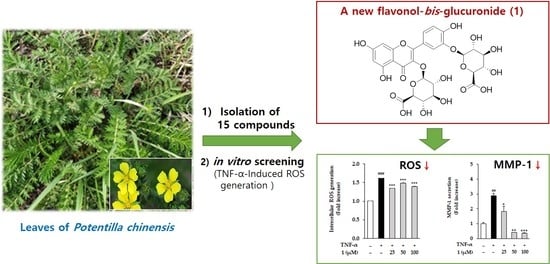
Potentilloside A, a New Flavonol-bis-Glucuronide from the Leaves of Potentilla chinensis, Inhibits TNF-α-Induced ROS Generation and MMP-1 Secretion
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation of 1 and Identification of 2–15
2.2. Effects of LPCE and Isolates 1–15 on HDF Cell Viability
2.3. Effects of LPCE and Isolates 1–15 on TNF-α-Induced ROS Generation
2.4. Effects of LPCE and Flavonol-bis-Glucuronides 1–4 on TNF-α-Induced MMP-1 Secretion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
Potentilloside A (1)
3.4. Acidic Hydrolysis of 1 and Sugar Identification
3.5. Cell Culture Conditions
3.6. Sample Preparations
3.7. Cell Viability
3.8. ROS Generation Assay
3.9. Enzyme-Linked Immunosorbent Assay (ELISA)
3.10. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naveen, K.V.; Kim, H.Y.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.H. Phyto-fabrication of biocompatible silver nanoparticles using Potentilla chinensis Ser leaves: Characterization and evaluation of its antibacterial activity. J. Nanostructure Chem. 2022, 12, 655–667. [Google Scholar] [CrossRef]
- Kang, C.H.; Han, S.H.; So, J.S. Anti-inflammatory effect of chloroform extract from Potentilla chinensis. KSBB J. 2013, 28, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Batool, Z.; Liu, R.; Sui, G.; Sheng, B.; Zheng, X.; Xu, D. Characterization and immunological activity of polysaccharides from Potentilla chinensis. Int. J. Biol. Macromol. 2020, 165, 683–690. [Google Scholar] [CrossRef]
- Tomczyk, M.; Latté, K.P. Potentilla—A review of its phytochemical and pharmacological profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Shen, Y.; Zhang, H.j.; Tang, H.; Lin, H.; Qiu, F. The chemical constituents of Potentilla chinensis. Pharm. Care Res. 2007, 7, 262. [Google Scholar]
- Liu, P.; Duan, H.; Pan, Q.; Zhang, Y.; Yao, Z. Triterpenes from herb of Potentilla chinesis. Zhongguo Zhong Yao Za Zhi 2006, 31, 1875–1879. [Google Scholar]
- Wan, G.; Tao, J.G.; Wang, G.D.; Liu, S.P.; Zhao, H.X.; Liang, Q.D. In vitro antitumor activity of the ethyl acetate extract of Potentilla chinensis in osteosarcoma cancer cells. Mol. Med. Rep. 2016, 14, 3634–3640. [Google Scholar] [CrossRef] [Green Version]
- Han, J.S.; Kim, J.G.; Le, T.P.L.; Cho, Y.B.; Lee, M.K.; Hwang, B.Y. Pentacyclic triterpenes with nitric oxide inhibitory activity from Potentilla chinensis. Bioorg. Chem. 2021, 108, 104659. [Google Scholar] [CrossRef]
- Cui, W.; Sun, C.; Ma, Y.; Wang, S.; Wang, X.; Zhang, Y. Neuroprotective effect of tormentic acid against memory impairment and neuro-inflammation in an Alzheimer’s disease mouse model. Mol. Med. Rep. 2020, 22, 739–750. [Google Scholar] [CrossRef]
- Wei, J.; Huang, Q.; Huang, R.; Chen, Y.; Lv, S.; Wei, L.; Liang, C.; Liang, S.; Zhuo, L.; Lin, X. Asiatic acid from Potentilla chinensis attenuate ethanol-induced hepatic injury via suppression of oxidative stress and Kupffer cell activation. Biol Pharm Bull. 2013, 36, 1980–1989. [Google Scholar] [CrossRef] [Green Version]
- Schinella, G.R.; Tournier, H.A.; Máñez, S.; de Buschiazzo, P.M.; del Carmen Recio, M.; Ríos, J.L. Tiliroside and gnaphaliin inhibit human low density lipoprotein oxidation. Fitoterapia 2007, 78, 1–6. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, Y.; Liu, J.; Jin, M.; Qin, N.; Chen, Y.; Niu, W.; Duan, H. AMPK/AS160 mediates tiliroside derivatives-stimulated GLUT4 translocation in muscle cells. Drug Des. Devel. Ther. 2018, 12, 1581–1587. [Google Scholar] [CrossRef]
