The Leaf Essential Oil of Myrtus communis subsp. tarentina (L.) Nyman: From Phytochemical Characterization to Cytotoxic and Antimigratory Activity in Human Prostate Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization
2.2. Micromorphological Investigation
2.3. Cytotoxic Activity
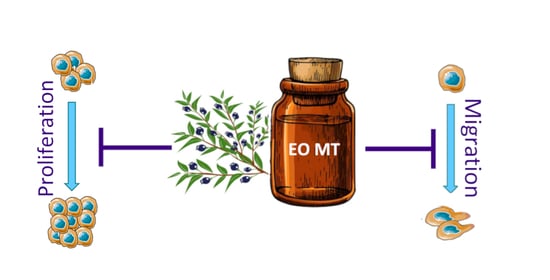
2.3.1. Cytotoxic and Proapoptotic Activity of EO MT in CRPC Cells
2.3.2. Antimigratory Activity of EO MT in CRPC Cells
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Antibodies
3.3. Cell Cultures
3.4. Preparation of Essential Oil
3.5. GC-MS Analysis of Essential Oil
3.6. Light Microscopy and Fluorescence Microscopy
3.7. MTT Viability Assay
3.8. Clonogenic Assay
3.9. Annexin V/Propidium Iodide Assay
3.10. Western Blot Assay
3.11. Migration Assay
3.12. Immunofluorescence
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sisay, M.; Gashaw, T. Ethnobotanical, ethnopharmacological, and phytochemical studies of Myrtus communis Linn: A popular herb in Unani system of medicine. J. Evid. Based Complement. Altern. Med. 2017, 22, 1035–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izgi, K.; Iskender, B.; Jauch, J.; Sezen, S.; Cakir, M.; Charpentier, M.; Canatan, H.; Sakalar, C. Myrtucommulone-A induces both extrinsic and intrinsic apoptotic pathways in cancer cells. J. Biochem. Mol. Toxicol. 2015, 29, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.; Charpentier, M.; Huch, V.; Jauch, J.; Bruhn, T.; Bringmann, G.; Quandt, D. Stereoisomeric composition of natural myrtucommulone A. J. Nat. Prod. 2015, 78, 2381–2389. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, I.; Blaesius, D.; Maxia, L.; Maxia, L.; Wesselborg, S.; Schulze-Osthoff, K.; Cinatl, J.; Michaelis, M.; Werz, O. Myrtucommulone from Myrtus communis induces apoptosis in cancer cells via the mitochondrial pathway involving caspase-9. Apoptosis 2008, 13, 119–131. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bugarin, D.; Grbovic, S.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Orčić, D.; Jovin, E.; Couladis, M. Essential oil of Myrtus communis L. as a potential antioxidant and antimutagenic agents. Molecules 2010, 15, 2759–2770. [Google Scholar] [CrossRef] [Green Version]
- Hayder, N.; Bouhlel, I.; Skandrani, I.; Kadri, M.; Steiman, R.; Guiraud, P.; Mariotte, A.M.; Ghedira, K.; Dijoux-Franca, M.G.; Chekir-Ghedira, L. In vitro antioxidant and antigenotoxic potentials of myricetin-3-O-galactoside and myricetin-3-O-rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicol. In Vitro 2008, 22, 567–581. [Google Scholar] [CrossRef]
- Harassi, Y.; Tilaoui, M.; Idir, A.; Frédéric, J.; Baudino, S.; Ajouaoi, S.; Mouse, H.A.; Zyad, A. Phytochemical analysis, cytotoxic and antioxidant activities of Myrtus communis essential oil from Morocco. J. Complement. Integr. Med. 2019, 16, 20180100. [Google Scholar] [CrossRef]
- Asllani, U. Chemical composition of albanian myrtle oil (Myrtus communis L.). J. Essent. Oil Res. 2000, 12, 140–142. [Google Scholar] [CrossRef]
- Berka-Zougali, B.; Ferhat, M.A.; Hassani, A.; Chemat, F.; Allaf, K.S. Comparative study of essential oils extracted from Algerian Myrtus communis L. leaves using microwaves and hydrodistillation. Int. J. Mol. Sci. 2012, 13, 4673–4695. [Google Scholar] [CrossRef] [Green Version]
- Bouzabata, A.; Casanova, J.; Bighelli, A.; Cavaleiro, C.; Salgueiro, L.; Tomi, F. The genus Myrtus L. in Algeria: Composition and biologicala of essential oils from M. communis and M. nivellei: A review. Chem. Biodivers. 2016, 13, 672–680. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Barra, A.; Angioni, A.; Sarritzu, E.; Pirisi, F.M. Chemical composition of volatiles in Sardinian myrtle (Myrtus communis L.) alcoholic extracts and essential oils. J. Agric. Food Chem. 2006, 54, 1420–1426. [Google Scholar] [CrossRef]
