A Scoping Review of Genus Viscum: Biological and Chemical Aspects of Alcoholic Extracts
Abstract
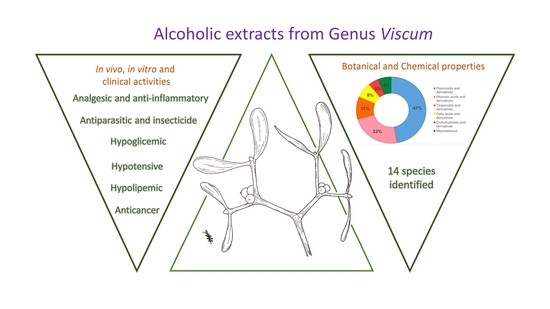
:1. Introduction
2. Results and Discussions
| Species | Subspecies/Var. | Parts Used | Extractive Solvent | Extractive Method | Harvest Month | Authors |
|---|---|---|---|---|---|---|
| Viscum album | subsp. album | Leaves, stems, berries | Methanol 80% | Bead mill (30 Hz) | September | [7] |
| Viscum album | n.d. | Leaves and twigs | Ethanol, methanol | Maceration at room temperature | n.d. | [12] |
| Viscum album | n.d. | n.d. | Methanol | Soxhlet extraction | July | [13] |
| Viscum coloratum | n.d. | n.d. | Ethanol 70% | Boiling | n.d. | [14] |
| Viscum album | n.d. | Stems | Ethanol | Maceration at room temperature | June | [15] |
| Viscum album | n.d. | Leaves and stems | Ethanol | Maceration | n.d. | [21] |
| Viscum album | subsp. abietis | Whole plant | Ethanol 70% | Room temperature maceration | January | [22] |
| Viscum album | n.d. | Leaves | Methanol | Maceration at room temperature | n.d. | [25] |
| Viscum album | n.d. | Leaves and stems | Ethanol | Percolation at room temperature | n.d. | [26] |
| Viscum album | n.d. | Leaves and flowers | Ethanol 95% | Cold maceration | October | [27] |
| Viscum album | n.d. | Leaves | Ethanol 96% | High temperature maceration | April | [28] |
| Viscum album | n.d. | Aerial parts | Methanol | Soxhlet extraction | n.d. | [29] |
| Viscum album | n.d. | Stem, leaves and fruits | Methanol | Maceration | April | [30] |
| Viscum album | var. coloratum | Stems | Ethanol 70% | Maceration | n.d. | [31] |
| Viscum album | subsp. album | Leaves | Ethanol 50% | Waring blender at room temperature | February | [32] |
| Viscum album | subsp. austriacum | Leaves, fruits and bodies | Ethanol 80% | Intermittent shaking | September, October | [33] |
| Viscum album | n.d. | Leaves, stems and flowers | Ethanol 95% | Maceration | March, April | [34] |
| Viscum album | n.d. | n.d. | Ethanol 45% | Maceration | n.d. | [35] |
| Viscum album | subsp. album | Leaves | Methanol | Maceration | July | [36] |
| Viscum album | n.d. | Leaves | Methanol | Maceration | September | [37] |
| Viscum album | n.d. | Leaves and stems | Ethanol/Methanol | Maceration | n.d | [38] |
| Viscum album | subsp. album, austriacum | Fruits | Methanol 80% | Ultrasound assisted maceration at room temperature | n.d. | [39] |
| Viscum album | n.d. | Leaves and branches | Ethanol 95%, methanol 5% | Magnetic stirring | March | [40] |
| Viscum album | n.d. | Leaves and stems | Ethanol | Cold chamber with circular agitation | February, November | [41] |
| Viscum album | n.d. | Fruits | Ethanol 80% | Ultrasonic bath | May | [42] |
| Viscum album | subsp. album, austriacum, abietis | n.d. | Ethanol 80% | Room temperature | April, June | [43] |
| Viscum album | subsp. album | Herbaceous parts | Methanol | Maceration at room temperature | September | [44] |
| Viscum album | n.d. | Whole plant | Methanol 80% | n.d. | August | [45] |
| Viscum album | subsp. album, austriacum | Aerial parts | Ethanol 80% | Maceration | n.d. | [46] |
| Viscum album | n.d. | Whole plant | Ethanol | Successive extraction | July, September | [47] |
| Viscum album | n.d. | Aerial parts | Ethanol 70% | Sonication on an ice-bath | n.d. | [48] |
| Viscum album | var. coloratum | n.d. | Ethanol 25%, 50%, 75%, 100% | Microwave power extraction | n.d. | [49] |
| Viscum album | n.d. | n.d. | Methanol | Soxhlet extraction | n.d. | [50] |
| Viscum album | n.d. | Leaves | Ethanol 80% | Maceration at room temperature | n.d. | [51] |
| Viscum album | var. coloratum | n.d. | Ethanol 70% | High temperature (70 °C) | January | [52] |
| Viscum album | subsp. album, abietis, austriacum | Whole plant | Ethanol 96% | Room temperature | n.d. | [53] |
| Viscum album | subsp. album | Whole plant | Ethanol 90% | Room temperature | n.d. | [54] |
| Viscum album | n.d. | n.d. | Ethanol 90% | Cold maceration | November, December | [55] |
| Viscum album | n.d. | n.d. | Ethanol 90% | Maceration | n.d. | [56] |
| Viscum album | n.d. | Aerial parts | Methanol, butanol | Soxhlet extraction | September | [57] |
| Viscum album | subsp. album | Leaves | Methanol 80% | Soxhlet extraction | March | [58] |
| Viscum album | n.d. | Leaves | Methanol 80% | Soxhlet extraction | March | [59] |
| Viscum album | var. coloratum | Whole plant | Methanol | Cold maceration | March | [60] |
| Viscum album | subsp. album | Leaves | Methanol 80% | Percolation | March | [61] |
| Viscum album | subsp. album, abietis, austriacum | Leaves and stems | Ethanol, butanol | n.d. | April, June | [62] |
| Viscum album | subsp. album | Leaves and stems | Ethanol 80% | Room temperature | June | [63] |
| Viscum album | subsp. album | Leaves | Methanol 80% | Maceration | n.d. | [64] |
| Viscum album | n.d. | Fruit | Methanol | Maceration | November | [65] |
| Viscum album | n.d. | Leaves and fruits | Ethanol 94.7–95.2% v/v | Maceration at room temperature | n.d. | [66] |
| Viscum album | n.d. | Leaves | Methanol | Warring blender | n.d. | [67] |
| Viscum album | n.d. | Leaves | Methanol | Maceration | n.d. | [68] |
| Viscum album | n.d. | Aerial parts | Ethanol 60% | High temperature maceration | n.d. | [69] |
| Viscum album | subsp. abietis | Leaves, stems | Methanol and ethanol 50%, 80% and 100% | Maceration, reflux, ultrasonic extraction | n.d. | [70] |
| Viscum album | n.d. | Leaves and stems | Methanol | Cold maceration | December | [71] |
| Viscum album | n.d. | Leaves and stems | Ethanol 98% | Ultra-Turrax | December, May, July | [72] |
| Viscum album | n.d. | Leaves and twigs | Methanol 80, 96% | Soxhlet extraction | n.d. | [73] |
| Viscum album | n.d. | n.d. | Methanol | Room temperature | n.d. | [74] |
| Viscum album | n.d. | Stem, leaves | Ethanol 95% | Reflux | June | [75] |
| Viscum album | subsp. album | Leaves | Methanol | Maceration in an incubatory shaker | February, July | [76] |
| Viscum album | subsp. album, austriacum | Leaves and stems | Ethanol 80%, methanol | Maceration | April, May, June | [77] |
| Viscum album | n.d. | n.d. | Ethanol 96% | Maceration at room temperature | n.d. | [78] |
| Viscum album | n.d. | Aerial parts | Methanol | Soxhlet extraction | September | [79] |
| Viscum album | var. coloratum | Leaves and twigs | Methanol | Successive extraction | n.d. | [80] |
| Viscum album | subsp. album, abietis | Leaves | Methanol | Rotary incubator | July | [81] |
| Viscum album | n.d. | Leaves and twigs | Methanol | Percolation | n.d. | [82] |
| Viscum album | n.d. | Leaves, stems | Ethanol 70% | Maceration | July | [83] |
| Viscum album | subsp. album, abietis, austriacum | Leaves, stems and twigs | Ethanol 80% | Room temperature | April, June | [84] |
| Viscum album | subsp. album | Leaves, stems and twigs | Methanol | High temperature maceration | April, June | [85] |
| Viscum album | n.d. | n.d. | Ethanol | Maceration | n.d. | [86] |
| Viscum album | n.d. | n.d. | Methanol | Household blender | April | [87] |
| Viscum album | n.d. | n.d. | Methanol | Soxhlet extraction | January | [88] |
| Viscum album | n.d. | Leaves | Methanol 70% | High temperature maceration | June | [89] |
| Viscum album | subsp. album, austriacum, abietis | Whole plant | Ethanol 80% | Room temperature maceration | July | [90] |
| Viscum album | n.d. | n.d. | Methanol | Ultrasonic bath (35 °C) | May | [91] |
| Viscum album | subsp. austriacum | Leaves and stems | Ethanol | Stirred for 72 h (25 °C) | n.d. | [92] |
| Viscum album | n.d. | Leaves, stems and berries | Methanol 80%, Ethanol 80% | Incubatory rotatory shaker (200 rpm) | December | [93] |
| Viscum album | n.d. | Leaves | Ethanol | Reflux | n.d. | [94] |
| Viscum album | n.d. | Root | Ethanol | Maceration | n.d. | [95] |
| Viscum album | n.d. | Aerial parts | Ethanol | Sonication | February, December | [96] |
| Viscum album | n.d. | Leaves | Ethanol | Soxhlet and ultrasound-assisted extraction | September | [97] |
| Viscum album | n.d. | Whole plant | Ethanol, methanol | Soxhlet | n.d. | [98] |
| Viscum album | subsp. album, austriacum, abietis | Leaves and stems | Methanol 80% | Accelerated solvent extractions | February 2016 to April 2017 | [99] |
| Viscum album | n.d. | Leaves and stems | Methanol 85% | Maceration | n.d. | [100] |
| Viscum album | n.d. | Leaves | Methanol | Maceration | April | [101] |
| Viscum album | n.d. | Leaves, fruits and seeds | Methanol | Ultra-Turrax and ultrasonic bath | December | [102] |
