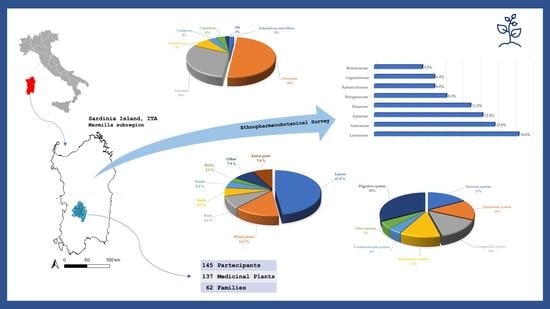
Ethnopharmacobotany and Diversity of Mediterranean Endemic Plants in Marmilla Subregion, Sardinia, Italy
Abstract
:1. Introduction
2. Results
| n° | Family | Taxa | Local Name | Part Used | Preparation | Folk Therapeutic Uses |
|---|---|---|---|---|---|---|
| 1 | Adoxaceae | Sambucus nigra L. | Sammucu | Le; Fl | De | Emollient and decongestant in case of edema |
| 2 | Amaranthaceae | Chenopodium vulvaria L. | Cadoni budésu | AP | Inf | Emmenagogue, anthysteric, antirheumatic |
| 3 | Amaryllidaceae | Narcissus tazetta L. | Narcisu | Le | De | Antispasmodic, sedative, emetocathartic, emmenagogue |
| 4 | Anacardiaceae | Pistacia lentiscus L. | Ollestincu | Re | IU | Painkiller, expectorant [32], intestinal astringent, stomachic, hemostatic |
| 5 | Apiaceae | Bupleurum fruticosum L. | Linna budescia | Le | De | Astringent, vulnerary |
| 6 | Daucus carota L. | Pistinaga | Se | De | Carminative and revitaminizing | |
| 7 | Eryngium campestre L. | Spin’e corra | Rt | De | Diuretic, cholagogue, emmenagogue | |
| 8 | Ferula communis L. | Fèurra | Fl | Co | Antirheumatic | |
| 9 | Foeniculum vulgare Mill. | Fenoghu | Fr; Rt | Inf | Adjuvant for slimming diets [33], expectorant | |
| Fr | IU | Against halitosis and anorexia | ||||
| 10 | Helosciadium crassipes W.D.J.Koch ex Rchb. | Appiu | Le | De | Against bronchial and pharyngeal catarrhs | |
| IU | Antiscorbutic | |||||
| 11 | Oenanthe fistulosa L. | Appiu burdu | Le | Fu | Sedative, expectorant | |
| 12 | Scandix pecten-veneris L. | Erba de agullas | Rt | De | Anti-inflammatory, astringent, eupeptic gastric dyspepsia, cystitis, nephritis, pyelitis | |
| 13 | Thapsia garganica L. | Feruledda | Lt | EU | Vesicatory, against lumbago, ischialgia, and rheumatic or neuralgic affections | |
| 14 | Apocynaceae | Nerium oleander L. | Launaxi | Le | De | Against skin lesion |
| 15 | Araliaceae | Hedera helix L. | Édera | Le | De | Against neuritis and neuralgia of gouty or rheumatic origin |
| 16 | Asparagaceae | Muscari comosum (L.) Mill. | Cibudda de colorus | Bu | De | Emollient |
| 17 | Prospero autumnale (L.) Speta | Lillixeddu | Cas | De | Diuretic, thin broncho-pulmonary secretion, increased arterial tension | |
| 18 | Ruscus aculeatus L. | Spinadoppis | AP | Inf | Diuretic | |
| 19 | Aspleniaceae | Asplenium onopteris L. | Filixi | AP | De | Expectorant, emollient, adjuvant bronchial affections |
| 20 | Asplenium trichomanes L. | Sfarzi de rana | AP | De | Expectorant emollient, adjuvant bronchial affections | |
| 21 | Asphodelaceae | Asphodelus ramosus L. | Kadrilloni | UP | Ca | Chilblain’s treatment, adsorbent in case of intoxication [24] |
| Fl | DI | |||||
| 22 | Asteraceae | Achillea millefolium L. | Erba de feridas | Fl | Inf | Cholagogue, crubs uterine bleeding relieves hemorrhoids and pulmonary disorders |
| 23 | Artemisia arborescens (Vaill.) L. | Sentsu | Le; Fl | Co | Against pleurisy, bronchitis, headaches | |
| Le | De | Diuretic | ||||
| 24 | Bellis perennis L. | Sittsia | WP | De | Against uterine hemorrhages | |
| Inf | Treatment of pleurisy and upper respiratory tract disease | |||||
| Co | Against bruises, sprains, and boils | |||||
| 25 | Calendula arvensis (Vaill.) L. | Erba de froris | Le | De | Antiphlogistic, astringent, cleansing, diuretic, emmenagogue, emetocathartic, sedative, topic, and sudorific | |
| 26 | Carlina gummifera (L.) Less. | Carducabiddu | Fl | De | Diuretic | |
| 27 | Cichorium intybus L. | Giccoria | Le | De | Laxative | |
| 28 | Cynara cardunculus L. | Cardureu | Le | De | Choleretic, cholagogue [34], diuretic, slightly laxative, stimulates liver functions and is useful in subacute and chronic icteric forms | |
| 29 | Helichrysum italicum (Roth) G.Don subsp. tyrrhenicum (Bacch., Brullo & Giusso) Herrando, J.M.Blanco, L.Sáez Galbany | Alluevògu | WP | Inf | Diaphoretic and pectoral | |
| 30 | Matricaria chamomilla L. | Kamomilla | Fl | De | Lenitive, decongestionant | |
| Inf | Digestive | |||||
| 31 | Senecio vulgaris L. | Coccoininni burdu | WP | De | Astringent and diuretic | |
| 32 | Boraginaceae | Anchusa azurea Mill. | Burraccia aresti | Le | Inf | Diaphoretic [35], expectorant |
| 33 | Borago officinalis L. | Pittsa carroga | Le | Inf; De | Intestinal laxative and purifying agent, diuretic | |
| 34 | Cynoglossum creticum Mill. | Fùndu gràssu | WP | Inf | Astringent, antituberculosis [36] | |
| 35 | Echium plantagineum L. | Erba de bòrcu | WP | De | Astringent, diaphoretic, diuretic, emollient | |
| 36 | Heliotropium europaeum L. | Erba de soli | Fl | Inf | Astringent, vulnerary | |
| 37 | Myosotis ramosissima Rochel | Origa de topi | WP | De | Astringent, ophthalmic, healing | |
| 38 | Brassicaceae | Capsella bursa-pastoris (L.) Medik. | Erba de feminas | AP | Inf | Anti-hemorrhagic, regulator of menstrual flow |
| 39 | Nasturtium officinale R. Br. | Nasturtu | Le | De | Diuretic | |
| IU | Anabolic and antiscorbutic | |||||
| EU | Anti-inflammatory | |||||
| 40 | Sinapis arvensis L. | Masaòccu | Se | De | Diuretic, laxative, eupeptic, rubefacient, stimulating the gastric mucosa | |
| 41 | Teesdalia coronopifolia (J.P.Bergeret) Thell | Cauliteddu | WP | De | Stomachic, antiscorbutic | |
| 42 | Cactaceae | Opuntia ficus- indica (L.) Mill. | Figu morisca | Cl | Co | Soothing, anti-inflammatory, against fissures and mammary inflammations |
| 43 | Caprifoliaceae | Dipsacus ferox Loisel | Cima de pastori | Le | De | Dermatosi desquamative, eczema, folliculitis, urticaria, psoriasis |
| 44 | Caryophyllaceae | Stellaria media (L.) Vill. | Erba de buddas | Le | Inf | Diaphoretic |
| 45 | Cistaceae |
Cistus creticus
L. subsp. eriocephalus (Viv.) Greuter & Burdet | Murdegu arrubiu | Le | Inf | Balsamic and revulsive |
| 46 | Convolvulaceae | Convolvulus arvensis L. | Melamida pitticca | Rt | Inf | Cathartic, drastic purgative [36], cholagogue, against skin affection |
| 47 | Crassulaceae | Sedum sp. pl. | Erba grassa | Le | De | Astringent, emollient, healing in skin ulcers |
| 48 | Umbilicus horizontalis (Guss.) DC. | Calixi de muru | Le | Inf | Diaphoretic, refreshing | |
| 49 | Umbilicus rupestris (Salisb.) Dandy. | Capeddu de muru | Le | Inf | Diaphoretic, refreshing | |
| Co | Against boils, distortion, hematomas, soothing in skin inflammatory states | |||||