- Usai, M.; Mulas, M.; Marchetti, M. Chemical composition of essential oils of leaves and flowers from five cultivars of myrtle (Myrtus communis L.). J. Essent. Oil Res. 2015, 27, 465–476. [Google Scholar] [CrossRef]
- Taamalli, A.; Iswaldi, I.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Zarrouk, M. UPLC–QTOF/MS for a rapid characterisation of phenolic compounds from Leaves of Myrtus communis L. Phytochem. Anal. 2013, 25, 89–96. [Google Scholar] [CrossRef]
- Yoshimura, M.; Amakura, Y.; Tokuhara, M.; Yoshida, T. Polyphenolic compounds isolated from the leaves of Myrtus communis. J. Nat. Med. 2008, 62, 366–368. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, L.; Morelli, I.; Maccioni, S.; Baldini, R. Phytochemical typologies in some populations of Myrtus communis L. on Caprione Promontory (East Liguria, Italy). Food Chem. 2004, 85, 599–604. [Google Scholar] [CrossRef]
- Senatore, F.; Formisano, C.; Napolitano, F.; Rigano, D.; Özcan, M. Chemical composition and antibacterial activity of essential oil of Myrtus communis L. growing wild in Italy and Turkey. J. Essent. Oil Bear. Plants 2006, 9, 162–169. [Google Scholar] [CrossRef]
- Siracusa, L.; Napoli, E.; Tuttolomondo, T.; Licata, M.; LaBella, S.; Gennaro, M.C.; Leto, C.; Sarno, M.; Sperlinga, E.; Ruberto, G. A two-year bio-agronomic and chemotaxonomic evaluation of wild sicilian myrtle (Myrtus communis L.) berries and leaves. Chem. Biodiv. 2019, 16, e1800575. [Google Scholar] [CrossRef]
- Maggio, A.; Loizzo, M.R.; Riccobono, L.; Bruno, M.; Tenuta, M.C.; Leporini, M.R. Comparative chemical composition and bioactivity of leaves essential oils from nine Sicilian accessions of Myrtus communis L. J. Essent. Oil Res. 2019, 31, 546–555. [Google Scholar] [CrossRef]
- Giuliani, C.; Bottoni, M.; Milani, F.; Todero, S.; Berera, P.; Maggi, F.; Santagostini, L.; Fico, G. Botanic Garden as a factory of molecules: Myrtus communis L. subsp. communis as a case study. Plants 2022, 11, 754. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia; Edagricole: Milan, Italy, 2017–2019; Volume 2, p. 878, key 4–637. [Google Scholar]
- Ghafouri, F.; Rahimmalek, M. Genetic structure and variation in different Iranian myrtle (Myrtus communis L.) populations based on morphological, phytochemical and molecular markers. Ind. Crops Prod. 2018, 123, 489–499. [Google Scholar] [CrossRef]
- Melito, S.; Bella, S.L.; Martinelli, F.; Cammalleri, I.; Tuttolomondo, T.; Leto, C.; Fadda, A.; Molinu, M.G.; Mulas, M. Morphological, chemical, and genetic diversity of wild myrtle (Myrtus communis L.) populations in Sicily. Turk. J. Agric. For. 2016, 40, 249–261. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Pagni, A.M.; Andreucci, A.C. Ontogeny of secretory cavities in vegetative parts of Myrtus communis L. (Myrtaceae): An example of schizolysigenous development. Isr. J. Plant Sci. 2003, 51, 193–198. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Andreucci, A.C.; Pagni, A.M.; Garbari, F. Structure and development of the elaiosome in Myrtus communis L. (Myrtaceae) seeds. Flora 2005, 200, 326–331. [Google Scholar] [CrossRef]
- Ciccarelli, D.; Garbari, F.; Pagni, A.M. The flower of Myrtus communis (Myrtaceae): Secretory structures, unicellular papillae, and their ecological role. Flora 2008, 203, 85–93. [Google Scholar] [CrossRef]
- Kalachanis, D.; Psaras, G.K. Structure and development of the secretory cavities of Myrtus communis leaves. Biol. Plant. 2005, 49, 105–110. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate cancer. Lancet 2021, 18, 398. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers. 2021, 7, 9. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic antitumor effect of alpha-pinene and beta-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). Drug Res. 2015, 65, 214–218. [Google Scholar]