| Viscum album | n.d. | Leaves | Methanol | Shaking incubator | n.d. | [103] |
| Viscum album | n.d. | n.d. | Methanol | Soxhlet extraction | January | [104] |
| Viscum angulatum | n.d. | Whole plant | Methanol | Cold maceration | November | [105] |
| Viscum angulatum | n.d. | n.d. | Ethanol, methanol | Cold shaking | n.d. | [106] |
| Viscum articulatum | n.d. | Whole plant | Ethanol | Continuous hot percolation | July, August | [107] |
| Viscum articulatum | n.d. | Aerial part | Methanol 95% | Successive extraction at room temperature | December, July | [108] |
| Viscum articulatum | n.d. | Whole plant | Ethanol 70% | Maceration | February | [109] |
| Viscum articulatum | n.d. | Whole plant | Ethanol | Soxhlet extraction | n.d. | [110] |
| Viscum articultum | n.d. | n.d. | Ethanol | Soxhlet extraction | n.d. | [111] |
| Viscum articulatum | n.d. | Whole plant | Methanol | Maceration at room temperature | n.d. | [112] |
| Viscum articulatum | n.d. | Whole plant | Methanol | Maceration at room temperature | October | [113] |
| Viscum articulatum | n.d. | Aerial parts | Methanol 95% | Maceration at room temperature | December | [114] |
| Viscum capense | n.d. | Stems | Methanol | Soxhlet extraction | n.d. | [115] |
| Viscum capitellatum | n.d. | Aerial parts | 80% ethanol, methanol | Decoction | September | [116] |
| Viscum capitellatum | n.d. | n.d. | Methanol | Cold maceration | n.d. | [117] |
| Viscum coloratum | n.d. | Leaves and twigs | Ethanol 75% | Boiling | n.d. | [118] |
| Viscum coloratum | n.d. | n.d. | Ethanol 70% | Maceration at high temperature | n.d. | [119] |
| Viscum coloratum | n.d. | Leaves and twigs | Butanol | n.d. | n.d. | [120] |
| Viscum coloratum | n.d. | n.d. | Methanol 50% | Heated water bath | n.d. | [121] |
| Viscum coloratum | n.d. | Whole plant | Ethanol 95% | Reflux | n.d. | [122] |
| Viscum coloratum | n.d. | Whole plant | Ethanol 50% | Sonication | n.d. | [123] |
| Viscum coloratum | n.d. | Leaves and stems | Methanol | Ultrasonic extraction | n.d. | [124] |
| Viscum combreticola | n.d. | Whole plant | Methanol | Incubatory rotatory shaker (70 rpm) | n.d. | [125] |
| Viscum congolensis | n.d. | n.d. | Ethanol | n.d. | n.d. | [126] |
| Viscum cruciatum | n.d. | Aerial parts | Methanol | Soxhlet extraction | February | [127] |
| Viscum cruciatum | n.d. | Aerial parts | Methanol | Soxhlet extraction | January | [128] |
| Viscum cruciatum | n.d. | Aerial parts | Ethanol 80% | Maceration | April | [129] |
| Viscum cruciatum | n.d. | n.d. | Methanol 80% | Shaking water bath | n.d. | [130] |
| Viscum cruciatum | n.d. | Aerial parts | Methanol | Maceration | n.d. | [23] |
| Viscum liquidambaricola | n.d. | Leaves and stems | Methanol | Ultrasonic extraction | n.d. | [131] |
| Viscum monoicum | n.d. | Whole plant | Ethanol | Maceration | October | [132] |
| Viscum multinerve | n.d. | Leaves and stems | Ethanol | Successive extraction at water bath | n.d. | [133] |
| Viscum orientale | n.d. | Aerial parts | Methanol | Maceration | July | [134] |
| Viscum orientale | n.d. | Leaves | Methanol 100% | Maceration | July | [135] |
| Viscum rotundifolium | n.d. | Leaves | Methanol | Shaking incubator | n.d. | [136] |
| Viscum schimperi | n.d. | Aerial parts | Methanol | Ultra-Turrax | May | [137] |
| Viscum schimperi | n.d. | Aerial parts | Methanol 100% | Ultra-Turrax | May | [138] |
| Viscum schimperi | n.d. | Aerial parts | Methanol | Ultra-Turrax | March | [139] |
| Viscum triflorum | n.d. | Leaves | Ethanol 96% | Ultrasonic bath | February | [140] |
2.1. In Vitro Studies
2.1.1. Antimicrobial and Antiviral Activities of Viscum sp. Alcoholic Extracts
2.1.2. Antiparasitic and Insecticide Properties of Viscum sp. Alcoholic Extracts
2.1.3. Cytotoxic and Cytostatic In Vitro Activities of Viscum sp. Alcoholic Extracts
2.1.4. Cell Migration and Metalloproteinases Inhibition Induced by Viscum sp. Alcoholic Extracts
2.1.5. Antiplatelet and Antihypertensive Activities of Viscum sp. Alcoholic Extracts
2.1.6. Anti-Inflammatory Effects Activities of Viscum sp. Alcoholic Extracts
2.1.7. Immunomodulatory Effects of Viscum sp. Alcoholic Extracts
2.1.8. Hypoglycemic/Hypolipidemic Activities of Viscum sp. Alcoholic Extracts
2.1.9. Cellular Antioxidant Effect of Viscum sp. Alcoholic Extracts
2.2. In Vivo Studies
2.2.1. Hypoglicemic Effects of Viscum sp. Alcoholic Extracts
2.2.2. Hypolipemic Effects of Viscum sp. Alcoholic Extracts
2.2.3. Anticancer Activities of Viscum sp. Alcoholic Extracts
2.2.4. Hypotensive Effects of Viscum sp. Alcoholic Extracts
2.2.5. Analgesic and Anti-Inflammatory Activities of Viscum sp. Alcoholic Extracts
2.2.6. Neuropharmacological Activities of Viscum sp. Alcoholic Extracts
2.2.7. Toxicity Activities of Viscum sp. Alcoholic Extracts
2.2.8. Other Activities of Viscum sp. Alcoholic Extracts
2.3. Ex Vivo Studies with Viscum sp. Alcoholic Extracts
2.4. Clinical Trial
2.5. Chemical Aspects of Viscum sp. Alcoholic Extracts
3. Material and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sardesai, M.M.; Gaikwad, S.P.; Yadav, S.R. Viscum sahyadricum (Viscaceae), a New Species from the Western Ghats of India. Edinb. J. Bot. 2019, 76, 369–376. [Google Scholar] [CrossRef]
- Zuber, D. Biological Flora of Central Europe: Viscum album L. Flora-Morphol. Distrib. Funct. Ecol. Plants 2004, 203, 181–203. [Google Scholar] [CrossRef]
- Bussing, A. Mistletoe: The Genus Viscum; Bussing, A., Ed.; Harwood Academic Publisher: Reading, UK, 2000; Volume 16. [Google Scholar]
- Boericke, W. Manual of the Homeopathic Materia Medica; Karl F Haug Verlag: Heidelberg, Germany, 1992. [Google Scholar]
- Ramm, H. Mistletoe through Cultural and Medical History: The All-Healing Plant Proves to Be a Cancer-Specific Remedy. Transl. Res. Biomed. 2015, 4, 1–10. [Google Scholar]
- Song, C.; Wei, X.Y.; Qiu, Z.D.; Gong, L.; Chen, Z.Y.; Ma, Y.; Shen, Y.; Zhao, Y.J.; Wang, W.H.; Lai, C.J.S.; et al. Exploring the Resources of the Genus Viscum for Potential Therapeutic Applications. J. Ethnopharmacol. 2021, 277, 114233. [Google Scholar] [CrossRef] [PubMed]
- Jäger, T.; Holandino, C.; de Melo, M.N.O.; Peñaloza, E.M.C.; Oliveira, A.P.; Garrett, R.; Glauser, G.; Grazi, M.; Ramm, H.; Urech, K.; et al. Metabolomics by UHPLC-Q-TOF Reveals Host Tree-Dependent Phytochemical Variation in Viscum album L. Plants 2021, 10, 1726. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Fan, R.; Zhang, Q.; Zhang, Z.; Wang, D.; Xia, Y.; Ma, Y.; Yu, Z.; Zhao, Y. Simultaneous Identification and Quantification of the Common Compounds of Viscum coloratum and Its Corresponding Host Plants by Ultra-High Performance Liquid Chromatography with Quadrupole Time-of-Flight Tandem Mass Spectrometry and Triple Quadrupole Mass. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061, 176–184. [Google Scholar] [CrossRef]
- Patel, B.P.; Singh, P.K. Viscum articulum Burm. f.: A Review on Its Phytochemistry, Pharmacology and Traditional Uses. J. Pharm. Pharmacol. 2018, 70, 159–177. [Google Scholar] [CrossRef]
- Schaller, G.; Urech, K.; Grazi, G.; Giannattasio, M. Viscotoxin Composition of the Three European Subspecies of Viscum album. Planta Med. 1998, 64, 677–678. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Quality of Life in Cancer Patients Treated with Mistletoe: A Systematic Review and Meta-Analysis. BMC Complement. Med. Ther. 2020, 20, 227. [Google Scholar] [CrossRef]
- Hussain, M.A.; Khan, M.Q.; Hussain, N.; Habib, T. Antibacterial and Antifungal Potential of Leaves and Twigs of Viscum album L. J. Med. Plants Res. 2011, 5, 5545–5549. [Google Scholar]
- Anatole, P.C.; Jeanne, N.; Marieta, C. Polyphenol Contents of Five of Medicinal Plants from Cameroon and Effects of Their Extracts on Antioxidant Capacities of Human Breast Cancer Cells. Toxicol. Environ. Chem. 2014, 96, 1120–1130. [Google Scholar] [CrossRef]
- Yoo, J.-M.; Yang, J.-H.; Kim, Y.S.; Yang, H.J.; Cho, W.-K.; Ma, J.Y. Inhibitory Effects of Viscum coloratum Extract on IgE/Antigen-Activated Mast Cells and Mast Cell-Derived Inflammatory Mediator-Activated Chondrocytes. Molecules 2016, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Radenkovic, M.; Ivetic, V.; Popovic, M.; Brankovic, S.; Gvozdenovic, L. Effects of Mistletoe (Viscum album L., Loranthaceae) Extracts on Arterial Blood Pressure in Rats Treated with Atropine Sulfate and Hexocycline. Clin. Exp. Hypertens. 2009, 31, 11–19. [Google Scholar] [CrossRef]
- International Plant Names Index. Available online: https://www.ipni.org/ (accessed on 29 January 2023).