| 50 | Cytinaceae | Cytinus hypocistis L. | Cabòne de murdegu | WP | Co | Astringent, tonic, hemostatic |
| 51 | Cytinus ruber Fourr. ex Fritsch. | Kobòne de murdegu | WP | Co | Astringent, tonic, hemostatic | |
| 52 | Dioscoreaceae | Dioscorea communis (L.) Caddick & Wilkin | Agina de margiani | Rt | De | Urinary anti-inflammatory [37], diuretic, emetocathartic, hemolytic, vulnerary |
| 53 | Ericaceae | Arbutus unedo L. | Olidoni | WP | IU | Urinary and intestinal anti-inflammatory |
| 54 | Erica arborea L. | Tuvara | Le | De | Uro-genital disorders | |
| 55 | Euphorbiaceae | Euphorbia helioscopia L. | Lua burda | Lt | IU | Strongly emetic and laxative |
| 56 | Fagaceae | Quercus ilex L. | Ilixi | Se | IU | Coffee substitute |
| 57 | Quercus pubescens Willd. | Orròli | Se | De | Against dysentery, gastralgia, eutrophic in lymphatic and tuberculous disease | |
| 58 | Gentianaceae | Blackstonia perfoliata (L.) Huds. | Centàurea groga | WP | Inf | Biter, stomachic |
| 59 | Centaurium maritimum (L.) Fritsch | Brundedda | WP | Inf | Digestive, cleansing, and healing | |
| 60 | Geraniaceae |
Geranium purpureum
Vill. | Erba de agullas | Le | De | Against infections and inflammation of the oral cavity |
| IU | Tuberculosis | |||||
| Po | Against brush | |||||
| 61 | Geranium robertianum L. | Erba de agullas | Le | De | Against infections and inflammation of the oral cavity | |
| IU | Tuberculosis | |||||
| Po | Against brush | |||||
| 62 | Hypericaceae | Hypericum perforatum L. | Erba de S. Giuanni | Re | -- | Against ulcerations and burns |
| Fl | Inf | Abdominal pain, bronchial and urinary inflammation | ||||
| 63 | Iridaceae | Crocus sativus L. | Zafanau | Fl | Inf | Antispasmodic [38], carminative, stimulant, emmenagogue, expectorant |
| 64 | Limniris pseudacorus (L.) Fuss. | Lillu grogu de arriu | Rt | De | Emetocathartic, epidermal astringent, and hemostatic | |
| 65 | Juncaceae | Luzula forsteri (Sm.) DC. | Erba lutza | Rt | De | Against gallstones |
| 66 | Lamiaceae | Ballota nigra L. | Marrupiu nieddu | Le | Inf | Antispasmodic, sedative, vermifuge |
| 67 | Clinopodium vulgare L. | Le | De | Carminative, stimulating, emmenagogue | ||
| 68 | Lavandula stoechas L. | Abioi | Le | De | Antiseptic [39], antispasmodic, diuretic, digestive system stimulant | |
| Ca | Against dislocations, wounds, sores, and ulcers | |||||
| 69 | Marrubium vulgare L. | Marrupiu | Fl | Inf | Tonic, intestinal purifier, weak action cardiac activity, thins the mucous secretions of the airways | |
| 70 | Melissa officinalis L. | Ment’i àbis | WP | De | Antispasmodic [40], choleretic, stimulating and tonic | |
| 71 | Mentha pulegium L. | Amenta | Le | Inf | Aromatic and refreshing, bechico, bronchodilator, against digestive system disorders | |
| 72 | Origanum vulgare L. | Origanu | Le; Fr | Co | Analgesic | |
| Le | IU | Dental pain reliever | ||||
| 73 | Prunella laciniata L. | Brunella | Le; Rt | Inf | Astringent, against respiratory and gastrointestinal diseases | |
| 74 | Salvia rosmarinus Schleid. | Spiccu | Le; | Inf | Carminative and digestive, hepato-stimulating, antiseptic and intestinal antispasmodic, against asthma and bronchitis | |
| 75 | Salvia sp. pl. | Salvia | Le | IU | Antiseptic, odontalgic, and against halitosis | |
| 76 | Stachys glutinosa L. | Scova de argolas | Le | De | Cholagogue, diuretic, hepatoprotective | |
| 77 | Teucrium chamaedrys L. | Camedriu | AP | De | Antipyretic, astringent, tonic | |
| 78 | Lauraceae | Laurus nobilis L. | Lauru | Le | De; Inf | Antiseptic, stimulant, febrifuge, sedative colic spasms |
| 79 | Leguminosae | Anagyris foetida L. | Tilibba | Le | Inf | Mild laxative, emetic emmenagogue |
| 80 | Ceratonia siliqua L. | Karrubba | Co | Against cough and tonsillitis | ||
| Se | De; Inf | For emollient baths | ||||
| AP | Inf | Anti-inflammatory of the throat and digestive system, astringent, antibacterial | ||||
| 81 | Lotus corniculatus L. | Truvulleddu | Fl | Inf | Sedative for anxiety, insomnia, depression, and tachycardia | |
| 82 | Lupinus gussoneaus J.Agardh. | Lupinu | Se | Inf | Vermifuge and hypoglycemic [41] | |
| 83 | Ononis spinosa L. subsp. antiquorum (L.) Arcang. | Stasibois | Rt | De | Increased diuresis, decreased inflammatory processes | |
| 84 | Linaceae | Linum usitatissimum L. subsp. angustifolium (Huds.) Thell. | Linu | Se | Ca | Revulsive in inflammations of the respiratory system |
| 85 | Lythraceae | Punica granatum L. | Arenada | Le | De | Antifungal and reduces sweating, antiviral [42,43] |
| 86 | Malvaceae | Malva sylvestris L. | Narbedda | Le | De | Against boils, chilblains |
| IU | Bronchial renal and intestinal anti-inflammatory, suppurative lesions, fistulas | |||||
| Co | Against boils, chilblains, suppurative lesions, fistulas, and hemorrhoids | |||||
| WP | Fu | Processes inflammatory in the throat | ||||
| 87 | Molluginaceae | Corrigiola litoralis L. | Le; Fl | De | Diuretic | |
| 88 | Moraceae | Ficus carica L. | Figu | Lt | EU | Eradication wart |
| 89 | Myrtaceae | Eucalyptus sp. pl. Dehn. | Occallitu | Le | Co | Stimulant for external and internal use |
| 90 | Myrtus communis L. | Mirtu | Le | De | Balsamic, expectorant and diuretic | |
| Fr | IU | Digestive and aperitif | ||||
| 91 | Oleaceae | Olea europaea L. | Ollastu | Le | De | In cases of biliary lithiasis |
| 92 | Phillyrea latifolia L. | Arrideli | Le | De | Diuretic, tonic astringent | |
| 93 | Onagraceae | Epilobium hirsutum L. | Frori de acqua | Rt | De | Preparation of astringent mouthwashes against mouth ulcers |
| 94 | Orchidaceae | Ophrys apifera Hud. | Orchidea aresti | Bu | De | Anti-inflammatory gastrointestinal, against childhood diarrhea, cystitis, and nephritis |
| 95 | Papaveraceae | Fumaria capreolata L. | Fumària bianca | WP | De | Bitter, diaphoretic, purifying, stimulating the secretions of the digestive system |
| 96 | Papaver rhoeas L. | Babbaòi | Fl | Inf | Bechic, diaphoretic, broncho sedative and narcotic-sedative | |
| 97 | Pinaceae | Pinus sp. pl. | Oppinu | Le | De | Colds and joint pains |
| 98 | Plantaginaceae | Linaria pelisseriana (L.) Mill. | Angulias | Fl; Le | Inf | Against angiocholitis with jaundice, intestinal atony, urinary tract disorders |