- Qiao, W.; Zhao, C.; Qin, N.; Zhai, H.Y.; Duan, H.Q. Identification of trans-tiliroside as active principle with anti-hyperglycemic, anti-hyperlipidemic and antioxidant effects from Potentilla chinesis. J. Ethnopharmacol. 2011, 135, 515–521. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine aspects of skin aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef] [Green Version]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J. Invest. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and function of the skin. In Nanoscience in Dermatology; Academic Press: Cambridge, MA, USA, 2016; pp. 1–14. [Google Scholar]
- Rakshit, M.; Gautam, A.; Toh, L.Z.; Lee, Y.S.; Lai, H.Y.; Wong, T.T.; Ng, K.W. Hydroxyapatite particles induced modulation of collagen expression and secretion in primary human dermal fibroblasts. Int. J. Nanomed. 2020, 15, 4943. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.; Kim, M. Structural and functional changes and possible molecular mechanisms in aged skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Pensalfini, M.; Rotach, M.; Hopf, R.; Bielicki, A.; Santoprete, R.; Mazza, E. How cosmetic tightening products modulate the biomechanics and morphology of human skin. Acta Biomater. 2020, 115, 299–316. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from plants protect against skin photoaging. Oxid. Med. Cell Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef] [Green Version]
- Svobodova, A.; Walterova, D.; Vostalova, J. Ultraviolet light induced alteration to the skin. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech. Repub. 2006, 150, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Phung, H.M.; Lee, S.; Hong, S.; Lee, S.; Jung, K.; Kang, K.S. Protective effect of polymethoxyflavones isolated from Kaempferia parviflora against TNF-α-induced human dermal fibroblast damage. Antioxidants 2021, 10, 1609. [Google Scholar] [CrossRef] [PubMed]
- Steenport, M.; Khan, K.F.; Du, B.; Barnhard, S.E.; Dannenberg, A.J.; Falcone, D.J. Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: Evidence for the role of TNF-α and cyclooxygenase-2. J. Immunol. 2009, 183, 8119–8127. [Google Scholar] [CrossRef] [PubMed]
- Starodubtseva, N.; Sobolev, V.; Soboleva, A.; Nikolaev, A.; Bruskin, S. Genes expression of metalloproteinases (MMP-1, MMP-2, MMP-9, and MMP-12) associated with psoriasis. Russ. J. Genet. 2011, 47, 1117–1123. [Google Scholar] [CrossRef]
- Shin, J.W.; Kwon, S.H.; Choi, J.Y.; Na, J.I.; Huh, C.H.; Choi, H.R.; Park, K.C. Molecular mechanisms of dermal aging and antiaging approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlova, A.A.; Whaley, A.K.; Ponkratova, A.O.; Balabas, O.A.; Smirnov, S.N.; Povydysh, M.N. Two new flavonol-bis-3, 7-glucuronides from Geum rivale L. Phytochem. Lett. 2021, 42, 41–44. [Google Scholar] [CrossRef]
- Harborne, J. Plant polyphenols—XIV.: Characterization of flavonoid glycosides by acidic and enzymic hydrolyses. Phytochemistry 1965, 4, 107–120. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Huang, R.; Zhu, W.; Zeng, X.; Zhao, J.; Feng, Y.; He, R. Simultaneous quantification of 17 bioactive constituents in Sarcandra glabra by liquid chromatography-electrospray ionisation-mass spectrometry. Anal. Methods 2014, 6, 7989–7995. [Google Scholar] [CrossRef]
- Granica, S.; Czerwińska, M.E.; Żyżyńska-Granica, B.; Kiss, A.K. Antioxidant and anti-inflammatory flavonol glucuronides from Polygonum aviculare L. Fitoterapia 2013, 91, 180–188. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, J.Y.; Park, P.S.; Bang, S.H.; Lee, K.M.; Lee, Y.R.; Jang, Y.H.; Kim, M.J.; Chun, W.; Heo, M.Y. Flavonoid glycosides as acetylcholinesterase inhibitors from the whole plants of Persicaria thunbergii. Nat. Prod. Sci. 2014, 20, 191–195. [Google Scholar]