- The World Flora on Line. Available online: http://www.worldfloraonline.org/ (accessed on 30 January 2023).
- Dauncey, E.A.; Irving, J.; Allkin, R.; Robinson, N. Common Mistakes When Using Plant Names and How to Avoid Them. Eur. J. Integr. Med. 2016, 8, 597–601. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid. Based Complement. Altern. Med. 2015, 595393. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Panossian, A.; Kocharian, A.; Matinian, K.; Amroyan, E.; Gabrielian, E.; Mayr, C.; Wagner, H. Pharmacological Activity of Phenylpropanoids of the Mistletoe, Viscum album L., Host: Pyrus Caucasica Fed. Phytomedicine 1998, 5, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Batista, J.V.D.C.; Matos, A.P.S.; Oliveria, A.P.; Ricci Júnior, E.; Freitas, Z.M.; Oliveira, C.A.; Toma, H.K.; Capella, M.A.M.; Rocha, L.M.; Weissenstein, U.; et al. Thermoresponsive Hydrogel Containing Viscum album Extract for Topic and Transdermal Use: Development, Stability and Cytotoxicity Activity. Pharmaceutics 2022, 14, 37. [Google Scholar] [CrossRef]
- Abo-Elghiet, F.; Ibrahim, M.H.; El Hassab, M.A.; Bader, A.; Abdallah, Q.M.A.; Temraz, A. LC/MS Analysis of Viscum cruciatum Sieber Ex Boiss. Extract with Anti-Proliferative Activity against MCF-7 Cell Line via G0/G1 Cell Cycle Arrest: An in-Silico and in-Vitro Study. J. Ethnopharmacol. 2022, 295, 115439. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Abualhasan, M.; Jaradat, N.; Abu-Hasan, N.; Almasri, M.; Taha, A.A.; Rabbaa, A.; Natsheh, N.; Shalalfeh, S.; Najib, M. Bioactivity of Viscum album Extracts from Olive and Almond Host Plants in Palestine. Pharmacogn. J. 2014, 6, 117–123. [Google Scholar] [CrossRef]
- Ergun, F.; Deliorman, D.; Ozcelik, B.; Abbasoglu, U. Screening of Antifungal Activities of Various Viscum album L. Samples. Gazi Universitesi Eczacilik Fakultesi Dergisi 1997, 14, 1–4. [Google Scholar]
- Ertürk, Ö. Antibacterial and Antifungal Effects of Alcoholic Extracts of 41 Medicinal Plants Growing in Turkey. Czech J. Food Sci. 2010, 28, 53–60. [Google Scholar] [CrossRef]
- Özogul, F.; Kus, B.; Kuley, E. The Impact of Strawflower and Mistletoe Extract on Quality Properties of Rainbow Trout Fillets. Int. J. Food Sci. Technol. 2013, 48, 2228–2238. [Google Scholar] [CrossRef]
- Sengul, M.; Yildiz, H.; Gungor, N.; Bulent, C.; Eser, Z.; Ercisli, S. Total Phenolic Content, Antioxidant and Antimicrobial Activities of Some Medicinal Plants. Pak. J. Pharm. Sci. 2009, 22, 102–106. [Google Scholar]
- Shah, S.S.; Rehman, Y.U.; Iqbal, A.; Rahman, Z.U.; Zhou, B.; Peng, M.; Li, Z. Phytochemical Screening and Antimicrobial Activities of Stem, Leaves and Fruit Extracts of Viscum album L. J. Pure Appl. Microbiol. 2017, 11, 1337–1349. [Google Scholar] [CrossRef]
- Kang, S.-N. Ethanol Extracts from Mistletoe (Viscum album L.) Act as Natural Antioxidants and Antimicrobial Agents in Uncooked Pork Patties during Refrigerated Storage. Asian-Australas. J. Anim. Sci. 2016, 29, 109–118. [Google Scholar] [CrossRef]
- Karagöz, A.; Onay, E.; Arda, N.; Kuru, A. Antiviral Potency of Mistletoe (Viscum album Ssp. Album) Extracts against Human Parainfluenza Virus Type 2 in Vero Cells. Phytother. Res. 2003, 17, 560–562. [Google Scholar] [CrossRef]
- Ozpinar, H.; Ozpinar, N.; Eruygur, N. Effect of Viscum album L. Ssp. Austriacum (WİESP.) Vollman on Metronidazole Resistant and Sensitive Strains of Trichomonas Vaginalis. S. Afr. J. Bot. 2019, 125, 81–85. [Google Scholar] [CrossRef]
- Ertürk, Ö. Antifeedant and Toxicity Effects of Some Plant Extracts on Thaumetopoae solitaria Frey. (Lep.: Thaumetopoeidae). Turk. J. Biol. 2006, 30, 51–57. [Google Scholar]
- Melo, M.N.O.; Oliveira, A.P.; Wiecikowski, A.F.; Carvalho, R.S.; de Lima Castro, J.; de Oliveira, F.A.G.; Pereira, H.M.G.; da Veiga, V.F.; Capella, M.M.A.; Rocha, L.; et al. Phenolic Compounds from Viscum album Tinctures Enhanced Antitumor Activity in Melanoma Murine Cancer Cells. Saudi Pharm. J. 2018, 26, 311–322. [Google Scholar] [CrossRef]
- Önay-Üçar, E.O.; Arda, N.; Aitken, A. Extract from Mistletoe, Viscum album L., Reduces Hsp27 and 14-3-3 Protein Expression and Induces Apoptosis in C6 Rat Glioma Cells. Genet. Mol. Res. 2012, 11, 2801–2813. [Google Scholar] [CrossRef] [PubMed]
- Önay-Üçar, E.; Erol, Ö.; Kandemir, B.; Mertoglu, E.; Karagoz, A.; Arda, N. Viscum album L. Extracts Protects HeLa Cells against Nuclear and Mitochondrial DNA Damage. Evid.-Based Complement. Altern. Med. 2012, 2012, 958740. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Khan, M.Q.; Hussain, N. Antimicrobial Screening of Viscum album L. In Proceedings of the 2011 2nd International Conference on Environmental Science and Technology, Singapore, 26–28 February 2011; 2011; Volume 6, pp. 203–208. [Google Scholar]
- Pietrzak, W.; Nowak, R.; Gawlik-Dziki, U.; Lemieszek, M.K.; Rzeski, W. LC-ESI-MS/MS Identification of Biologically Active Phenolic Compounds in Mistletoe Berry Extracts from Different Host Trees. Molecules 2017, 22, 624. [Google Scholar] [CrossRef]
- Vlad, D.C.; Popescu, R.; Dumitrascu, V.; Cimporescu, A.; Vlad, C.S.; Vágvölgyi, C.; Krisch, J.; Dehelean, C.; Horhat, F.G. Phytocomponents Identification in Mistletoe (Viscum album) Young Leaves and Branches, by GC-MS and Antiproliferative Effect on HEPG2 and MCF7 Cell Lines. Farmacia J. 2016, 64, 82–87. [Google Scholar]
- Zelovitis, I.; Peschos, D.; Ragos, V.; Vlachou, A.-M.; Kontargiris, E.; Dhima, I.; Scaltsoyiannes, A.; Simos, Y.V.; Chatzidimitriou, M.; Zachariou, C.; et al. Viscum album L. & Abies Alba Borisii Regis Effects on Platelet Aggregation and Tumor Metastasis. J. Appl. Pharm. Sci. 2019, 9, 122–128. [Google Scholar] [CrossRef]
- Alpsoy, L.; Uzonur, I.; Sakcali, M.S.; Karaman, S. Antioxidant and Antimutagenic Activities of Viscum album Fruit Ethanolic Extract in Human Lymphocytes. Afr. J. Biotechnol. 2010, 9, 2539–2543. [Google Scholar]
- Yesilada, E.; Deliorman, D.; Ergun, F.; Takaishi, Y.; Ono, Y. Effects of the Turkish Subspecies of Viscum album on Macrophage-Derived Cytokines. J. Ethnopharmacol. 1998, 61, 195–200. [Google Scholar] [CrossRef]
- Fidan, I.; Ozkan, S.; Gurbuz, I.; Yesilyurt, E.; Erdal, B.; Yolbakan, S.; Imir, T. The Efficiency of Viscum album Ssp. Album and Hypericum Perforatum on Human Immune Cells In Vitro. Immunopharmacol. Immunotoxicol. 2008, 30, 519–528. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Maher, S.; Begum, A.; Abbaskhan, A.; Ali, S.; Khan, A.; Shafique-ur-Rehman; Atta-ur-Rahman. Characterization and Antiglycation Activity of Phenolic Constituents from Viscum album (European Mistletoe). Chem. Pharm. Bull. 2010, 58, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Orhan, N.; Hoşbaş, S.; Orhan, D.D.; Aslan, M.; Ergun, F. Enzyme Inhibitory and Radical Scavenging Effects of Some Antidiabetic Plants of Turkey. Iran. J. Basic Med. Sci. 2014, 17, 426–432. [Google Scholar] [PubMed]
- Lee, Y.M.; Kim, Y.S.; Lee, Y.; Kim, J.; Sun, H.; Kim, J.H.; Kim, J.S. Inhibitory Activities of Pancreatic Lipase and Phosphodiesterase from Korean Medicinal Plant Extracts. Phytother. Res. 2012, 26, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Bouchmaa, N.; Aazza, S.; Gaamoussi, F.; Lyoussi, B. Antioxidant, Anti-Inflammatory and Anti-Acetylcholinesterase Activities of Eleven Extracts of Moroccan Plants. Fresenius Environ. Bull. 2014, 23, 1375–1388. [Google Scholar]