| 99 | Plantago coronopus L. | Erba sterria | WP | Sy; De | Astringent, blood coagulant | |
| 100 | Veronica anagallis-aquatica L. | Murutzu aresti | WP | IU | Diuretic, antiscorbutic, purifying | |
| 101 | Poaceae | Avena barbata Pott. Ex Link. | Enargu | AP | De | Emollient in bronchial inflammatory processes |
| Le; Rt | Inf | Diuretic | ||||
| 102 | Cynodon dactylon (L.) Pers. | Cannajoni | WP | De | Anti-inflammatory of the digestive tract and the uro-genital system | |
| 103 | Lolium rigidium Gaudin | Allorgu | Le | De | Antineuralgic, astringent, sedative | |
| 104 | Polygonaceae | Polygonum aviculare L. | Erba de zentu nùus | AP | Inf | Astringent in case of internal bleeding, mild laxative, and blood purifier |
| 105 | Rumex crispus L. | Melagra | Le | De | Astringent for boils, abscesses, myalgias, sprains | |
| 106 | Primulaceae | Lysimachia arvensis (L.) | Erba de puddas | Le | De | Expectorant [44], diaphoretic, diuretic, cholagogue |
| Ca | Against sores, ulcers, skin affection [11] | |||||
| 107 | Lysimachia foemina (Mill.) U.Manns & Anderb. | Erba de puddas | Le | De | Expectorant, diaphoretic, diuretic, cholagogue | |
| Co | Against sores, ulcers, skin affections | |||||
| 108 | Pteridaceae | Adiantum capillus-veneris L. | Fartsia | AP | De | Expectorant, emollient, adjuvant bronchial affections |
| 109 | Ranunculaceae | Anemone hortensis L. | Anemoni | Le | Inf | Rubefacient vesicatory, against skin rashes, joint rheumatism, sciatica |
| 110 | Clematis flammula L. | Bintisillu | Le | Inf | Rubefacient, vesicatory, against rheumatism, gout | |
| 111 | Clematis vitalba L. | Pipiringiu | Rt; AP | TU | Analgesic for diseased teeth or horns of animals | |
| 112 | Ficaria verna Huds. | Landiri de terra | Rt | De | Analgesic, anti-hemorrhoidal, hemostatic | |
| 113 | Ranunculus macrophyllus Desf. | Cadedda | Le | Ca | Revulsive and rubefacient against rheumatic forms, in arthrosis and sciatica | |
| 114 | Resedaceae | Reseda luteola L. | Erba de gallu | Le | Inf | Diaphoretic, diuretic, stomachic |
| 115 | Rhamnaceae | Ziziphus jujuba Mill. | Isaba | Fr | De | Respiratory tract anti-inflammatory |
| 116 | Rosaceae | Agrimonia eupatoria L. | Erba mela | Le | De | Astringent, mouthwash, against inflammation of the digestive system and against liver and kidney disorders |
| 117 | Crataegus laevigata (Poir.) DC. | Travigu | Le; Fl; Fr | De | Vasodilator, hypotensive, antiarrhythmic, sedative [45] | |
| 118 | Crataegus monogyna Jacq. | Soarviu | Fl | De | Against hypertension, cardiac, cardiac neurosis and angina pectoris, antispasmodic ad against anxiety and insomnia | |
| Ba | De | febrifuge | ||||
| 119 | Potentilla reptans L. | Erba de cincu follas | Le | Inf | Astringent, stomachic, antiscorbutic, febrifuge | |
| 120 | Poterium sanguisorba L. | Pimpinella | WP | Inf | Astringent, and against acute and chronic intestinal diseases | |
| 121 | Prunus spinosa L. | Prunizedda | Fl | De | Laxative and diuretic, intestinal astringent | |
| Ba | Inf | Intestinal astringent | ||||
| 122 | Rosa canina L. | Arrosa burda | Le | Inf | Analgesic | |
| Fl | De | Astringent, tonic [46], ophthalmic | ||||
| Fr | Inf | Against urinary tract diseases, and in cases of diabetes | ||||
| 123 | Rubus ulmifolius Schott. | Arrù | Fr | De | Refreshing and light laxative | |
| Le | De | Mouthwash preparations for astringent and anti-inflammatory gargling | ||||
| 124 | Rubiaceae | Galium aparine L. | Appiciga | WP | Inf | Antispasmodic, slightly diuretic, astringent, against digestive system disorders and skin disease |
| 125 | Rutaceae | Ruta chalepensis L. | Arruda | Le | IU; Oil | Against odontalgia, oral cavity infections |
| 126 | Scrophulariaceae | Scrophularia trifoliata L. | Suisùi | Le | Inf | Emetic, purgative [47], against the manifestations of Grave’s disease and related cardiac disorders |
| 127 | Verbascum creticum (L.) Kuntze | Cadumbu | Le | De | Emollient, decongestant, anti-inflammatory of the intestinal mucosa | |
| 128 | Smilacaceae | Smilax aspera L. | Tittione | Le | De | Diaphoretic [25,48] |
| 129 | Solanaceae | Hyoscyamus niger L. | Nasturru | Le; Se | De | Against trigeminal neuralgia, attenuation of senile tremor in Parkinson’s disease, antispasmodic, local anesthetic and analgesic [49] |
| 130 | Solanum nigrum L. | Margaridraza | Le | Inf | Anti-inflammatory [50], emetocathartic, spasmolytic, against skin affections and analgesic | |
| 131 | Tamaricaceae | Tamarix africana Poir. | Tramattsu | Ba | De | Astringent, diaphoretic |
| 132 | Thymelaeaceae | Daphne gnidium L. | Truiscu | Le | Inf | Diaphoretic, emetocathartic, rubefacient, vesicatory [51] |
| 133 | Thymelaea hirsuta (L.) Endl. | Scova de forru | Fl | Inf | Rhinitis and asthma [52] | |
| 134 | Ulmaceae | Ulmus minor Mill. | Ulumu | Le; Ba | De | Intestinal astringent [24] |
| 135 | Urticaceae | Urtica dioica L. | Occiau | Le | De; Inf | Astringent [53], hemostatic, hypoglycemic, depurative, diuretic, against headaches and digestive and heart problems |
| Co | Pain reliever | |||||
| 136 | Urtica pilulifera L. | Occiau femina | Le | De | Astringent, hemostatic, hypoglycemic, urtication for revulsive purposes in cases of paralysis and joint rheumatism | |
| 137 | Violaceae | Viola alba Besser subsp. dehnhardtii (Ten.) W.Becker | Violedda | Le | De | Emollient, expectorant |
3. Discussion
4. Material and Methods
4.1. Historical and Ethnographic Background
4.2. Environmental Background
4.3. Data Collection
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2003, 31, 343–366. [Google Scholar] [CrossRef] [Green Version]
- D’Odorico, P.; Laio, F.; Ridolfi, L.; Lerdau, M.T. Biodiversity enhancement induced by environmental noise. J. Theor. Biol. 2008, 255, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Mills, M.M.; Arrigo, K.R.; Berman-Frank, I.; Bopp, L.; Boyd, P.W.; Galbraith, E.D.; Geider, R.J.; Guieu, C.; Jaccard, S.L.; et al. Processes and patterns of cceanic nutrient limitation. Nat. Geosci. 2013, 6, 701–710. [Google Scholar] [CrossRef] [Green Version]
- Zaman, W.; Ahmad, M.; Zafar, M.; Amina, H.; Lubna; Saqib, S.; Ullah, F.; Ayaz, A.; Bahadur, S.; Park, S. Diversity of medicinal plants used as male contraceptives: An initiative towards herbal contraceptives. Indian J. Tradit. Knowl. (IJTK) 2022, 21, 616–624. [Google Scholar] [CrossRef]
