 1998; p. 59.
- FIFRA Scientific Advisory Panel. Bt Plant-Pesticides Risk and Benefit Assessments; Report No. 2000-07; US Environmental Protection Agency: Washington, DC, USA, 2001; p. 78.
- U.S. Environmental Protection Agency (EPA). Terms and Conditions for Bt Corn Registrations 30 Sept 2010; Office of Pesticide Programs: Washington, DC, USA, 2010. Available online: http://www3.epa.gov/pesticides/chem_search/reg_actions/pip/bt-corn-terms-conditions.pdf (accessed on 16 May 2022).
- Liang, J.G.; Zhang, D.D.; Li, D.Y.; Zhao, S.Y.; Wang, C.Y.; Xiao, Y.T.; Xu, D.; Yang, Y.Z.; Li, G.P.; Wang, L.L.; et al. Expression profiles of Cry1Ab protein and its insecticidal efficacy against the invasive fall armyworm for Chinese domestic GM maize DBN9936. J. Integr. Agric. 2021, 20, 792–803. [Google Scholar] [CrossRef]
- Zhang, D.D.; Wu, K.M. The bioassay of Chinese domestic Bt-Cry1Ab and Bt-(Cry1Ab+Vip3Aa) maize against the fall armyworm, Spodoptera frugiperda. Plant Prot. 2019, 45, 54–60. (In Chinese) [Google Scholar]
- He, L.M.; Zhao, S.Y.; Gao, X.W.; Wu, K.M. Ovipositional responses of Spodoptera frugiperda on host plants provide a basis for using Bt-transgenic maize as trap crop in China. J. Integr. Agric. 2020, 19, 2–12. [Google Scholar] [CrossRef]
- Wang, W.W.; Zhang, D.D.; Zhao, S.Y.; Wu, K.M. Susceptibilities of the Invasive Fall Armyworm (Spodoptera frugiperda) to the Insecticidal Proteins of Bt maize in China. Toxins 2022, 14, 507. [Google Scholar] [CrossRef]
- Yang, X.M.; Zhao, S.Y.; Liu, B.; Gao, Y.; Hu, C.X.; Li, W.J.; Yang, Y.Z.; Li, G.P.; Wang, L.L.; Yang, X.Q.; et al. Bt maize can provide non-chemical pest control and enhance food safety in China. Plant Biotechnol. J. 2022. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Yang, X.M.; Liu, D.Z.; Sun, X.X.; Li, G.P.; Wu, K.M. Performance of the domestic Bt-corn event expressing pyramided Cry1Ab and Vip3Aa19 against the invasive Spodoptera frugiperda (J. E. Smith) in China. Pest Manag. Sci. 2022. [Google Scholar] [CrossRef]
- Tong, P.Y. Maize Plant District in China; Chinese Agricultural Science and Technology Press: Beijing, China, 1992. (In Chinese) [Google Scholar]
- Wang, Z.Y.; Wang, X.M. Current status and management strategies for corn pests and diseases in China. Plant Prot. 2019, 45, 1–11. (In Chinese) [Google Scholar]
- Erasmus, R.; Pieters, R.; Du, P.H.; Hilbeck, A.; Trtikova, M.; Erasmus, A.; Van den Berg, J. Introgression of a cry1Ab transgene into open pollinated maize and its effect on Cry protein concentration and target pest survival. PLoS ONE 2019, 14, e0226476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Peng, S.B.; Cui, K.H.; Nie, L.X.; Huang, J.L. Field performance of Bt transgenic crops: A review. Aust. J. Crop. Sci. 2014, 8, 18–26. [Google Scholar]
- Ramirez-Romero, R.; Desneux, N.; Chaufaux, J.; Kaiser, L. Bt-maize effects on biological parameters of the non-target aphid Sitobion avenae (Homoptera: Aphididae) and Cry1Ab toxin detection. Pestic. Biochem. Physiol. 2008, 91, 110–115. [Google Scholar] [CrossRef]
- Bakhsh, A.; Shahzad, K.; Husnain, T. Variation in the spatio-temporal expression of insecticidal genes in cotton. Czech J. Genet. Plant Breed 2011, 47, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Székács, A.; Weiss, G.; Quist, D.; Takács, E.; Darvas, B.; Meier, M.; Swain, T.; Hilbeck, A. Inter-laboratory comparison of Cry1Ab toxin quantification in MON 810 maize by enzyme-immunoassay. Food Agric. Immunol. 2012, 23, 99–121. [Google Scholar] [CrossRef]
- Trtikova, M.; Wikmark, O.G.; Zemp, N.; Widmer, A.; Hilbeck, A. Transgene expression and Bt protein content in transgenic Bt maize (MON810) under optimal and stressful environmental conditions. PLoS ONE 2015, 10, e0123011. [Google Scholar] [CrossRef]