- Cho, J.Y.; Yoon, I.; Jung, D.H.; Hyun, S.H.; Lee, K.H.; Moon, J.H.; Park, K.H. Jaboticabin and flavonoids from the ripened fruit of black rasberry (Rubus coreanum). Food Sci. Biotechnol. 2012, 21, 1081–1086. [Google Scholar] [CrossRef]
- He, D.; Huang, Y.; Ayupbek, A.; Gu, D.; Yang, Y.; Aisa, H.A.; Ito, Y. Separation and purification of flavonoids from black currant leaves by high-speed countercurrent chromatography and preparative HPLC. J. Liq. Chromatogr. Relat. Technol. 2010, 33, 615–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukamoto, S.; Tomise, K.; Aburatani, M.; Onuki, H.; Hirorta, H.; Ishiharajima, E.; Ohta, T. Isolation of cytochrome P450 inhibitors from strawberry fruit, Fragaria ananassa. J. Nat. Prod. 2004, 67, 1839–1841. [Google Scholar] [CrossRef]
- Van der Woude, H.; Boersma, M.G.; Vervoort, J.; Rietjens, I.M. Identification of 14 quercetin phase II mono-and mixed conjugates and their formation by rat and human phase II in vitro model systems. Chem. Res. Toxicol. 2004, 17, 1520–1530. [Google Scholar] [CrossRef]
- Bhatarrai, G.; Seong, S.H.; Jung, H.A.; Choi, J.S. Isolation and quantitative analysis of BACE1 inhibitory compounds from Cirsium maackii flower. Nat. Prod. Sci. 2019, 25, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Bai, N.; He, K.; Roller, M.; Zheng, B.; Chen, X.; Shao, Z.; Peng, T.; Zheng, Q. Active compounds from Lagerstroemia speciosa, insulin-like glucose uptake-stimulatory/inhibitory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Agric. Food Chem. 2008, 56, 11668–11674. [Google Scholar] [CrossRef]
- Tanaka, T.; Nonaka, G.I.; Nishioka, I. Tannins and related compounds. C.: Reaction of dehydrohexahydroxydiphenic acid esters with bases, and its application to the structure determination of pomegranate tannins, granatins A and B. Chem. Pharm. Bull. 1990, 38, 2424–2428. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Ren, X.; Sun, S.; Wang, X.; Xu, X.; Li, X.; Wang, X.; Li, X.; Yan, X.; Li, R. Structure, biological activities and metabolism of flavonoid glucuronides. Mini Rev. Med. Chem. 2022, 22, 322–354. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Beak, S.Y.; Choi, I.; Sung, J.S. Quercetin and its metabolites protect hepatocytes against ethanol-induced oxidative stress by activation of Nrf2 and AP-1. Food Sci. Biotechnol. 2018, 27, 809–817. [Google Scholar] [CrossRef]
- Shang, X.; Tan, J.N.; Du, Y.; Liu, X.; Zhang, Z. Environmentally-friendly extraction of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves with deep eutectic solvents and evaluation of their antioxidant activities. Molecules 2018, 23, 2110. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Zhou, X.; Zhao, C.; Chen, H.; Zhao, Y.; Gong, X. Anti-inflammatory, antiviral and quantitative study of quercetin-3-O-β-d-glucuronide in Polygonum perfoliatum L. Fitoterapia 2011, 82, 805–810. [Google Scholar] [CrossRef]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3′-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef]
- Ben Hmidene, A.; Hanaki, M.; Murakami, K.; Irie, K.; Isoda, H.; Shigemori, H. Inhibitory activities of antioxidant flavonoids from Tamarix gallica on amyloid aggregation related to Alzheimer’s and Type 2 Diabetes Diseases. Biol. Pharm. Bull. 2017, 40, 238–241. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschkeg, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure activity correlations with related alkaloids. Bioorg. Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Yuan, K.; Zhu, J.X.; Si, J.P.; Cai, H.K.; Ding, X.D.; Pan, Y.J. Studies on chemical constituents and antibacterial activity from n-butanol extract of Sarcandra glabra. Zhongguo Zhong Yao Za Zhi 2008, 33, 1843–1846. [Google Scholar]