- Potterat, O.; Hamburger, M. Drug discovery and development with plant-derived compounds. Prog. Drug Res. 2008, 65, 45–118. [Google Scholar] [CrossRef] [PubMed]
- Lautié, E.; Russo, O.; Ducrot, P.; Boutin, J.A. Unraveling plant natural chemical diversity for drug discovery Purposes. Front. Pharmacol. 2020, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Usher, P.J. Traditional ecological knowledge in environmental assessment and management. Arctic 2000, 53, 183–193. [Google Scholar] [CrossRef]
- Biró, É.; Babai, D.; Bódis, J.; Molnár, Z. Lack of knowledge or loss of knowledge? Traditional ecological knowledge of population dynamics of threatened plant species in east-central Europe. J. Nat. Conserv. 2014, 22, 318–325. [Google Scholar] [CrossRef]
- Abidullah, S.; Rauf, A.; Zaman, W.; Ullah, F.; Ayaz, A.; Batool, F.; Saqib, S. Consumption of wild food plants among tribal communities of Pak-Afghan border, near Bajaur, Pakistan. Acta Ecol. Sin. 2021; in press. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Lopez-Alvarado, J.; Farris, E. Ecology and evolution of plants in the Mediterranean basin: Perspectives and challenges. Plants 2022, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D. Plant Evolution in the Mediterranean Insights for Conservation, 2nd ed.; Oxford University Press: Oxford, UK, 2020; ISBN 9780198835158. [Google Scholar]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Bacchetta, G.; Fenu, G.; Mattana, E. A checklist of the exclusive vascular flora of Sardinia with priority rankings for conservation. Anales del Jardin Botanico de Madrid 2012, 69, 81–89. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Lazari, D.; Maloupa, E. Sustainable use of mediterranean medicinal-aromatic plants. In Feed Additives: Aromatic Plants and Herbs in Animal Nutrition and Health; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2020; pp. 57–74. ISBN 9780128147016. [Google Scholar]
- Cappadone, C.; Mandrone, M.; Chiocchio, I.; Sanna, C.; Malucelli, E.; Bassi, V.; Picone, G.; Poli, F. Antitumor potential and phytochemical profile of plants from Sardinia (Italy), a hotspot for biodiversity in the Mediterranean basin. Plants 2020, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atzeni, C.; Mocci, S. Paesaggi rurali in Sardegna: “Interferenze progettuali nella regione storica della Marmilla”; University Press: Firenze, Italy, 2017; Volume 5, pp. 117–124. [Google Scholar]
- Puggioni, G.; Atzeni, F. Comuni in estinzione gli scenari dello spopolamento in Sardegna; Centro Regionale di Programmazione: Cagliari, Italy, 2013. [Google Scholar]
- Casazza, G.; Giordani, P.; Benesperi, R.; Foggi, B.; Viciani, D.; Filigheddu, R.; Farris, E.; Bagella, S.; Pisanu, S.; Mariotti, M.G. Climate change hastens the urgency of conservation for range-restricted plant species in the central-northern Mediterranean region. Biol. Conserv. 2014, 179, 129–138. [Google Scholar] [CrossRef]
- Loi, M.C.; Maxia, L.; Maxia, A. Ethnobotanical comparison between the villages of Escolca and Lotzorai (Sardinia, Italy). J. Herbs Spices E Med. Plants 2008, 11, 67–84. [Google Scholar] [CrossRef]
- Ballero, M.; Floris, R.; Poli, F. Le piante utilizzate nella medicina popolare nel territorio di Laconi (Sardegna Centrale). Boll. Soc. Sarda Sci. Nat. 1997, 31, 207–229. [Google Scholar]
- Ballero, M.; Floris, R.; Poli, F. Plants in folk medicine in the territory of Perdasdefogu (Central Sardinia, Italy). Boll. Della Soc. À Sarda Di Sci. Nat. 1997, XXXI, 207–229. [Google Scholar]
- Maxia, A.; Lancioni, M.C.; Balia, A.N.; Alborghetti, R.; Pieroni, A.; Loi, M.C. Medical ethnobotany of the Tabarkins, a northern Italian (Ligurian) minority in South-Western Sardinia. Genet. Resour. Crop. Evol. 2008, 55, 911–924. [Google Scholar] [CrossRef]
- Palmese, M.T.; Uncini Manganelli, R.E.; Tomei, P.E. An ethno-pharmacobotanical survey in the Sarrabus district (South-East Sardinia). Fitoterapia 2001, 72, 619–643. [Google Scholar] [CrossRef]
- Bruni, A.; Ballero, M.; Poli, F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J. Ethnopharmacol. 1997, 57, 97–124. [Google Scholar] [CrossRef]
- Ballero, M.; Floris, R.; Sacchetti, G.; Poli, F. Ricerche etnobotaniche nel comune di Ussassai (Sardegna Centro-Orientale). Atti Soc. Tosc. Sci. Nat. Mem. Ser. B 1998, 105, 83–87. [Google Scholar]
- Ballero, M.; Manas, G.; Poli, F.; Bruni, A. Le piante utilizzate nella medicina popolare nel comune di Arzana (Sardegna Orientale). Plant Biosyst. 2009, 128, 442. [Google Scholar] [CrossRef]
- Poulain, M.; Pes, G.M.; Grasland, C.; Carru, C.; Ferrucci, L.; Baggio, G.; Franceschi, C.; Deiana, L. Identification of a geographic area characterized by extreme longevity in the Sardinia Island: The AKEA Study. Exp. Gerontol. 2004, 39, 1423–1429. [Google Scholar] [CrossRef]
- Poulain, M.; Herm, A.; Pes, G. The blue zones: Areas of exceptional longevity around the world. Vienna Yearb. Popul. Res. 2013, 11, 87–108. [Google Scholar] [CrossRef]
- Farris, E.; Carta, M.; Circosta, S.; Falchi, S.; Papuga, G.; de Lange, P. The indigenous vascular flora of the forest domain of Anela (Sardinia, Italy). PhytoKeys 2018, 113, 97–143. [Google Scholar] [CrossRef]
- Fois, M.; Farris, E.; Calvia, G.; Campus, G.; Fenu, G.; Porceddu, M.; Bacchetta, G. The endemic vascular flora of Sardinia: A dynamic checklist with an overview of biogeography and conservation Status. Plants 2022, 11, 601. [Google Scholar] [CrossRef]
- Axiotis, E.; Halabalaki, M.; Skaltsounis, L.A. An ethnobotanical study of medicinal plants in the Greek islands of north Aegean region. Front. Pharmacol. 2018, 9, 409. [Google Scholar] [CrossRef]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed. Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [Green Version]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Zengin, G.; Mahomoodally, M.F.; Leporini, L.; Locatelli, M.; Cacciagrano, F.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Multiple pharmacological approaches on hydroalcoholic extracts from different parts of Cynoglossum creticum Mill. (Boraginaceae). Plant Biosyst. 2019, 153, 633–639. [Google Scholar] [CrossRef]
- Esmail Al-snafi, A. The pharmacology of Anchusa italica and Anchusa strigosa. A review. Int. J. Pharm. Pharm. Sci. 2014, 6, 7–10. [Google Scholar] [CrossRef]