- Székács, A.; Lauber, É.; Takács, E.; Darvas, B. Detection of Cry1Ab toxin in the leaves of MON 810 transgenic maize. Anal. Bioanal. Chem. 2010, 396, 2203–2211. [Google Scholar] [CrossRef]
- Reisig, D.D.; DiFonzo, C.; Dively, G.; Farhan, Y.; Gore, J.; Smith, J. Best Management practices to delay the evolution of Bt resistance in lepidopteran pests without high susceptibility to Bt toxins in North America. J. Econ. Entomol. 2022, 115, 26–36. [Google Scholar] [CrossRef]
- Li, G.P.; Ji, T.J.; Sun, X.X.; Jiang, Y.Y.; Wu, K.M.; Feng, H.Q. Susceptibility evaluation of invaded Spodoptera frugiperda population in Yunnan province to five Bt proteins. Plant Prot. 2019, 45, 15–20. (In Chinese) [Google Scholar]
- Li, G.P.; Feng, H.Q.; Ji, T.J.; Huang, J.R.; Tian, C.H. What type of Bt corn is suitable for a region with diverse lepidopteran pests: A laboratory evaluation. GM Crops Food 2021, 12, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.M. Monitoring and management strategy for Helicoverpa armigera resistance to Bt cotton in China. J. Invertebr. Pathol. 2007, 95, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Xu, D.; Cong, S.; Jiang, Y.; Huang, Y.; Wang, J.; Wu, H.; Wang, L.; Wu, K.; Carrière, Y.; et al. Hybridizing transgenic Bt cotton with non-Bt cotton counters resistance in pink bollworm. Proc. Natl. Acad. Sci. USA 2017, 114, 5413–5418. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.N.; Andow, D.A.; Buschman, L.L. Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomol. Exp. Appl. 2011, 140, 1–16. [Google Scholar] [CrossRef]
- Caprio, M.A.; Kurtz, R.; Catchot, A.; Kerns, D.; Reisig, D.; Gore, J.; Reay-Jones, F.P.F. The corn–cotton agroecosystem in the midsouthern United States: What insecticidal event pyramids should be used in each crop to extend Vip3A durability. J. Econ. Entomol. 2019. 112, 2894–2906.
- DiFonzo, C. Handy Bt Trait Table for U.S. Corn Production. 2021. Available online: https://www.texasinsects.org/bt-corn-trait-table.html (accessed on 12 September 2021).
- U.S. Environmental Protection Agency (EPA). Biopesticides Registration Action Document Cry1Ab and Cry1F Bacillus thuringiensis (Bt) Corn Plant-Incorporated Protectants; U.S. Environmental Protection Agency Office of Pesticide Programs Biopesticides and Pollution Prevention Division: Washington, DC, USA, 2010. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/pip/cry1f-cry1ab-brad.pdf (accessed on 16 December 2021).
- U.S. Environmental Protection Agency (EPA). Biopesticide Registration Action Document: Bacillus thuringiensis Cry1A.105 and Cry2Ab2 Insecticidal Proteins and the Genetic Material Necessary for Their Production in Corn [PC Codes 006515 (Cry2Ab2), 006514 (Cry1A.105)]; U.S. Environmental Protection Agency Office of Pesticide Programs Biopesticides and Pollution Prevention Division: Washington, DC, USA, 2010. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/pip/mon-89034-brad.pdf (accessed on 16 December 2021).
- U.S. Environmental Protection Agency (EPA). Biopesticide Registration Action Document Bacillus thuringiensis Vip3Aa20 Insecticidal Protein and the Genetic Material Necessary for Its Production (via Elements of Vector pNOV1300) in Event MIR162 Maize (OECD Unique Identifier: SYN-IR162-4) PC Code: 006599; U.S. Environmental Protection Agency Office of Pesticide Programs Biopesticides and Pollution Prevention Division: Washington, DC, USA, 2010. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/registration/decision_PC-006599_3-Apr-09.pdf (accessed on 16 December 2021).