- Ma, Q.; Jiang, J.G.; Zhang, X.M.; Zhu, W. Identification of luteolin 7-O-β-d-glucuronide from Cirsium japonicum and its anti-inflammatory mechanism. J. Funct. Foods. 2018, 46, 521–528. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, X.M.; Jiang, J.G.; Zhu, W. Apigenin-7-O-β-d-glucuronide inhibits modified low-density lipoprotein uptake and foam cell formation in macrophages. J. Funct. Foods. 2017, 35, 615–621. [Google Scholar] [CrossRef]
- Gumbinger, H.; Winterhoff, H.; Wylde, R.; Sosa, A. On the influence of the sugar moiety on the antigonadotropic activity of luteoline glycosides. Planta Med. 1992, 58, 49–50. [Google Scholar] [CrossRef]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2020, 160, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Polefka, T.G.; Meyer, T.A.; Agin, P.P.; Bianchini, R.J. Effects of solar radiation on the skin. J. Cosmet. Dermatol. 2012, 11, 134–143. [Google Scholar] [CrossRef]
- Supp, D.M.; Boyce, S.T. Engineered skin substitutes: Practices and potentials. Clin. Dermatol. 2005, 23, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Ali, J.; Baboota, S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 2018, 57, 646–660. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. Plus. 2020, 8, 100041. [Google Scholar] [CrossRef] [PubMed]
- Oliver, R.F.; Jahoda, C.A. Dermal-epidermal interactions. Clin. Dermatol. 1988, 6, 74–82. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.M. Intrinsic and extrinsic regulation of human skin melanogenesis and pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef] [Green Version]
- Fussell, J.C.; Kelly, F.J. Oxidative contribution of air pollution to extrinsic skin ageing. Free Radic. Biol. Med. 2020, 151, 111. [Google Scholar] [CrossRef]
- Pourang, A.; Tisack, A.; Ezekwe, N.; Torres, A.E.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Effects of visible light on mechanisms of skin photoaging. Photodermatol. Photoimmunol. Photomed. 2022, 38, 191–196. [Google Scholar] [CrossRef]
- Borg, M.; Brincat, S.; Camilleri, G.; Schembri-Wismayer, P.; Brincat, M.; Calleja-Agius, J. The role of cytokines in skin aging. Climacteric 2013, 16, 514–521. [Google Scholar] [CrossRef]
- Lee, K.-J.; Park, K.H.; Hahn, J.-H. Alleviation of ultraviolet-B radiation-induced photoaging by a TNFR antagonistic peptide, TNFR2-SKE. Mol. Cells 2019, 42, 151–160. [Google Scholar]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Sreedhar, A.; Aguilera-Aguirre, L.; Singh, K.K. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020, 11, 444. [Google Scholar] [CrossRef]
- Ochoa, C.D.; Wu, R.F.; Terada, L.S. ROS signaling and ER stress in cardiovascular disease. Mol. Asp. Med. 2018, 63, 18–29. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Medi. 1997, 337, 1419–1429. [Google Scholar] [CrossRef]
- Ding, Y.; Jiratchayamaethasakul, C.; Lee, S.H. Protocatechuic aldehyde attenuates UVA-induced photoaging in human dermal fibroblast cells by suppressing MAPKs/AP-1 and NF-κB signaling pathways. Int. J. Mol. Sci. 2020, 21, 4619. [Google Scholar] [CrossRef]
- Naruishi, K.; Nagata, T. Biological effects of interleukin-6 on gingival fibroblasts: Cytokine regulation in periodontitis. J. Cell Physiol. 2018, 233, 6393–6400. [Google Scholar] [CrossRef]

 ), 1H−13C HMBC (
), 1H−13C HMBC ( ) and 1H−1H NOESY (
) and 1H−1H NOESY ( ) correlations of compound 1.