- Amraoui, N.; Mayouf, N.; Charef, N.; Baghiani, A.; Arrar, L. Antioxidant, anti-inflammatory and anti-arthritic activities of methanol extract of Tamus communis L. roots. Trop. J. Pharm. Res. 2019, 18, 1499–1506. [Google Scholar] [CrossRef]
- Mohtashami, L.; Amiri, M.S.; Ramezani, M.; Emami, S.A.; Simal-Gandara, J. The Genus Crocus L.: A review of ethnobotanical uses, phytochemistry and pharmacology. Ind. Crops Prod. 2021, 171, 113923. [Google Scholar] [CrossRef]
- Bousta, D.; Farah, A. A Phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytosci. 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Bounihi, A.; Hajjaj, G.; Alnamer, R.; Cherrah, Y.; Zellou, A. In vivo potential anti-inflammatory activity of Melissa officinalis L. Essential Oil. Adv. Pharmacol. Sci. 2013, 2013, 101759. [Google Scholar] [CrossRef] [Green Version]
- Cabello-Hurtado, F.; Keller, J.; Ley, J.; Sanchez-Lucas, R.; Jorrín-Novo, J.V.; Aïnouche, A. Proteomics for exploiting diversity of Lupin seed storage proteins and their use as nutraceuticals for health and welfare. J. Proteom. 2016, 143, 57–68. [Google Scholar] [CrossRef]
- Sanna, C.; Marengo, A.; Acquadro, S.; Caredda, A.; Lai, R.; Corona, A.; Tramontano, E.; Rubiolo, P.; Esposito, F.; Ferreira, U. In vitro anti-HIV-1 reverse transcriptase and integrase properties of Punica granatum L. leaves, bark, and peel extracts and their main compounds. Plants 2021, 10, 2124. [Google Scholar] [CrossRef]
- Acquadro, S.; Civra, A.; Cagliero, C.; Marengo, A.; Rittà, M.; Francese, R.; Sanna, C.; Bertea, C.; Sgorbini, B.; Lembo, D.; et al. Punica granatum leaf ethanolic extract and ellagic acid as inhibitors of Zika virus infection. Planta Med. 2020, 86, 1363–1374. [Google Scholar] [CrossRef]
- Hussain, K.; Shahazad, A.; Zia-Ul-Hussnain, S. An Ethnobotanical survey of important wild medicinal plants of Hattar district Haripur, Pakistan. Ethnobot. Leafl. 2008, 12, 29–35. [Google Scholar]
- Verma, S.K.; Jain, V.; Verma, D.; Khamesra, A. Crataegus oxyacantha—A cardioprotective herb. J. Herb. Med. Toxicol. 2007, 1, 65–71. [Google Scholar]
- Šavikin, K.; Zdunić, G.; Menković, N.; Živković, J.; Ćujić, N.; Tereščenko, M.; Bigović, D. Ethnobotanical study on traditional use of medicinal plants in south-western Serbia, Zlatibor District. J. Ethnopharmacol. 2013, 146, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Maxia, A.; Fenu, G.; Loi, M.C. So Uncommon and so singular, but underexplored: An updated overview on ethnobotanical uses, biological properties and phytoconstituents of Sardinian endemic plants. Plants 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Cervellati, R.; Loi, M.C.; Innocenti, G. Evaluation of in vitro antioxidant properties of some traditional Sardinian medicinal plants: Investigation of the high antioxidant aapacity of Rubus ulmifolius. Food Chem. 2008, 106, 745–749. [Google Scholar] [CrossRef]
- Esmail Al-Snafi, A.; Author, C. Therapeutic importance of Hyoscyamus species grown in Iraq (Hyoscyamus albus, Hyoscyamus niger and Hyoscyamus reticulates)—A Review. J. Pharm. 2018, 8, 18–32. [Google Scholar]
- Amiruddin, Z.; Kumar Gopalan, H.; Zainal, H.; Hidayu, N.; Pojan, M.; Atiqah, N.; Aris, A.; Roslan Sulaiman, M. Antinociceptive, anti-inflammatory and antipyretic effects of Solanum nigrum chloroform extract in animal models. Yakugaku Zasshi 2006, 126, 1171–1178. [Google Scholar] [CrossRef]
- Moshiashvili, G.; Tabatadze, N.; Mshvildadze, V. The Genus Daphne: A review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2020, 143, 104540. [Google Scholar] [CrossRef]
- Marmouzi, I.; Bouchmaa, N.; Kharbach, M.; Ezzat, S.M.; Merghany, R.M.; Berkiks, I.; el Jemli, M. Thymelaea genus: Ethnopharmacology, chemodiversity, and bioactivities. S. Afr. J. Bot. 2021, 142, 175–192. [Google Scholar] [CrossRef]
- Ummara, U.; Bokhari, T.Z.; Altaf, A.; Younis, U.; Dasti, A.A. Pharmacological study of Shogran valley flora, Pakistan. Int. J. Sci. Eng. Res. 2013, 4, 1–9. [Google Scholar]
- Atanu, F.O.; Ebiloma, U.G.; Ajayi, E.I. A review of the pharmacological aspects of Solanum nigrum Linn. Biotechnol. Mol. Biol. Rev. 2011, 6, 1–7. [Google Scholar]
- Medical Economics Company. PDR for Herbal Medicines; Medical Economics: Cranbury, NJ, USA, 2000; ISBN 1563633612. [Google Scholar]
- Saxena, S.; Rao, P.B. Pharmacological and phytochemical assessment of Anagallis arvensis L. leaf extracts. Asian J. Chem. 2021, 33, 1831–1841. [Google Scholar] [CrossRef]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Khouchlaa, A.; El Menyiy, N.; Guaouguaou, F.E.; El Baaboua, A.; Charfi, S.; Lakhdar, F.; El Omari, N.; Taha, D.; Shariati, M.A.; Rebezov, M.; et al. Ethnomedicinal use, phytochemistry, pharmacology, and toxicology of Daphne gnidium: A Review. J. Ethnopharmacol. 2021, 275, 114124. [Google Scholar] [CrossRef] [PubMed]
- Serrano, R. Toxic plants: Knowledge, medicinal uses and potential human health risks. Environ. Ecol. Res. 2018, 6, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Duke, S.O.; Cantrell, C.L.; Meepagala, K.M.; Wedge, D.E.; Tabanca, N.; Schrader, K.K. Natural toxins for use in pest management. Toxins 2010, 2, 1943–1962. [Google Scholar] [CrossRef] [Green Version]
- Sidore, C.; Busonero, F.; Maschio, A.; Porcu, E.; Naitza, S.; Zoledziewska, M.; Mulas, A.; Pistis, G.; Steri, M.; Danjou, F.; et al. Genome sequencing elucidates Sardinian genetic architecture and augments association analyses for lipid and blood inflammatory markers. Nat. Genet. 2015, 47, 1272–1281. [Google Scholar] [CrossRef] [Green Version]
- Shapira, Y.; Agmon-Levin, N.; Shoenfeld, Y. Defining and analyzing geoepidemiology and human autoimmunity. J. Autoimmun. 2010, 34, J168–J177. [Google Scholar] [CrossRef]
- Orrù, V.; Steri, M.; Sidore, C.; Marongiu, M.; Serra, V.; Olla, S.; Sole, G.; Lai, S.; Dei, M.; Mulas, A.; et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat. Genet. 2020, 52, 1036–1045. [Google Scholar] [CrossRef]
- Orrù, V.; Steri, M.; Sole, G.; Sidore, C.; Virdis, F.; Dei, M.; Lai, S.; Zoledziewska, M.; Busonero, F.; Mulas, A.; et al. Genetic variants regulating immune cell levels in health and disease. Cell 2013, 155, 242. [Google Scholar] [CrossRef] [Green Version]