- Kim, J.Y.; Do, J.-R.; Kwon, J.-H.; Kim, H.-K. Physiological Properties of Viscum album Var. Coloratum Extracts by Response Surface Methodology. J. Appl. Biol. Chem. 2010, 53, 330–336. [Google Scholar] [CrossRef]
- Adaramoye, O.; Amanlou, M.; Habibi-Rezaei, M.; Pasalar, P.; Ali, M.-M. Methanolic Extract of African Mistletoe (Viscum album) Improves Carbohydrate Metabolism and Hyperlipidemia in Streptozotocin-Induced Diabetic Rats. Asian Pac. J. Trop. Med. 2012, 5, 427–433. [Google Scholar] [CrossRef]
- Nwaegerue, E.; Nweke, I.N.; Ezeala, C.C.; Unekwe, P.C. Glucose Lowering Effect of Leaf Extracts of Viscum album in Normal and Diabetic Rats. J. Res. Med. Sci. 2007, 12, 235–240. [Google Scholar]
- Ko, B.-S.; Kang, S.; Moon, B.R.; Ryuk, J.A.; Park, S. A 70% Ethanol Extract of Mistletoe Rich in Betulin, Betulinic Acid, and Oleanolic Acid Potentiated β-Cell Function and Mass and Enhanced Hepatic Insulin Sensitivity. Evid.-Based Complement. Altern. Med. 2016, 2016, 7836823. [Google Scholar] [CrossRef]
- Orhan, D.D.; Aslan, M.; Sendogdu, N.; Ergun, F.; Yesilada, E. Evaluation of the Hypoglycemic Effect and Antioxidant Activity of Three Viscum album Subspecies (European Mistletoe) in Streptozotocin-Diabetic Rats. J. Ethnopharmacol. 2005, 98, 95–102. [Google Scholar] [CrossRef]
- Avci, G.; Kupeli, E.; Eryavuz, A.; Yesilada, E.; Kucukkurt, I. Antihypercholesterolaemic and Antioxidant Activity Assessment of Some Plants Used as Remedy in Turkish Folk Medicine. J. Ethnopharmacol. 2006, 107, 418–423. [Google Scholar] [CrossRef]
- Sevastre, B.; Olah, N.K.; Hanganu, D.; Sárpataki, O.; Taulescu, M.; Mǎnǎlǎchioae, R.; Marcus, I.; Cǎtoi, C. Viscum album L. Alcoholic Extract Enhance the Effect of Doxorubicin in Ehrlich Carcinoma Tumor Cells. Rom. Biotechnol. Lett. 2012, 17, 6975–6981. [Google Scholar]
- Stan, R.; Hangan, A.; Dican, L.; Sevastre, B.; Hanganu, D.; Catoi, C.; Sarpataki, O.; Ionescu, C. Comparative Study Concerning Mistletoe Viscotoxins Antitumor Activity. Acta Biol. Hung. 2013, 64, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, D.; Kaushik, D.; Kumar, S. Screening of Neuropharmacological Activities of Viscum album and Estimation of Major Flavonoid Constituents Using TLC Densitometry. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 179–186. [Google Scholar]
- Şekeroǧlu, V.; Aydin, B.; Şekeroǧlu, Z.A. Viscum album L. Extract and Quercetin Reduce Cyclophosphamide-Induced Cardiotoxicity, Urotoxicity and Genotoxicity in Mice. Asian Pac. J. Cancer Prev. 2011, 12, 2925–2931. [Google Scholar]
- Şekeroĝlu, Z.A.; Şekeroĝlu, V. Effects of Viscum album L. Extract and Quercetin on Methotrexate-Induced Cyto-Genotoxicity in Mouse Bone-Marrow Cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 746, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.O.; Park, S.Y.; Hong, K.H.; Kang, L.W.; Choe, K.H.; Kim, Y.K. Bleeding Time Prolongation Effect of Methanol Extract of Viscum album Var. Coloratum. Nat. Prod. Sci. 2002, 8, 152–154. [Google Scholar]
- Karagöz, A.; Kesici, S.; Vural, A.; Usta, M.; Tezcan, B.; Semerci, T.; Teker, E. Cardioprotective Effects of Viscum album L. ssp. Album (Loranthaceae) on Isoproterenol-Induced Heart Failure via Regulation of the Nitric Oxide Pathway in Rats. Anatol. J. Cardiol. 2016, 16, 923–930. [Google Scholar] [CrossRef]
- Ergun, F.; Deliorman, D.; Akar, F.; Ark, M.; Kanzik, I. Screening of Various Viscum album L. Samples for Their Possible Vascular Effects. Gazi Universitesi Eczacilik Fakultesi Dergisi 1995, 12, 153–158. [Google Scholar]
- Deliorman, D.; Çaliş, I.; Ergun, F.; Dogan, B.S.U.; Buharalioglu, C.K.; Kanzik, I. Studies on the Vascular Effects of the Fractions and Phenolic Compounds Isolated from Viscum album ssp. Album. J. Ethnopharmacol. 2000, 72, 323–329. [Google Scholar] [CrossRef]
- Suveren, E.; Baxter, G.F.; Iskit, A.B.; Turker, A.U. Cardioprotective Effects of Viscum album L. Subsp. Album (European Misletoe) Leaf Extracts in Myocardial Ischemia and Reperfusion. J. Ethnopharmacol. 2017, 209, 203–209. [Google Scholar] [CrossRef]
- Khan, T.; Ali, S.; Qayyum, R.; Hussain, I.; Wahid, F.; Shah, A.J. Intestinal and Vascular Smooth Muscle Relaxant Effect of Viscum album Explains Its Medicinal Use in Hyperactive Gut Disorders and Hypertension. BMC Complement. Altern. Med. 2016, 16, 251. [Google Scholar] [CrossRef] [PubMed]
- Poruthukaren, K.J.; Palatty, P.L.; Baliga, M.S.; Suresh, S. Clinical Evaluation of Viscum album Mother Tincture as an Antihypertensive: A Pilot Study. J. Evid.-Based Complement. Altern. Med. 2014, 19, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Oluwaseun, A.A.; Ganiyu, O. Antioxidant Properties of Methanolic Extracts of Mistletoes (Viscum album) from Cocoa and Cashew Trees in Nigeria. Afr. J. Biotechnol. 2008, 7, 3138–3142. [Google Scholar]
- Osunlana, O.R.; Bello, M.O.; Johnson, J.A.; Afolabi, O.B. Antioxidant, Compositional Evaluation and Blood Pressure Modulating Potentials of Bryophyllum pinnatum (Lam.), Viscum album (L.) and Artocarpus altilis (Parkinson) Leave Extracts. Potravin. Slovak J. Food Sci. 2018, 12, 422–430. [Google Scholar] [CrossRef]
- Papuc, C.; Crivineanu, M.; Goran, G.; Nicorescu, V.; Durdun, N. Free Radicals Scavenging and Antioxidant Activity of European Mistletoe (Viscum album) and European Birthwort (Aristolochia clematitis). Revista Chimie 2010, 61, 619–622. [Google Scholar]
- Pietrzak, W.; Nowak, R.; Olech, M. Effect of Extraction Method on Phenolic Content and Antioxidant Activity of Mistletoe Extracts from Viscum album Subsp. Abietis. Chem. Pap. 2014, 68, 976–982. [Google Scholar] [CrossRef]
- Vicas, S.; Prokisch, J.; Rugina, D.; Socaciu, C. Hydrophilic and Lipophilic Antioxidant Activities of Mistletoe (Viscum album) as Determined by FRAP Method. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 112–116. [Google Scholar]
- Vicaş, S.I.; Ruginǎ, D.; Socaciu, C. Comparative Study about Antioxidant Activities of Viscum album from Different Host Trees, Harvested in Different Seasons. J. Med. Plants Res. 2011, 5, 2237–2244. [Google Scholar]
- Condrat, D.; Szabo, M.-R.; Crişan, F.; Lupea, A.-X. Antioxidant Activity of Some Phanerogam Plant Extracts. Food Sci. Technol. Res. 2009, 15, 95–98. [Google Scholar] [CrossRef]
- Amer, B.; Juvik, O.J.; Francis, G.W.; Fossen, T. Novel GHB-Derived Natural Products from European Mistletoe (Viscum album). Pharm. Biol. 2013, 51, 981–986. [Google Scholar] [CrossRef]
- Cao, D.; Wang, L.-Q.; Han, X.-M.; Guan, H.-R.; Lei, M.; Wei, Y.-H.; Cheng, L.; Yang, P.-M.; Sun, Z.-L.; Gao, W.; et al. Two Symmetrical Unsaturated Acids Isolated from Viscum album. Chin. J. Nat. Med. 2019, 17, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Önay-Uçar, E.; Karagöz, A.; Arda, N. Antioxidant Activity of Viscum album Ssp. Album. Fitoterapia 2006, 77, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Orhan, D.D.; Senol, F.S.; Hosbas, S.; Orhan, I.E. Assessment of Cholinesterase and Tyrosinase Inhibitory and Antioxidant Properties of Viscum album L. Samples Collected from Different Host Plants and Its Two Principal Substances. Ind. Crops Prod. 2014, 62, 341–349. [Google Scholar] [CrossRef]