- Burkness, E.C.; Dively, G.; Patton, T.; Morey, A.C.; Hutchison, W.D. Novel Vip3A Bacillus thuringiensis (Bt) maize approaches high dose efficacy against Helicoverpa zea (Lepidoptera: Noctuidae) under field conditions: Implications for resistance management. GM Crops. 2010, 1, 337–343. [Google Scholar] [CrossRef]
- Sousa, F.F.; Mendes, S.M.; Santos-Amaya, O.F.; Araújo, O.G.; Oliveira, E.E.; Pereira, E.J. Life-history traits of Spodoptera frugiperda populations exposed to low-dose Bt maize. PLoS ONE 2016, 11, e0156608. [Google Scholar] [CrossRef] [Green Version]
- Horner, T.A.; Dively, G.P.; Herbert, D.A. Development, survival and fitness performance of Helicoverpa zea (Lepidoptera: Noctuidae) in MON810 Bt field corn. J. Econ. Entomol. 2003, 96, 914–924. [Google Scholar] [CrossRef]
- Li, G.P.; Huang, J.R.; Ji, T.J.; Tian, C.H.; Zhong, J.; Feng, H.Y.; Feng, H.Q. The Artificial Diet for Rearing Ostrinia furnacalis and Its Application. China Patent CN108813114A, 30 July 2021. [Google Scholar]
- Li, G.P.; Ji, T.J.; Huang, J.R.; Tian, C.H.; Zhong, J.; Feng, H.Y.; Feng, H.Q. The Artificial Diet for Rearing Conogethes punctiferalis and Its Application. China Patent CN108740611A, 29 October 2021. [Google Scholar]
- Macrae, T.C.; Baur, M.E.; Boethel, D.J.; Fitzpatrick, B.J.; GAO, A.G.; Gamundi, J.C.; Harrison, L.A.; Kabuye, V.T.; Mcpherson, R.M.; Miklos, J.A.; et al. Laboratory and field evaluations of transgenic soybean exhibiting high-dose expression of a synthetic Bacillus thuringiensis Cry1AGene for control of Lepidoptera. J. Econ. Entomol. 2005, 98, 577–587. [Google Scholar] [CrossRef]
- Bernardi, O.; Malvestiti, G.S.; Dourado, P.M.; Wladecir, S.O.; Martinelli, S.; Berger, G.U.; Head, G.P.; Omoto, C. Assessment of the high-dose concept and level of control provided by MON87701 × MON89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag. Sci. 2012, 68, 1083–1109. [Google Scholar] [CrossRef]





| Maize Growth Stage | Tissue | Specific Sampling Requirements |
|---|---|---|
| V6–V8 (6–8 leaves have visible collars) | Leaf | The youngest leaf that emerged and was at least 20 cm in length was cut from the leaf tip. |
| V12 (12 leaves have visible collars) | Leaf | The youngest leaf that emerged and was at least 20 cm in length was cut from the leaf tip. |
| VT (The lowest branch of the tassel is visible, but silks are not) | Tassel | One tassel was extracted from each corn plant |
| R1 (Silk is visible) | Silk | The ear was bagged, and the ear with the bag was removed from the plant and moved to a pollen-free environment, and the silk were cut from the ear. |
| R4 (Kernel contents are pasty as starch accumulates) | Grain | Thirty young grains were collected from each ear. |
| Insect Species | Date | Collecting Location | Insect Number Collected | Insect Stage | Host Plants |
|---|---|---|---|---|---|
| S. frugiperda | January 2019 | Ruili City, Yunnan Province (23°58′ N, 97°48′ E) | 150–200 | 3–5 instar larvae | Maize |
| H. armigera | August 2016 | Yuanyang County, Henan Province (35°13′ N, 113°42′ E) | 150–200 | 4–5 instar larvae | Maize |
| O. furnacalis | May to June 2015 | Yuanyang County, Henan Province (35°13′ N, 113°42′ E) | 150–200 | 4–5 instar larvae | Maize |
| C. punctiferalis | September 2016 | Yuanyang County, Henan Province (35°13′ N, 113°42′ E) | 250–300 | 3–5 instar larvae | Maize and sorghum |
| M. separata | August 2016 | Lingbao County, Henan Province (34°36′ N, 110°48′ E) | 300–350 | 4–5 instar larvae | Maize |
| L. loreyi | May 2019 | Xinyang City, Henan Province (32°17′ N, 114°01′ E) | 100–150 | 4–5 instar larvae | Maize |
| A. lepigone | May to June 2016 | Yuanyang County, Henan Province (35°13′ N, 113°42′ E) | 100–120 | Adults | Captured by light trap |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Ji, T.; Zhao, S.; Feng, H.; Wu, K. High-Dose Assessment of Transgenic Insect-Resistant Maize Events against Major Lepidopteran Pests in China. Plants 2022, 11, 3125. https://doi.org/10.3390/plants11223125
Li G, Ji T, Zhao S, Feng H, Wu K. High-Dose Assessment of Transgenic Insect-Resistant Maize Events against Major Lepidopteran Pests in China. Plants. 2022; 11(22):3125. https://doi.org/10.3390/plants11223125
Chicago/Turabian StyleLi, Guoping, Tingjie Ji, Shengyuan Zhao, Hongqiang Feng, and Kongming Wu. 2022. "High-Dose Assessment of Transgenic Insect-Resistant Maize Events against Major Lepidopteran Pests in China" Plants 11, no. 22: 3125. https://doi.org/10.3390/plants11223125
APA StyleLi, G., Ji, T., Zhao, S., Feng, H., & Wu, K. (2022). High-Dose Assessment of Transgenic Insect-Resistant Maize Events against Major Lepidopteran Pests in China. Plants, 11(22), 3125. https://doi.org/10.3390/plants11223125