- Lancioni, M.C.; Ballero, M.; Mura, L.; Maxia, A. Usi alimentari e terapeutici nella tradizione popolare del Goceano (Sardegna Centrale). Atti Soc. Toscana Sci. Nat. Mem. Ser. B 2007, 114, 45–56. [Google Scholar]
- Mattalia, G.; Sõukand, R.; Corvo, P.; Pieroni, A. Wild food thistle gathering and pastoralism: An inextricable link in the biocultural landscape of Barbagia, central Sardinia (Italy). Sustainability 2020, 12, 5105. [Google Scholar] [CrossRef]
- Sanna, C.; Ballero, M.; Maxia, A. Le piante medicinali utilizzate contro le patologie epidermiche in Ogliastra (Sardegna centro-orientale). Atti Soc. Toscana Sci. Nat. Mem. Ser. B 2006, 113, 73–82. [Google Scholar]
- Tomassini, L.; Foddai, S.; Nicoletti, M. Iridoids from Dipsacus Ferox (Dipsacaceae). Biochem. Syst. Ecol. 2004, 32, 1083–1085. [Google Scholar] [CrossRef]
- Chun, J.M.; Lee, A.Y.; Nam, J.Y.; Lim, K.S.; Choe, M.S.; Lee, M.Y.; Kim, C.; Kim, J.S. Effects of Dipsacus asperoides extract on monosodium iodoacetate–induced osteoarthritis in rats based on gene expression profiling. Front. Pharmacol. 2021, 12, 293. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Chen, L.; Yan, J. Traditional Uses, Processing methods, phytochemistry, pharmacology and quality control of Dipsacus asper Wall. Ex C.B. Clarke: A review. J. Ethnopharmacol. 2020, 258, 112912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Shi, Y.P. Phytochemicals and biological activities of Dipsacus species. Chem. Biodivers 2011, 8, 414–430. [Google Scholar] [CrossRef]
- Atzei, A.D. Le Piante Nella Tradizione Popolare Della Sardegna; Carlo Delfino Editore: Sassari, Italy, 2003. [Google Scholar]
- Piozzi, F.; Bruno, M. Diterpenoids in the essential oils from the genus Stachys. Nat. Prod. 2009, 3, 120–125. [Google Scholar]
- Serrilli, A.M.; Ramunno, A.; Piccioni, F.; Serafini, M.; Ballero, M.; Bianco, A. Monoterpenoids from Stachys glutinosa L. Nat. Prod. Res. 2006, 20, 648–652. [Google Scholar] [CrossRef]
- Ruiu, S.; Anzani, N.; Orrü, A.; Floris, C.; Caboni, P.; Alcaro, S.; MacCioni, E.; Distinto, S.; Cottiglia, F. Methoxyflavones from Stachys glutinosa with binding affinity to opioid receptors: In silico, in vitro, and in vivo studies. J. Nat. Prod. 2015, 78, 69–76. [Google Scholar] [CrossRef]
- Pintore, G.; Chessa, M.; Manconi, P.; Zanetti, S.; Deriu, A.; Tirillini, B. Chemical composition and antimicrobial activities of essential oil of Stachys glutinosa from Sardinia. Nat. Prod. Commun. 2006, 1, 1133–1136. [Google Scholar] [CrossRef]
- Guzzo, F.; Russo, R.; Sanna, C.; Celaj, O.; Caredda, A.; Corona, A.; Tramontano, E.; Fiorentino, A.; Esposito, F.; D’abrosca, B. Chemical characterization and anti-HIV-1 activity assessment of iridoids and flavonols from Scrophularia trifoliata. Molecules 2021, 26, 4777. [Google Scholar] [CrossRef] [PubMed]
- Giner, R.M.; Villalba, M.L.; Recio, M.C.; Máñez, S.; Cerdá-Nicolás, M.; Ríos, J.L. Anti-inflammatory glycoterpenoids from Scrophularia auriculata. Eur. J. Pharmacol. 2000, 389, 243–252. [Google Scholar] [CrossRef]
- Pasdaran, A.; Hamedi, A. The Genus Scrophularia: Source of iridoids and terpenoids with a diverse biological activity. Pharm. Biol. 2017, 55, 2211–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renda, G.; Kadıoğlu, M.; Kılıç, M.; Korkmaz, B.; Kırmızıbekmez, H. Anti-inflammatory secondary metabolites from Scrophularia kotschyana. Hum Exp Toxicol 2021, 40, S676–S683. [Google Scholar] [CrossRef] [PubMed]
- Azadmehr, A.; Afshari, A.; Baradaran, B.; Hajiaghaee, R.; Rezazadeh, S.; Monsef-Esfahani, H. Suppression of nitric oxide production in activated murine peritoneal macrophages in vitro and ex vivo by Scrophularia striata ethanolic extract. J. Ethnopharmacol. 2009, 124, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Azadmehr, A.; Maliji, G.; Hajiaghaee, R.; Shahnazi, M.; Afaghi, A. Inhibition of pro-inflammatory cytokines by ethyl acetate extract of Scrophularia striata. Trop. J. Pharm. Res. 2013, 11, 893–897. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.; Dadmehr, M.; Sharifi, Y.; Manshouri, S.; Kamali, M.; Vahidi Emami, Z.; Rasizadeh, R.; Seif, F. The potential effects of Scrophularia striata Boiss on COVID-19. Immunoregulation 2022, 4, 69–72. [Google Scholar] [CrossRef]
- Ballero, M.; Fresu, I. Le piante di uso officinale nella Barbagia di Seui (Sardegna centrale). Fitoterapia 1993, 64, 141–150. [Google Scholar]
- Ornano, L.; Venditti, A.; Sanna, C.; Ballero, M.; Maggi, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Bianco, A. Chemical composition and biological activity of the essential oil from Helichrysum microphyllum Cambess. Ssp. tyrrhenicum Bacch., Brullo e Giusso growing in La Maddalena Archipelago, Sardinia. J. Oleo Sci. 2015, 64, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Venditti, A.; Lattanzi, C.; Ornano, L.; Maggi, F.; Sanna, C.; Ballero, M.; Alvino, A.; Serafini, M.; Bianco, A. A new glucosidic phthalide from Helichrysum microphyllum subsp. tyrrhenicum from La Maddalena Island (Sardinia, Italy). Nat. Prod. Res. 2016, 30, 789–795. [Google Scholar] [CrossRef]
- Melito, S.; Petretto, G.L.; Podani, J.; Foddai, M.; Maldini, M.; Chessa, M.; Pintore, G. Altitude and climate influence Helichrysum italicum subsp. microphyllum essential oils composition. Ind. Crops Prod. 2016, 80, 242–250. [Google Scholar] [CrossRef]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A.; Ballero, M.; Sterner, O.; Fiebich, B.L.; Munoz, E. Arzanol. An anti-inflammatory and anti-HIV-1 phloroglucinol α-pyrone from Helichrysum italicum Ssp. microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Marchetti, M.; Campagna, P.; Usai, M. Antimicrobial activity and chemical composition of essential oil from Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso collected in South-West Sardinia. Saudi J. Biol. Sci. 2019, 26, 897. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Barra, A.; Arlorio, M.; Coisson, J.D.; Russo, M.T.; Pirisi, F.M.; Satta, M.; Cabras, P. Chemical composition, plant genetic differences, and antifungal activity of the essential oil of Helichrysum italicum G. Don Ssp. microphyllum (Willd) Nym. J. Agric. Food Chem. 2003, 51, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Taglialatela-Scafati, O.; Pollastro, F.; Chianese, G.; Minassi, A.; Gibbons, S.; Arunotayanun, W.; Mabebie, B.; Ballero, M.; Appendino, G. Antimicrobial phenolics and unusual glycerides from Helichrysum Italicum subsp. microphyllum. J. Nat. Prod. 2013, 76, 346–353. [Google Scholar] [CrossRef]