- Condrat, D.; Crisan, F.; Szabo, M.-R.; Chambree, D.-R.; Lupea, A.-X. Flavonoids in Angiosprmatophyta and Spermatophyta Species and Their Antioxidant Activity. Revista Chimie 2009, 60, 1129–1134. [Google Scholar]
- Kaur, A.; Kumar, D.; Kumar, S. Isolation, Characterization and Estimation of Marker Compound of Viscum album L. Indian Drugs 2016, 53, 20–25. [Google Scholar] [CrossRef]
- Fukunaga, T.; Kajikawa, I.; Nishiya, K.; Takeya, K.; Itokaw, H. Studies on the Constituents of the Japanese Mistletoe, Viscum album L: Var: Coloratum Ohwi Grown on Different Host Trees. Chem. Pharm. Bull. 1989, 37, 1300–1303. [Google Scholar] [CrossRef]
- Arda, N.; Önay, E.; Koz, Ö.; Kirmizigül, S. Monosaccharides and Polyols from Mistletoes (Viscum album L.) Growing on Two Different Host Species. Biol. Sect. Cell. Mol. Biol. 2003, 58, 1037–1041. [Google Scholar]
- Fukunaga, T. Studies on the Constituents of the European Mistletoe, Viscum album L. Chem. Pharm. Bull. 1988, 36, 1185–1189. [Google Scholar] [CrossRef]
- Vicaş, S.I.; RuginǍ, D.; Leopold, L.; Pintea, A.; Socaciu, C. HPLC Fingerprint of Bioactive Compounds and Antioxidant Activities of Viscum album from Different Host Trees. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 48–57. [Google Scholar] [CrossRef]
- Deliorman, D.; Çaliş, I.; Ergun, F.; Tamer, U. The Comparative Studies on Phenylpropanoid Glycosides of Viscum album Subspecies by High Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 1999, 22, 3101–3114. [Google Scholar] [CrossRef]
- Deliorman, D.; Ergun, F. High Performance Liquid Chromatographic Determination of Syringin in Viscum album L. ssp. Album Samples Collected from Different Host Plants. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 3033–3042. [Google Scholar] [CrossRef]
- Hanousek Čiča, K.; Rupert, M.; Koczoń, P.; Derewiaka, D.; Gajdoš-Kljusurić, J.; Petravić-Tominac, V.; Mrvčić, J.; Stanzer, D. Characterisation of Flavour Compounds in Biska—A Herbal Spirit Produced with Mistletoe. J. Inst. Brew. 2019, 125, 143–154. [Google Scholar] [CrossRef]
- Janakat, S.; Al-Thnaibat, O. Antilipoperoxidative Effect of Three Edible Plants Extracts: Viscum album, Arum Dioscoridis and Eminium spiculatum. J. Food Qual. 2008, 31, 1–12. [Google Scholar] [CrossRef]
- Pieme, C.A.; Penlap, V.N.; Ngogang, J.; Costache, M. In Vitro Cytotoxicity and Antioxidant Activities of Five Medicinal Plants of Malvaceae Family from Cameroon. Environ. Toxicol. Pharmacol. 2010, 29, 223–228. [Google Scholar] [CrossRef]
- Trifunschi, S.I.; Munteanu, M.F.; Orasan-Alic, V.-E.; Gligor-Dinca, F.S.; Gligor, R. A Comparative Study of the Antioxidant Activity of Garlic and Mistletoe Extracts. Revista Chimie 2016, 67, 460–462. [Google Scholar]
- Holandino, C.; de Melo, M.N.O.; Oliveira, A.P.; da Batista, J.V.C.; Capella, M.A.M.; Garrett, R.; Grazi, M.; Ramm, H.; Torre, C.D.; Schaller, G.; et al. Phytochemical Analysis and in Vitro Anti-Proliferative Activity of Viscum album Ethanolic Extracts. BMC Complement. Med. Ther. 2020, 20, 215. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green Synthesis of Silver Nanoparticles Using Natural Extracts with Proven Antioxidant Activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef]
- García-García, J.D.; Anguiano-Cabello, J.C.; Arredondo-Valdés, R.; Del Toro, C.A.C.; Martínez-Hernández, J.L.; Segura-Ceniceros, E.P.; Govea-Salas, M.; González-Chávez, M.L.; Ramos-González, R.; Esparza-González, S.C.; et al. Phytochemical Characterization of Phoradendron bollanum and Viscum album Subs. Austriacum as Mexican Mistletoe Plants with Antimicrobial Activity. Plants 2021, 10, 1299. [Google Scholar] [CrossRef]
- Majeed, M.; Pirzadah, T.B.; Mir, M.A.; Hakeem, K.R.; Alharby, H.F.; Alsamadany, H.; Bamagoos, A.A.; Rehman, R.U. Comparative Study on Phytochemical Profile and Antioxidant Activity of an Epiphyte, Viscum album L. (White Berry Mistletoe), Derived from Different Host Trees. Plants 2021, 10, 1191. [Google Scholar] [CrossRef]
- Pozdnyakov, D.I.; Leont’evna Adzhiahmetova, S.; Chervonnaya, N.M.; Voronkov, A.V.; Oganesyan, E.T. Some Aspects of the Adaptogenic Potential of European Mistletoe (Viscum album L.) Extracts under Variable Physical Performance. J. Med. Plants 2021, 20, 60–78. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, Y.S.; Jung, D.Y.; Kim, N.Y.; Lim, H.; Kim, H.P. Viscum album and Its Constituents Downregulate Mmp-13 Expression in Chondrocytes and Protect Cartilage Degradation. Nat. Prod. Sci. 2021, 27, 151–160. [Google Scholar]
- Szurpnicka, A.; Wrońska, A.K.; Bus, K.; Kozińska, A.; Jabłczyńska, R.; Szterk, A.; Lubelska, K. Phytochemical Screening and Effect of Viscum album L. on Monoamine Oxidase A and B Activity and Serotonin, Dopamine and Serotonin Receptor 5-HTR1A Levels in Galleria mellonealla (Lepidoptera). J. Ethnopharmacol. 2022, 298, 115604. [Google Scholar] [CrossRef]
- Milošević, M.D.; Mašković, P.Z.; Stanković, V.D.; Paunović, M.G.; Mitić, M.N.; Matić, M.M.; Ognjanović, B.I. Protective Effects of Viscum album L. Leaf Extract on Chlorpyrifos-Induced Hepatotoxicity in Wistar Rats. J. King Saud Univ. Sci. 2022, 34, 101957. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Ghollasimood, S.; Ahmadpour, N.; Homaeigohar, S. Biosynthesis of the ZnO/SnO2 Nanoparticles and Characterization of Their Photocatalytic Potential for Removal of Organic Water Pollutants. J. Photochem. Photobiol. A Chem. 2022, 425, 113662. [Google Scholar] [CrossRef]
- Pietrzak, W.; Nowak, R. Impact of Harvest Conditions and Host Tree Species on Chemical Composition and Antioxidant Activity of Extracts from Viscum album L. Molecules 2021, 26, 3741. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Naderi Farsani, M.; Ghafarifarsani, H.; Hoseinifar, S.H.; van Doan, H. The Effects of Dietary Supplementation of Mistletoe (Viscum album) Extract on the Growth Performance, Antioxidant, and Innate, Immune Responses of Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2021, 536, 736385. [Google Scholar] [CrossRef]
- Erdaş, M.; Akyüz, F.; Can, B.; Özkoç, M.; Öz, S.; Dönmez, D.B. In Vivo Effects of Viscum album and Probiotics against Carbon Tetrachloride-Induced Liver Injury. J. Bioenerg. Biomembr. 2021, 53, 139–148. [Google Scholar] [CrossRef]
- Stefanucci, A.; Zengin, G.; Llorent-Martinez, E.J.; Dimmito, M.P.; Della Valle, A.; Pieretti, S.; Ak, G.; Sinan, K.I.; Mollica, A. Viscum album L. Homogenizer-Assisted and Ultrasound-Assisted Extracts as Potential Sources of Bioactive Compounds. J. Food Biochem. 2020, 44, e13377. [Google Scholar] [CrossRef]
- Yildiz, Ü.C.; Kiliç, C.; Gürgen, A.; Yildiz, S. Possibility of Using Lichen and Mistletoe Extracts as Potential Natural Wood Preservative. Maderas. Ciencia Tecnología 2020, 22, 179–188. [Google Scholar] [CrossRef]
- Pieme, C.A.; Ngogang, J.; Costache, M. In Vitro Antiproliferative and Antioxidant Activities of Methanol Extracts of Urena lobata and Viscum album against Breast Cancer Cell Lines. Toxicol. Environ. Chem. 2012, 94, 987–999. [Google Scholar] [CrossRef]