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. α-Pinene inhibits human prostate cancer growth in a mouse xenograft model. Chemotherapy 2018, 63, 1–7. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Qi, F.; Yan, Q.; Zheng, Z.; Liu, J.; Chen, Y.; Zhang, G. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J. BUON 2018, 23, 346–352. [Google Scholar]
- Yin, L.; Sun, Z.; Ren, Q.; Su, X.; Zhang, D. Methyl eugenol induces potent anticancer effects in RB355 human retinoblastoma cells by inducing autophagy, cell cycle arrest and inhibition of PI3K/mTOR/Akt signalling pathway. J. BUON 2018, 23, 1174–1178. [Google Scholar]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds alpha-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis pharmacology: The usual suspects and a few promising leads. Adv. Pharmacol. 2016, 80, 67–134. [Google Scholar]
- Roozitalab, G.; Yousefpoor, Y.; Abdollahi, A.; Safari, M.; Rasti, F.; Osanloo, M. Antioxidative, anticancer, and antibacterial activities of a nanoemulsion-based gel containing Myrtus communis L. essential oil. Chem. Pap. 2022, 76, 4261–4271. [Google Scholar] [CrossRef]
- Caputo, L.; Capozzolo, F.; Amato, G.; De Feo, V.; Fratianni, F.; Vivenzio, G.; Nazzaro, F. Chemical composition, antibiofilm, cytotoxic, and anti-acetylcholinesterase activities of Myrtus communis L. leaves essential oil. BMC Complement. Med. Ther. 2022, 22, 142. [Google Scholar] [CrossRef]
- Dolghi, A.; Coricovac, D.; Dinu, S.; Pinzaru, I.; Dehelean, C.A.; Grosu, C.; Chioran, D.; Merghes, P.E.; Sarau, C.A. Chemical and antimicrobial characterization of Mentha piperita L. and Rosmarinus officinalis L. essential oils and in vitro potential cytotoxic effect in human colorectal carcinoma cells. Molecules 2022, 27, 6106. [Google Scholar] [CrossRef] [PubMed]
- Vukic, M.D.; Vukovic, N.L.; Obradovic, A.D.; Galovičová, L.; Čmiková, N.; Kačániová, M.; Matic, M.M. Chemical composition and biological activity of Tanacetum balsamita essential oils obtained from different plant organs. Plants 2022, 11, 3474. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Graziano, A.; Bruno, M.; Cardile, V.; Rigano, D. Apoptosis induction of essential oils from Artemisia arborescens L. in human prostate cancer cells. J. Ethnopharmacol. 2022, 303, 115929. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, S.; Vourda, A.; Syggelos, S.; Gyftopoulos, K. Cell plasticity and prostate cancer: The role of epithelial-mesenchymal transition in tumor progression, invasion, metastasis and cancer therapy resistance. Cancers 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielińska, W.; Hałas-Wiśniewska, M.; Grzanka, A. Involvement of actin and actin-binding proteins in carcinogenesis. Cells 2020, 9, 2245. [Google Scholar] [CrossRef]
- Niu, Q.L.; Sun, H.; Liu, C.; Li, J.; Liang, C.X.; Zhang, R.R.; Ge, F.R.; Liu, W. Croton tiglium essential oil compounds have anti-proliferative and pro-apoptotic effects in A549 lung cancer cell lines. PLoS ONE 2020, 15, e0231437. [Google Scholar] [CrossRef]
- Liu, K.; Deng, W.; Hu, W.; Cao, S.; Zhong, B.; Chun, J. Extraction of ‘Gannanzao’ orange peel essential oil by response surface methodology and its effect on cancer cell proliferation and migration. Molecules 2019, 24, 499. [Google Scholar] [CrossRef] [Green Version]
- Xing, X.; Ma, J.H.; Fu, Y.; Zhao, H.; Ye, X.X.; Han, Z.; Jia, F.J.; Li, X. Essential oil extracted from Erythrina corallodendron L. leaves inhibits the proliferation, migration, and invasion of breast cancer cells. Medicine 2019, 98, e17009. [Google Scholar] [CrossRef]