- Allen, D.; Bilz, M.; Leaman, D.J.; Miller, R.M.; Timoshyna, A.; Window, J. European Red List of Medicinal Plants; Publications Office of the European Union: Luxembourg, 2014; p. 63. [Google Scholar] [CrossRef]
- Orsenigo, S.; Fenu, G.; Gargano, D.; Montagnani, C.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Carta, A.; Castello, M.; et al. Red list of threatened vascular plants in Italy. Plant Biosyst. 2021, 155, 310–335. [Google Scholar] [CrossRef]
- Garau, C. Perspectives on cultural and sustainable rural tourism in a smart region: The case study of Marmilla in Sardinia (Italy). Sustainability 2015, 7, 6412. [Google Scholar] [CrossRef] [Green Version]
- Canu, S.; Rosati, L.; Fiori, M.; Motroni, A.; Filigheddu, R.; Farris, E. Bioclimate map of Sardinia (Italy). J. Maps 2015, 11, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Médail, F. Plant biogeography and vegetation patterns of the Mediterranean Islands. Bot. Rev. 2022, 88, 63–129. [Google Scholar] [CrossRef]
- Bacchetta, G.; Bagella, S.; Biondi, E.; Farris, E.; Filigheddu, R.S.; Mossa, L. Vegetazione forestale e serie di vegetazione della Sardegna (con rappresentazione cartografica alla scala 1:350.000). Fitosociologia 2009, 46, 3–82. [Google Scholar]
- Farris, E.; Secchi, Z.; Filigheddu, R.S. Phytosociological study of the shrub and pre-forest communities of the effusive substrata of NW Sardinia. Fitosociologia 2007, 2, 55–81. [Google Scholar]
- Weckerle, C.S.; de Boer, H.J.; Puri, R.K.; van Andel, T.; Bussmann, R.W.; Leonti, M. Recommended standards for conducting and reporting ethnopharmacological field studies. J. Ethnopharmacol. 2018, 210, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M. The relevance of quantitative ethnobotanical indices for ethnopharmacology and ethnobotany. J. Ethnopharmacol. 2022, 288, 115008. [Google Scholar] [CrossRef]
- Pignatti, S. Flora d’Italia, 2nd ed.; Edagricole-New Business Media: Bologna, Italy, 2017. [Google Scholar]







| n° | Family | Taxa | L.F. | Chorological Types |
|---|---|---|---|---|
| 1 | Adoxaceae | Sambucus nigra L. | P caesp | Europ.-Caucas. |
| 2 | Amaranthaceae | Chenopodium vulvaria L. | T scap | Europ. |
| 3 | Amaryllidaceae | Narcissus tazetta L. | G bulb | Steno-Medit. |
| 4 | Anacardiaceae | Pistacia lentiscus L. | P caesp (P scap) | S-Medit.–Macarones. |
| 5 | Apiaceae | Bupleurum fruticosum L. | NP | Steno-Medit. |
| 6 | Daucus carota L. | H bienn (T scap) | Subcosmop. | |
| 7 | Eryngium campestre L. | H scap | Euri-Medit. | |
| 8 | Ferula communis L. | H scap | S-Medit (Euri-) | |
| 9 | Foeniculum vulgare Mill. | H bienn/H scap | Euri-Medit. | |
| 10 | Helosciadium crassipes W.D.J.Koch ex Rchb. | H rept/ I rad | Steno-Medit. | |
| 11 | Oenanthe fistulosa L. | H scap | Eurasiat. | |
| 12 | Scandix pecten-veneris L. | T scap | Subcosmop. | |
| 13 | Thapsia garganica L. | H scap | Steno-Medit. | |
| 14 | Apocynaceae | Nerium oleander L. | P caesp (P scap) | S-Medit. |
| 15 | Araliaceae | Hedera helix L. | P lian | Submedit.–Subatl. |
| 16 | Asparagaceae | Muscari comosum (L.) Mill. | G bulb | Euri-Medit. |
| 17 | Prospero autumnale (L.) Speta | G bulb | Euri-Medit. | |
| 18 | Ruscus aculeatus L. | G rhiz/Ch frut | Euri-Medit. | |
| 19 | Aspleniaceae | Asplenium onopteris L. | H ros | Steno-Medit.-Macarones |
| 20 | Asplenium trichomanes L. | H ros | Cosmop.-Temp | |
| 21 | Asphodelaceae | Asphodelus ramosus L. | G tub | Steno-Medit. |
| 22 | Asteraceae | Achillea millefolium L. | H scap | Euro-Siber. |
| 23 | Artemisia arborescens (Vaill.) L. | NP/P caesp | S-Medit. | |
| 24 | Bellis perennis L. | H ros | Circumbor. | |
| 25 | Calendula arvensis (Vaill.) L. | T scap (H bienn) | Euri-Medit. | |
| 26 | Carlina gummifera (L.) Less. | H ros | S-Medit. | |
| 27 | Cichorium intybus L. | H scap | Cosmop. | |
| 28 | Cynara cardunculus L. | H scap | Steno-Medit. | |
| 29 | Helichrysum italicum (Roth) G.Don subsp. tyrrhenicum (Bacch., Brullo & Giusso) Herrando, J.M.Blanco, L.Sáez Galbany | Ch suffr | Endemism | |
| 30 | Matricaria chamomilla L. | T scap | Subcosmop. | |
| 31 | Senecio vulgaris L. | T scap | Cosmop. | |
| 32 | Boraginaceae | Anchusa azurea Mill. | H scap | Euri-Medit. |
| 33 | Borago officinalis L. | T scap | Euri-Medit. | |
| 34 | Cynoglossum creticum Mill. | H bienn | Euri-Medit. | |
| 35 | Echium plantagineum L. | T scap/H bienn | Euri-Medit. | |
| 36 | Heliotropium europaeum L. | T scap | Euri-Medit.–Turan. | |
| 37 | Myosotis ramosissima Rochel | T scap | Europ.–W-Asiat. | |
| 38 | Brassicaceae | Capsella bursa-pastoris (L.) Medik. | H bienn | Cosmop.(sinantrop.) |
| 39 | Nasturtium officinale R.Br. | H scap | Cosmop. | |
| 40 | Sinapis arvensis L. | T scap | Steno-Medit. | |
| 41 | Teesdalia coronopifolia (J.P.Bergeret) Thell | T scap | Euri-Medit | |
| 42 | Cactaceae | Opuntia ficus- indica (L.) Mill. | P succ | Messico (Neotropic.). |
| 43 | Caprifoliaceae | Dipsacus ferox Loisel | H bienn | Endemism |
| 44 | Caryophyllaceae | Stellaria media (L.) Vill. | T rept/ H bienn | Cosmopol. |
| 45 | Cistaceae | Cistus creticus L. subsp. eriocephalus (Viv.) Greuter & Burdet | NP | Steno-Medit. |
| 46 | Convolvulaceae | Convolvulus arvensis L. | G rhiz | Cosmop. |
| 47 | Crassulaceae | Sedum sp. pl. | ||
| 48 | Umbilicus horizontalis (Guss.) DC. | G bulb | Steno-Medit | |
| 49 | Umbilicus rupestris (Salisb.) Dandy. | G bulb | Steno-Medit | |
| 50 | Cytinaceae | Cytinus hypocistis (L.) L. | G rad | Medit.-Macarones. |
| 51 | Cytinus ruber Fourr. ex Fritsch | G rad | W-Medit | |
| 52 | Dioscoreaceae | Dioscorea communis (L.) Caddick & Wilkin | G rad | Euri-Medit. |
| 53 | Ericaceae | Arbutus unedo L. | P caesp (P scap) | Steno-Medit. |
| 54 | Erica arborea L. | P caesp (NP) | Steno-Medit.-Atlant. | |
| 55 | Euphorbiaceae | Euphorbia helioscopia L. | T scap | Cosmopol. |
| 56 | Fagaceae | Quercus ilex L. | P scap (P caesp.) | Steno–Medit. |
| 57 | Quercus pubescens Willd. | P scap | Europ.-Subpontica) | |
| 58 | Gentianaceae | Blackstonia perfoliata (L.) Huds. | T scap | Euri-Medit. |
| 59 | Centaurium maritimum (L.) Fritsch | T scap | Steno-Medit. | |