- Jadhav, R.B.B.; Bhatnagar, S.P.P.; Surana, S.J.J. Diuretic Activity of Squamate mistletoe, Viscum angulatum. Pharm. Biol. 2010, 48, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, R.S.; Sardesai, M.M.; Mishra, V. Anticancer Potential of Leafless Mistletoe (Viscum angulatum) from Western Ghats of India. Int. J. Pharm. Sci. Res. 2018, 9, 1902–1907. [Google Scholar] [CrossRef]
- Garg, S.; Patil, U.K.; Shrivastava, T.P. Antimicrobial Potential of Viscum articulum Brm., An Ethnomedicinal Plant. Res. J. Pharm. Technol. 2013, 6, 649–651. [Google Scholar]
- Nag, M.; Mukherjee, P.K.; Biswas, R.; Chanda, J.; Kar, A. Evaluation of Antimicrobial Potential of Some Indian Ayurvedic Medicinal Plants. Pharmacogn. J. 2016, 8, 525–533. [Google Scholar] [CrossRef]
- Chamlagai, D.; Singh, B. Study of In Vitro Anti-Inflammatory Activity of Ethnomedicinal Plants of Sikkim Viscum articulum and Acorus Calamus. Asian J. Pharm. Clin. Res. 2016, 9, 119–122. [Google Scholar]
- Devi, B.N.; Salma, S.; Lavanya, V. Phytochemical Evaluation and In Vitro Antidiabetic Activity of Ethanolic Extract of Viscum articulum. Int. J. Pharm. Sci. Rev. Res. 2020, 60, 99–104. [Google Scholar]
- Vadnere, G.; Pathan, A.; Kulkarni, B. Hypolipidemic Potential of Viscum articulum Extracts in Albino Mice. Indian J. Pharmacol. 2013, 45, S166. [Google Scholar]
- Jadhav, N.; Patil, C.R.; Chaudhari, K.B.; Wagh, J.P.; Surana, S.J.; Jadhav, R.B. Diuretic and Natriuretic Activity of Two Mistletoe Species in Rats. Pharmacogn. Res. 2010, 2, 50–57. [Google Scholar] [CrossRef]
- Bachhav, S.S.; Bhutada, M.S.; Patil, S.D.; Baser, B.; Chaudhari, K.B. Effect of Viscum articulum Burm. (Loranthaceae) in Nω- Nitro-l-Arginine Methyl Ester Induced Hypertension and Renal Dysfunction. J. Ethnopharmacol. 2012, 142, 467–473. [Google Scholar] [CrossRef]
- Nag, M.; Kar, A.; Chanda, J.; Mukherjee, P.K. RP-HPLC Analysis of Methanol Extract of Viscum articulum—A Plant from Ayurveda. J. Ayurveda Integr. Med. 2020, 11, 277–280. [Google Scholar] [CrossRef]
- Amabeoku, G.J.; Leng, M.J.; Syce, J.A. Antimicrobial and Anticonvulsant Activities of Viscum capense. J. Ethnopharmacol. 1998, 61, 237–241. [Google Scholar] [CrossRef]
- Petrus, A.J.A. Antioxidative Constitution of the Mistletoe, Viscum capitellatum Smith. Asian J. Chem. 2011, 23, 3014–3020. [Google Scholar]
- Jadhav, V.S.; Ghawate, V.B.; Jadhav, R.B.; Surana, S.J.; Kalaskar, M.G. Neuropharmacological Activities of Methanolic Extract of Leaves of Viscum capitellatum Smith. Bull. Pharm. Sci. 2022, 45, 129–137. [Google Scholar] [CrossRef]
- Yang, G.-E.; Chen, B.; Zhang, Z.; Gong, J.; Bai, H.; Li, J.; Wang, Y.; Li, B. Cytotoxic Activities of Extracts and Compounds from Viscum coloratum and Its Transformation Products by Rhodobacter sphaeroides. Appl. Biochem. Biotechnol. 2009, 152, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-M.; Park, K.I.; Ma, J.Y. Anticolitic Effect of Viscum coloratum through Suppression of Mast Cell Activation. Am. J. Chin. Med. 2019, 47, 203–221. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Zhao, Y.; Gao, X.; Bi, K.; Yu, Z. HPLC Determination and Pharmacokinetic Study of Homoeriodictyol-7-O-Beta-D-Glucopyranoside in Rat Plasma and Tissues. Biol. Pharm. Bull. 2007, 30, 617–620. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Z.; Fan, R.; Gao, X.; Yu, M.; Li, H.; Wei, H.; Bi, K. Simultaneous Determination of Ten Flavonoids from Viscum coloratum Grown on Different Host Species and Different Sources by LC-MS. Chem. Pharm. Bull. 2011, 59, 1322–1328. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Hou, J.P.; Huang, L.; Khan, A.; Xing, F.F.; Zhang, X.H.; Han, D.F.; Yan, S.L.; Cao, G.D.; Jiao, Q.Y.; et al. Chemical Constituents of Viscum coloratum (Kom.) Nakai and Their Cytotoxic Activities. Nat. Prod. Res. 2022, 36, 1927–1933. [Google Scholar] [CrossRef]
- Zhang, R.Z.; Zhao, J.T.; Wang, W.Q.; Fan, R.H.; Rong, R.; Yu, Z.G.; Zhao, Y.L. Metabolomics-Based Comparative Analysis of the Effects of Host and Environment on Viscum coloratum Metabolites and Antioxidative Activities. J. Pharm. Anal. 2022, 12, 243–252. [Google Scholar] [CrossRef]
- Song, C.; Wang, W.; Xue, Z.; Peng, H.; Yang, B. Characterization of the Interaction between Viscum coloratum (Mistletoe) and Its Host by Ultra-High Performance Liquid Chromatography–Quadrupole–Time-of-Flight–Mass Spectrometry (UPLC/Q-TOF-MS)-Based Metabolomics. Anal. Lett. 2022, 55, 2574–2589. [Google Scholar] [CrossRef]
- Moyo, B.; Tavengwa, N.T.; Madala, N.E. Diverse Chemical Modifications of the Chlorogenic Acid Composition of Viscum combreticola Engl.: A Premise for the State of Readiness against Excessive Sunlight Exposure. J. Photochem. Photobiol. B 2022, 233, 112501. [Google Scholar] [CrossRef] [PubMed]
- Bahizire, K.; Bagalwa, M.; Bashwira, S.; Basabose, K.; Bagalwa, B. In Vitro Phytochemical Screening and Anthelmintic Activity of Viscum congolensis and Galiniera coffeoides against Adult Earthworm Alma Emini. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 753–757. [Google Scholar]
- Gómez, M.A.; Sáenz, M.T.; García, M.D.; Ahumada, M.C.; De La Puerta, R. Cytostatic Activity against HEp-2 Cells of Methanol Extracts from Viscum cruciatum Sieber Parasitic on Crataegus monogyna Jacq. and Two Isolated Principles. Phytother. Res. 1997, 11, 240–242. [Google Scholar] [CrossRef]
- Martín-Cordero, C.; López-Lázaro, M.; Agudo, M.A.A.; Navarro, E.; Trujillo, J.; Ayuso, M.J.J.; Martin-Cordero, C.; Lopez-Lazaro, M.; Agudo, M.A.A.; Navarro, E.; et al. A Cytotoxic Diarylheptanoid from Viscum cruciatum. Phytochemistry 2001, 58, 567–569. [Google Scholar] [CrossRef]
- Gilani, A.H.; Mehmood, M.H.; Janbaz, K.H.; Khan, A.-U.; Saeed, S.A. Gut Modulatory and Antiplatelet Activities of Viscum cruciatum. Pharm. Biol. 2009, 47, 955–961. [Google Scholar] [CrossRef]
- Alali, F.Q.; Tawaha, K.; El-Elimat, T.; Syouf, M.; El-Fayad, M.; Abulaila, K.; Nielsen, S.J.; Wheaton, W.D.; Falkinham, J.O.; Oberlies, N.H. Antioxidant Activity and Total Phenolic Content of Aqueous and Methanolic Extracts of Jordanian Plants: An ICBG Project. Nat. Prod. Res. 2007, 21, 1121–1131. [Google Scholar] [CrossRef]
- Wei, X.Y.; Guo, W.J.; Chen, Z.Y.; Qiu, Z.D.; Guo, J.; Cui, G.H.; Wang, Y.N.; Gong, L.; Chen, L.Y.; Lai, C.J.S.; et al. Chemical-Activity-Based Quality Marker Screening Strategy for Viscum articulum. Biomed. Chromatogr. 2021, 35, e5175. [Google Scholar] [CrossRef]
- Ahmmed, F.; Kumar Sarkar, K.; Hossain, L.; Hossin, A.; Mondal, H.; Islam, J.; Ahmed, I. Phytochemical and Pharmacological Investigation of Viscum monoicum (Whole Plant). Pharmacologyonline 2013, 1, 237–243. [Google Scholar]
- Lin, C.-C.; Lin, J.-M.; Chang, C.-H.; Hattori, M.; Namba, T. Pharmacological Studies on the Crude Drug ‘Hwang-Jin-Guey’ from Taiwan (1). Phytother. Res. 1994, 8, 193–200. [Google Scholar] [CrossRef]
- Khatun, A.; Rahman, M.; Haque, T.; Rahman, M.M.; Akter, M.; Akter, S.; Jhumur, A. Cytotoxicity Potentials of Eleven Bangladeshi Medicinal Plants. Sci. World J. 2014, 2014, 913127. [Google Scholar] [CrossRef]