- Cussenot, O.; Berthon, P.; Berger, R.; Mowszowicz, I.; Faille, A.; Hojman, F.; Teillac, P.; Le Duc, A.; Calvo, F. Immortalization of human adult normal prostatic epithelial cells by liposomes containing large T-SV40 gene. J. Urol. 1991, 146, 881–886. [Google Scholar] [CrossRef]




| N. | Class | Component | LRI a | RI b | % | SD |
|---|---|---|---|---|---|---|
| 1 | MH | α-pinene | 914 | 937 | 17.73 | 0.19 |
| 2 | MH | β-pinene | 975 | 979 | 0.38 | 0.09 |
| 3 | MO | Unidentified | 1029 | - | 11.96 | 0.49 |
| 4 | MH | γ-terpinene | 1050 | 1060 | 1.05 | 0.25 |
| 5 | MH | Terpinolene | 1077 | 1088 | 1.32 | 0.39 |
| 6 | MO | Linalool | 1093 | 1099 | 6.16 | 0.20 |
| 7 | MO | Pinocarveol | 1130 | 1139 | 0.42 | 0.03 |
| 8 | MO | α-pinocarvone | 1147 | 1164 | 0.16 | 0.02 |
| 9 | MO | 4-terpineol | 1160 | 1177 | 1.28 | 0.13 |
| 10 | MO | α-terpineol | 1172 | 1189 | 7.36 | 0.38 |
| 11 | MO | linalyl acetate | 1248 | 1257 | 1.54 | 0.06 |
| 12 | MO | trans-geraniol | 1256 | 1255 | 1.09 | 0.20 |
| 13 | MO | trans-pinocarvyl acetate | 1291 | 1297 | 0.15 | 0.02 |
| 14 | MO | methyl geranate | 1312 | 1324 | 1.76 | 0.12 |
| 15 | MO | exo-2-hydroxycineole acetate | 1332 | 1344 | 0.22 | 0.00 |
| 16 | MO | α-terpinyl acetate | 1340 | 1350 | 1.02 | 0.05 |
| 17 | MO | C-acetylsyncarpic acid | 1350 | 1369 | 1.53 | 0.06 |
| 18 | MO | geranyl acetate | 1370 | 1382 | 5.92 | 0.06 |
| 19 | MO | methyl eugenol | 1395 | 1402 | 4.34 | 0.62 |
| 20 | SH | β-caryophyllene | 1410 | 1419 | 5.17 | 0.20 |
| 21 | NH | 5-hydroxy-2,2,6,6-tetramethyl-4-propionylcyclohex-4-ene-1,3-dione | 1444 | 1464 | 0.50 | 0.09 |
| 22 | SH | α-humulene | 1451 | 1454 | 12.39 | 1.14 |
| 23 | NH | trans-β-ionone | 1473 | 1486 | 0.34 | 0.01 |
| 24 | SH | α-farnesene | 1493 | 1508 | 0.28 | 0.01 |
| 25 | NH | Durohydroquinon c | 1507 | - | 7.26 | 0.65 |
| 26 | SO | caryophyllene oxide | 1576 | 1581 | 1.59 | 0.07 |
| 27 | SO | Unidentified | 1591 | - | 0.78 | 0.04 |
| 28 | SO | humulene epoxide II | 1602 | 1606 | 3.19 | 0.24 |
| 29 | Unidentified | 1610 | 1.46 | 0.13 | ||
| Monoterpene hydrocarbons | 20.49 | 0.54 | ||||
| Oxygenated monoterpenes | 32.95 | 1.63 | ||||
| Sesquiterpene hydrocarbons | 17.84 | 1.32 | ||||
| Oxygenated sesquiterpenes | 4.78 | 0.31 | ||||
| Non-terpene hydrocarbons | 8.10 | 0.75 | ||||
| Non identified compounds | 14.19 | 0.34 | ||||
| Total compounds | 98.36 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliani, C.; Moretti, R.M.; Bottoni, M.; Santagostini, L.; Fico, G.; Montagnani Marelli, M. The Leaf Essential Oil of Myrtus communis subsp. tarentina (L.) Nyman: From Phytochemical Characterization to Cytotoxic and Antimigratory Activity in Human Prostate Cancer Cells. Plants 2023, 12, 1293. https://doi.org/10.3390/plants12061293
Giuliani C, Moretti RM, Bottoni M, Santagostini L, Fico G, Montagnani Marelli M. The Leaf Essential Oil of Myrtus communis subsp. tarentina (L.) Nyman: From Phytochemical Characterization to Cytotoxic and Antimigratory Activity in Human Prostate Cancer Cells. Plants. 2023; 12(6):1293. https://doi.org/10.3390/plants12061293
Chicago/Turabian StyleGiuliani, Claudia, Roberta Manuela Moretti, Martina Bottoni, Laura Santagostini, Gelsomina Fico, and Marina Montagnani Marelli. 2023. "The Leaf Essential Oil of Myrtus communis subsp. tarentina (L.) Nyman: From Phytochemical Characterization to Cytotoxic and Antimigratory Activity in Human Prostate Cancer Cells" Plants 12, no. 6: 1293. https://doi.org/10.3390/plants12061293
APA StyleGiuliani, C., Moretti, R. M., Bottoni, M., Santagostini, L., Fico, G., & Montagnani Marelli, M. (2023). The Leaf Essential Oil of Myrtus communis subsp. tarentina (L.) Nyman: From Phytochemical Characterization to Cytotoxic and Antimigratory Activity in Human Prostate Cancer Cells. Plants, 12(6), 1293. https://doi.org/10.3390/plants12061293