| 60 | Geraniaceae | Geranium purpureum Vill. | T scap | Euri-Medit. |
| 61 | Geranium robertianum L. | T scap/ H bienn | Subcosmop. | |
| 62 | Hypericaceae | Hypericum perforatum L. | H scap | Subcosmop. |
| 63 | Iridaceae | Crocus sativus L. | G bulb | W-Asiat. |
| 64 | Limniris pseudacorus (L.) Fuss. | G rhiz | Eurasiat. Temp. | |
| 65 | Juncaceae | Luzula forsteri (Sm.) DC. | H caesp | Euri-Medit. |
| 66 | Lamiaceae | Ballota nigra L. | H scap | Euri-Medit. |
| 67 | Clinopodium vulgare L. | H scap | Circumbor. | |
| 68 | Lavandula stoechas L. | NP | Steno-Medit. | |
| 69 | Marrubium vulgare L. | H scap | Subcosmop. | |
| 70 | Melissa officinalis L. | H scap | Euri-Medit. | |
| 71 | Mentha pulegium L. | H scap | Subcosmop. | |
| 72 | Origanum vulgare L. | H scap | Eurasiat. | |
| 73 | Prunella laciniata (L.) L. | H scap | Euri-Medit | |
| 74 | Salvia rosmarinus Schleid. | NP | Steno-Medit. | |
| 75 | Salvia sp. pl. | --- | --- | |
| 76 | Stachys glutinosa L. | Ch frut | Endemism | |
| 77 | Teucrium chamaedrys L. | Ch suff | Euri-Medit | |
| 78 | Lauraceae | Laurus nobilis L. | P caesp (P scap) | Steno-Medit. |
| 79 | Leguminosae | Anagyris foetida L. | P caesp | S. Medit. |
| 80 | Ceratonia siliqua L. | P caesp/ P scap | S.Medit. | |
| 81 | Lotus corniculatus L. | H scap | Cosmopol. | |
| 82 | Lupinus gussoneaus J.Agardh. | T scap | Steno–Medit. | |
| 83 | Ononis spinosa L. subsp. antiquorum (L.) Arcang. | Ch suffr | Euri-Medit. | |
| 84 | Linaceae | Linum usitatissimum L. subsp. angustifolium (Huds.) Thell. | H bienn/H scap (T scap) | Euri-Medit.-Subatl. |
| 85 | Lythraceae | Punica granatum L. | P scap | SW-Asiat. |
| 86 | Malvaceae | Malva sylvestris L. | H scap (T scap) | Subcosmop. |
| 87 | Molluginaceae | Corrigiola litoralis L. | T scap | Medit.–Atlant. |
| 88 | Moraceae | Ficus carica L. | P scap | Medit.–Turan. |
| 89 | Myrtaceae | Eucalyptus sp. pl. Dehn. | P scap | Australia (coltivate) |
| 90 | Myrtus communis L. | P caesp | Steno-Medit. | |
| 91 | Oleaceae | Olea europaea L. | P caesp/P scap | Steno-Medit. |
| 92 | Phillyrea latifolia L. | P caesp (P scap) | Steno-Medit. | |
| 93 | Onagraceae | Epilobium hirsutum L. | H scap | Subcosmop. |
| 94 | Orchidaceae | Ophrys apifera Huds. | G bulb | Medit.-Atlant. (Euri.) |
| 95 | Papaveraceae | Fumaria capreolata L. | T scap | Euri-Medit. |
| 96 | Papaver rhoeas L. | T scap | E-Medit. | |
| 97 | Pinaceae | Pinus sp. pl. | --- | --- |
| 98 | Plantaginaceae | Linaria pelisseriana (L.) Mill. | T scap | Medit.-Atlant. |
| 99 | Plantago coronopus L. | T scap/H bienn/H ros | Euri-Medit. | |
| 100 | Veronica anagallis-aquatica L. | H scap (T scap) | Cosmop. | |
| 101 | Poaceae | Avena barbata Pott ex Link | T scap | Euri–Medit.–Turan. |
| 102 | Cynodon dactylon (L.) Pers. | G rhiz/H rept | Termo-Cosmop. | |
| 103 | Lolium rigidium Gaudin | T scap. | Paleosubtrop. | |
| 104 | Polygonaceae | Polygonum aviculare L. | T rept | Cosmop. |
| 105 | Rumex crispus L. | H scap | Subcosmop. | |
| 106 | Primulaceae | Lysimachia arvensis (L.) | T rept | Subcosmop. |
| 107 | Lysimachia foemina (Mill.) U.Manns & Anderb. | T rept | Subcosmop. | |
| 108 | Pteridaceae | Adiantum capillus-veneris L. | G rhiz | Pantropic. e -subtropic. |
| 109 | Ranunculaceae | Anemone hortensis L. | G rhiz | S-Medit. |
| 110 | Clematis flammula L. | P lian (H scap) | Euri–Medit. | |
| 111 | Clematis vitalba L. | P lian | Europ.-Caucas. | |
| 112 | Ficaria verna Huds. | G bulb/H scap | Eurasiat. | |
| 113 | Ranunculus macrophyllus Desf. | H scap | SW-Medit. | |
| 114 | Resedaceae | Reseda luteola L. | H scap/T scap | Circumbor |
| 115 | Rhamnaceae | Ziziphus jujuba Mill. | P caesp/P scap | SE-Asiat. |
| 116 | Rosaceae | Agrimonia eupatoria L. | H scap | Subcosmop. |
| 117 | Crataegus laevigata (Poir.) DC. | P caesp (P scap) | Centroeurop. | |
| 118 | Crataegus monogyna Jacq. | P caesp (P scap) | Paleotemp. | |
| 119 | Potentilla reptans L. | H ros | Subcosmop. | |
| 120 | Poterium sanguisorba L. | H scap | Subcosmop. | |
| 121 | Prunus spinosa L. | P caesp | Europ.-Caucas. | |
| 122 | Rosa canina L. | NP | Paleotemp. | |
| 123 | Rubus ulmifolius Schott | P caesp | Euri-Medit. | |
| 124 | Rubiaceae | Galium aparine L. | T scap | Eurasiat. |
| 125 | Rutaceae | Ruta chalepensis L. | Ch suffr | S-Medit. |
| 126 | Scrophulariaceae | Scrophularia trifoliata L. | H scap | Endemism |
| 127 | Verbascum creticum (L.) Kuntze | H bienn | SW-Medit. | |
| 128 | Smilacaceae | Smilax aspera L. | P lian (NP, G rhiz) | Paleosubtrop. |
| 129 | Solanaceae | Hyoscyamus niger L. | T scap/H bienn | Eurasiat. |
| 130 | Solanum nigrum L. | T scap | Cosmop. Sinantrop. | |
| 131 | Tamaricaceae | Tamarix africana Poir. | P scap./caesp | Steno-Medit.-Occid. |
| 132 | Thymelaeaceae | Daphne gnidium L. | P caesp | Steno-Medit.–Macarones. |
| 133 | Thymelaea hirsuta (L.) Endl. | NP/Ch suffr | S-Medit.–W-Asiat. | |
| 134 | Ulmaceae | Ulmus minor Mill. | P caesp./P scap. | Europ.–Caucas. |
| 135 | Urticaceae | Urtica dioica L. | H scap | Subcosmop. |
| 136 | Urtica pilulifera L. | T scap (H bienn) | S-Medit | |
| 137 | Violaceae | Viola alba Besser subsp. dehnhardtii (Ten.) W.Becker | H ros | Euri-Medit. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocco, E.; Maccioni, D.; Sanjust, E.; Falconieri, D.; Farris, E.; Maxia, A. Ethnopharmacobotany and Diversity of Mediterranean Endemic Plants in Marmilla Subregion, Sardinia, Italy. Plants 2022, 11, 3165. https://doi.org/10.3390/plants11223165
Cocco E, Maccioni D, Sanjust E, Falconieri D, Farris E, Maxia A. Ethnopharmacobotany and Diversity of Mediterranean Endemic Plants in Marmilla Subregion, Sardinia, Italy. Plants. 2022; 11(22):3165. https://doi.org/10.3390/plants11223165
Chicago/Turabian StyleCocco, Emma, Delia Maccioni, Enrico Sanjust, Danilo Falconieri, Emmanuele Farris, and Andrea Maxia. 2022. "Ethnopharmacobotany and Diversity of Mediterranean Endemic Plants in Marmilla Subregion, Sardinia, Italy" Plants 11, no. 22: 3165. https://doi.org/10.3390/plants11223165
APA StyleCocco, E., Maccioni, D., Sanjust, E., Falconieri, D., Farris, E., & Maxia, A. (2022). Ethnopharmacobotany and Diversity of Mediterranean Endemic Plants in Marmilla Subregion, Sardinia, Italy. Plants, 11(22), 3165. https://doi.org/10.3390/plants11223165