- Khatun, A.; Rahman, M.; Rahman, M.; Hossain, H.; Jahan, I.A.; Nesa, L. Antioxidant, Antinociceptive and CNS Activities of Viscum orientale and High Sensitive Quantification of Bioactive Polyphenols by UPLC. Front. Pharmacol. 2016, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Malada, P.M.; Mogashoa, M.M.; Masoko, P. The Evaluation of Cytotoxic Effects, Antimicrobial Activity, Antioxidant Activity and Combination Effect of Viscum rotundifolium and Mystroxylon aethiopicum. S. Afr. J. Bot. 2022, 147, 790–798. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Alghamdi, D.O.; Al-Salem, M.S.; Alattas, M.; El-Bassossy, H.M.; Alahdal, A.M.; Shehata, I.A.; Abdel-Sattar, E. Effect of Viscum schimperi on Advanced Glycation Endproducts Formation. Pak. J. Pharm. Sci. 2016, 29, 2307–2316. [Google Scholar] [PubMed]
- Abdallah, H.M.; Farag, M.A.; Abdel-Naim, A.B.; Ghareib, S.A.; Abdel-Sattar, E.A. Mechanistic Evidence of Viscum schimperi (Viscaceae) Antihyperglycemic Activity: From a Bioactivity-Guided Approach to Comprehensive Metabolite Profiling. Phytother. Res. 2015, 29, 1737–1743. [Google Scholar] [CrossRef]
- Abdel-Sattar, E.A.; Elberry, A.A.; Harraz, F.M.; Ghareib, S.A.; Nagy, A.A.; Gabr, S.A. Antihyperglycemic and Hypolipidaemic Effects of the Methanolic Extract of Saudi Mistletoe (Viscum schimperi Engl.). J. Adv. Res. 2011, 2, 171–177. [Google Scholar] [CrossRef]
- Anne, A.; Peter, M.; Henning, A. Angiotensin-Converting Enzyme Inhibitory Activity of Viscum triflorum Is Host Plant-Dependent. Pharm. Biol. 2011, 49, 302–305. [Google Scholar] [CrossRef]
- European Medicines Agency. Assessment Report on Viscum album L., Herba. 2013. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-viscum-album-l-herba_en.pdf (accessed on 6 March 2023).
- Bonamin, L.V.; Carvalho, A.C.D.E.; Waisse, S. Viscum album (L.) in Experimental Animal Tumors: A Meta-Analysis. Exp. Ther. Med. 2017, 13, 2723–2740. [Google Scholar] [CrossRef]
- Marvibaigi, M.; Supriyanto, E.; Amini, N.; Abdul Majid, F.A.; Jaganathan, S.K. Preclinical and Clinical Effects of Mistletoe against Breast Cancer. BioMed Res. Int. 2014, 2014, 785479. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxidative Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]




| Species | Assay | Microorganism/Virus | Main Results | Authors |
|---|---|---|---|---|
| V. album | Disc diffusion assay | Alcaligenes feacalis, Acinetobacter lwoffi, Bacillus cereus, Bacillus subtilis, Cladosporium herbarum, Escherichia coli, Klebsiella pneumonia subsp. pneumonia, Pseudomonas aeruginosa, Providencia alcaliaciens, Penicillium roquefortii, Proteus vulgaris, Staphylococcus hominis | MeOH extract had activity against 12 out of 32 microorganisms tested (IZ 1–11 mm). | [29] |
| V. album | Colony formation assay | n.d | CFU changed during the storage period of the 14 days, and it was lower than the positive control. Log CFU in 14 days: positive control 5.27 ± 0.41 × 5.06 ± 0.18 of the extract 1%. | [31] |
| V. album | Disc diffusion assay | Aspergillus flavus, Bacillus subtilis, Bordetella bronchisiptica, Enterococcus faecium, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa, Pseudomonas syringae, Saccharomyces cerevisae, Staphylococcus aureus | EtOH and MeOH extracts (100 mg/mL) presented different inhibitory activity according to plant parts used: leaves IZ 15–20 mm, branches IZ 9–24 mm. Saccharomyces cerevisiae and Aspergillus flavus were not sensitive to extracts in the tested conditions. | [12] |
| V. album | Agar dilution method | Candida guilliermondii, Cryptococcus neoformans, Microsporum canis, Tricophyton mentagropytes | EtOH extracts were potent against all tested microorganisms with minimum inhibitory dilution between 0.04 and 3.13%. Activity was host tree dependent. | [26] |
| V. album | Disc diffusion assay | Agrobacterium tumefaciens, Bacillus atrophaeus, Bacillus subtilis, Candida albicans, Erwinia carotovora, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, Staphylococcus aureus | MeOH and BuOH extracts showed activity against all Gram-positive and Gram-negative bacteria and fungus tested and were dependent of the plant part used and the microorganism evaluated: stems IZ 15–35 mm; leaves IZ 15–30 mm; fruits IZ 15–35 mm. | [30] |
| V. album | Agar dilution method | Lactic acid bacteria, Enterobacteriaceae | Latic acid bacteria counts in negative control and V. album group were 2.67 and 2.44 log CFU g−1 at 6 days in the storage period. Enterobacteriaceae count in control and V. album group reached 7.05 and 7.54 log CFU g−1 at 23 days, respectively. | [28] |
| V. album | Disc diffusion assay and MIC in 24 well plate | Aspergillus niger, Bacillus subtilis, Candida albicans, Esherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | EtOH extract showed antibacterial and antifungal activities against all the strains tested, IZ 1–11 mm depending on the microorganism and MIC 0.5–1.0 μg/mL. | [27] |
| V. album | Plaque assay | Human parainfluenza virus type 2 (HPIV-2) | EtOH extract (25 µg/mL) did not affect the growth rate or viability in VERO cells and was inactive in HPIV-2 plaque formation on Vero cells. | [32] |
| V. album | Fungal decay test in wood | Coniophora puteana | MeOH extract at 18,75% showed the lowest wood weight loss (7.97%). | [103] |
| V. articulatum | Disc diffusion assay | Esherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | EtOH extract (200 µg/mL) presented inhibitory activity against the microorganisms tested, IZ 9–20 mm depending on the microorganism evaluated. | [107] |
| V. articulatum | Disc diffusion assay, broth micro-dilution assay for MIC and MBC | Bacillus cereus, Bacillus subtilis, Esherichia coli, Pseudomonas aureugenosa, Staphylococcus aureus, Staphylococcus typhi | MeOH extract (50–2000 mg/disc) inhibited Staphylococcus aureus and Esherichia coli (MIC of 728 and 920 µg/mL, respectively), with MBC of 1456 µg/mL and 1840 µg/mL and IZ 11.4 ± 0.05 and 10.1 ± 0.06 to Staphylococcus aureus and Esherichia coli, respectively. | [108] |
| V. capense | Disc diffusion assay | Candida albicans, Staphylococcus aureus, Pseudomonas aeruginosa | MeOH extract (40µL) showed inhibition only on S. aureus growth with IZ 12.8 mm | [115] |
| V. monoicum | Disc diffusion assay | Esherichia coli, Salmonella paratyphi, Salmonella typhi, Shigella dysenteriae, Staphylococcus aureus, Staphylococcus epidermis | EtOH extract at 250 and 500 µg/disc presented an tibacterial activity against all tested bacteria with IZ of the 6.35 to 9.10 mm and 10.25 to 13.00 mm, respectively. | [132] |
| V. rotundifolium | Micro-dilution assay | Staphylococcus aureus, Enterococcus faecalis, Pseudomonas aeruginosa, Escherichia coli | MeOH extract had MIC values range from 0.31 to 2.5 mg/mL. | [136] |
| Species | Assay | Microorganism | Main Results | Authors |
|---|---|---|---|---|
| V. album | Movement of flagella and undulating membranes | Metronidazole-resistant Trichomonas vaginalis; metronidazole-sensitive Trichomonas vaginalis | EtOH fractions (1.25–10 mg/mL) presented MLD (minimum lethal dose) varying from 5 to 10 mg/mL. | [33] |
| V. album | Absolute deterrence coefficient and toxicity to Larvae | Thaumetopoae solitaria | EtOH extract (1000 mg) showed antifeedant (36.71) effect. Toxicity effect reached the LC50 value of 60%. Thus, it can be used as a cheaper alternative for chemical pesticides and diminish environmental pollution. | [34] |
| V. congolensis | Petri dish with extracts | Alma emini worms | EtOH extract (19 mg/mL) reached 100% of mortality after 24 h. LC50 (1.65 mg/mL). | [126] |