) correlations of compound 1.



| Position a | 1 | 5 (Quercetin-3-O-β-d-Glucuronide) | ||
|---|---|---|---|---|
| δH Multi (J in Hz) | δC | δH Multi (J in Hz) | δC | |
| 2 | 155.9 | 156.3 | ||
| 3 | 133.3 | 133.2 | ||
| 4 | 177.3 | 177.3 | ||
| 5 | 161.2 | 161.3 | ||
| 6 | 6.23 d (2.0) | 98.9 | 6.21 d (2.0) | 98.9 |
| 7 | 164.4 | 164.3 | ||
| 8 | 6.48 d (2.0) | 94.0 | 6.41 d (2.0) | 93.7 |
| 9 | 156.4 | 156.4 | ||
| 10 | 104.0 | 104.0 | ||
| 1′ | 121.0 | 120.9 | ||
| 2′ | 7.75 d (2.5) | 116.8 | 7.55 d (2.5) | 116.2 |
| 3′ | 144.6 | 145.0 | ||
| 4′ | 150.2 | 148.7 | ||
| 5′ | 6.92 d (8.5) | 115.9 | 6.84 d (8.5) | 115.3 |
| 6′ | 7.89 dd (8.5, 2.5) | 125.5 | 7.60 dd (8.5, 2.5) | 121.8 |
| Glu-1″ | 5.46 d (7.5) | 101.2 | 5.49 d (7.5) | 101.2 |
| Glu-2″ | 3.368 b | 73.1 | 3.24–3.40 m b | 73.9 |
| Glu-3″ | 3.244 b | 75.8 | 3.24–3.40 m b | 76.0 |
| Glu-4″ | 3.370 b | 71.4 | 3.24–3.40 m b | 71.5 |
| Glu-5″ | 3.55 d (10.0) | 75.9 | 3.57 d (10.0) | 76.0 |
| Glu-6″ | 170.3 | 170.0 | ||
| Glu-1‴ | 5.08 d (7.5) | 101.5 | ||
| Glu-2‴ | 3.240 b | 73.8 | ||
| Glu-3‴ | 3.365 b | 75.3 | ||
| Glu-4‴ | 3.43 t (9.5) | 71.4 | ||
| Glu-5‴ | 3.94 d (9.5) | 75.4 | ||
| Glu-6‴ | 170.1 | |||
| OH-5 | 12.52 s | 12.54 s | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.Y.; Choi, Y.J.; Son, S.-R.; Yoon, Y.-S.; Lee, S.-H.; Lee, K.-T.; Lee, S.; Jang, D.S. Potentilloside A, a New Flavonol-bis-Glucuronide from the Leaves of Potentilla chinensis, Inhibits TNF-α-Induced ROS Generation and MMP-1 Secretion. Plants 2022, 11, 3318. https://doi.org/10.3390/plants11233318
Lee SY, Choi YJ, Son S-R, Yoon Y-S, Lee S-H, Lee K-T, Lee S, Jang DS. Potentilloside A, a New Flavonol-bis-Glucuronide from the Leaves of Potentilla chinensis, Inhibits TNF-α-Induced ROS Generation and MMP-1 Secretion. Plants. 2022; 11(23):3318. https://doi.org/10.3390/plants11233318
Chicago/Turabian StyleLee, So Young, Yea Jung Choi, So-Ri Son, Young-Seo Yoon, Sun-Hee Lee, Kyung-Tae Lee, Sullim Lee, and Dae Sik Jang. 2022. "Potentilloside A, a New Flavonol-bis-Glucuronide from the Leaves of Potentilla chinensis, Inhibits TNF-α-Induced ROS Generation and MMP-1 Secretion" Plants 11, no. 23: 3318. https://doi.org/10.3390/plants11233318
APA StyleLee, S. Y., Choi, Y. J., Son, S. -R., Yoon, Y. -S., Lee, S. -H., Lee, K. -T., Lee, S., & Jang, D. S. (2022). Potentilloside A, a New Flavonol-bis-Glucuronide from the Leaves of Potentilla chinensis, Inhibits TNF-α-Induced ROS Generation and MMP-1 Secretion. Plants, 11(23), 3318. https://doi.org/10.3390/plants11233318