| Species | Assay | Cell Line | Main Results | Authors |
|---|---|---|---|---|
| V. album | MTT | Human breast adenocarcinoma (MDA-MB-231) | MeOH extract (50–1000 mg/L) presented IC50 of 12.75 mg/L | [13] |
| V. album | MTT; Apoptotic activity; FITC Annexxin V and propidium iodide (PI) | Murine melanoma (B16F10), human chronic myelogenous leukaemia (K562), monkey kidney (MA-104) | EtOH extracts presented a dose-response activity (1–5% v/v), with higher potential against B16F10 and K562 than MA-104. Annexin-V/FITC demonstrated early and late apoptotic markers on cancer cells. | [35] |
| V. album | MTT; Apoptotic activity; caspase-3 assay | Rat glioma (C6) | MeOH extract presented IC50 = 270 µg/mL on C6 cells. It decreased the expression of Hsp27 (73%), 14-3-3β (124%), 14-3-3γ (23%) and 14-3-3ζ (84%), thus downregulating the expression of chaperone proteins and inducing apoptosis via caspase-3. | [36] |
| V. album | MTT | Human cervical carcinoma (HeLa) | MeOH extracts (host tree Tilia argentea, Acer campestre and Robinia pseudoacacia) decreased the viability of HeLa cells in a dose-dependent manner (IC50 93, 165, 85 µg/mL, respectively). Extracts from Robinia and Tilia host trees completely prevented nuclear and mitochondrial DNA damage. | [37] |
| V. album | MTT, BrdU immunoassay, lactate dehydrogenase (LDH) toxicology assay | Human colon adenocarcinoma (LS180), Human colon epithelial (CCD 841 CoTr) | MEOH extract had antiproliferative activity in a dose-dependent manner, reaching 30% of reduction at 100µg/mL. IC50 were 1 mg/mL, 164 µg/mL, 202 µg/mL, from host tree Pinus sylvestris, Tilia cordata, Populus nigra, respectively. BrdU assay demonstrated no effect on the DNA synthesis. LDH test showed nontoxic effect, indicating protective properties. | [39] |
| V. album | MTT | Human breast adenocarcinoma (MCF-7), human hepato- cellular carcinoma (HepG2) | EtOH and MeOH extracts (15–75 mg/mL) presented antiproliferative activity in a dose dependent manner. | [40] |
| V. album | MTT | Human hepatocyte carcinoma (HepG-2) | MeOH extract (50–1000 µg/mL) decreased cell viability in a concentration-dependent manner (CC50 of 456.5 µg/mL) | [88] |
| V. album | MTT and cytometry | Human acute lymphoblastic leucemia (Molt-4), Mouse sarcoma (Yoshida) | EtOH extract of V. album from Abies alba presented IC50 of 0.07 ± 0.01% v/v and 0.05 ± 0.03% v/v (Molt-4 and Yoshida, respectively). Necrotic potential of Abietis, Malus and Quercus was observed. | [90] |
| V. album | MTT | Breast cancer (MB-MDA 435) | MeOH extract (50–1000 mg/L) presented IC50 of 172 mg/L, presenting significant antiproliferative property | [104] |
| V. angulatum | MTT | Human breast adenocarcinoma (MDA-MB-231) | MeOH extract (0.1–100 µg/mL) from the host tree O. dioica decreased cell proliferation in a dose dependent manner (LC50 79.33 µg/mL) while the one from F. indica host tree presented no activity (LC50 500.82 µg/mL). | [106] |
| V. coloratum | MTT | Human ovarian carcinoma (HO-8910), human hepatocarcinoma (SMMC-7721), urinary bladder carcinoma (T24), human liver carcinoma (HepG2), human glioma (SHG) | Five fractions from EtOH extract (IC50 18.6–465.3 µg/mL for HO-8910; IC50 32.1–904.9 µg/mL for SMMC-7721) and isolated compounds (IC50 6.7–28.3 µg/mL for HO-8910; IC50 12.1–50.0 µg/mL for SMMC-7721) were cytotoxic. Isolated 3-epi-betulinic acid, oleanolic acid and erythrodiol exhibited the most significant activity. | [118] |
| V. coloratum | DNP-HAS and EZ-Cytox assay | Basophilic leukaemia cell line (RBL-2H3) | EtOH extract was not cytotoxic to IgE-sensitized cells (RBL-2H3). | [14] |
| V. coloratum | EZ-Cytox assay | Human colorectal adenocarcinoma (Caco-2) | EtOH extract (100–200 µg/mL) was not cytotoxic to the cell-derived inflammatory mediator (MDIM)-activated Caco-2 cells. | [119] |
| V. cruciatum | Bradford colorimetric method | Human laryngeal carcinoma (HEp-2) | MeOH extract (30 µg/mL) exhibited moderate cytostatic activity compared to 6-mercaptopurine positive control (36.04%). | [127] |
| V. cruciatum | Sulforhodamine B | Human renal adenocarcinoma (TK-10), human breast adenocarcinoma (MCF-7), human melanoma (UACC-62) | Hirsutanone isolated from MeOH extract presented GI50 values of 6.8, 1.9 and 4.8 µg/ml, when tested for TK-10, MCF-7 and UACC-62, respectively. Etoposide was used as a positive control (GI50 values for TK-10 cells, MCF-7 cells and UACC-62 cells were 8.1, 0.33 and 0.97 mg/ml, respectively). | [128] |
| V. cruciatum | Sulforhodamine B | Breast cancer cell line MCF-7 | MeOH extract after 72 h presented IC50 73 µg/mL. | [23] |
| V. orientale | Brine shrimp lethality | Brine shrimp nauplii | MeOH extract (10–320 µg/mL) showed potent cytotoxicity with LC50 of 21.63 μg/mL. | [134] |
| Species | Assay | Cell Line | Main Results | Authors |
|---|---|---|---|---|
| V. album | Metalloproteinase inhibition | IL-1β-stimulated chondrocyte cells (SW1353) | EtOH extract inhibited MMP-13 expression at 20–200 μg/mL (64.3%, 70.3% and 80.0% inhibition at 50, 100 and 200 μg/mL, respectively) | [95] |
| V. coloratum | Wound healing and transwell migration | MDIM-stimulated chondrocyte cells (SW1353) | EtOH extract reduced cell migration and inhibited the expression, secretion and/or activity of MMP-1, MMP-3 or MMP-13 in MDIM-stimulated SW1353 cells demonstrating anti-osteoarthritic properties | [14] |
| Species | Assay | Material | Main Results | Authors |
|---|---|---|---|---|
| V. album | Prothrombin time (PT) and activated partial thromboplastin time (PTT) | Human citrated whole blood | MeOH extract from V. album leaves from olive and almond host trees presented prolongation of prothrombin time (PT) and activated partial thromboplastin time (aPTT), important indicators of coagulation. | [25] |
| V. album | Inhibition of platelet aggregation and effects on arachidonic acid | Human citrated whole blood | EtOH isolated phenylpropanoids (0.001–1.0 µM) inhibited ADP-induced platelet aggregation. Arachidonic acid metabolism and biosynthesis of leukotrienes were not affected. Two di-glycosides (10 µM) inhibited 20–25% leukotriene B4 release. It suggests antitumor activity related to inhibition of protein kinase C by phenylpropanoids. | [21] |
| V. album | Platelet aggregation | Rabbits and human platelets | EtOH extract (1–10 mg/mL) exhibited a dose dependent inhibition on platelet aggregation (IC50 2.3–3.4 mg/mL). | [41] |
| V. cruciatum | Platelet aggregation | Human platelet rich plasma | EtOH extract (0.3, 0.6 and 1.2 mg/mL) inhibited the adrenaline (15%, 35%, 59%) and ADP (33%, 41%, 75%) induced human platelet aggregation. | [129] |
| V. triflorum | Angiotensin-converting enzyme (ACE) inhibitory activity | Rabbit lung ACE | EtOH extract presented no significant activity on ACE inhibition when compared to captopril positive control IC50 12.0 ± 2.6 nM. | [140] |
| Species | Assay | Material/Cell | Main Results | Authors |
|---|---|---|---|---|
| V. album | Inhibitory biosynthesis of IL-1α, IL-1β, TNF-α | Human whole blood | EtOH extract (1–30 µg/mL) exhibited none to insignificant inhibitory activity on cytokines | [43] |
| V. articulatum | Hypotonicity-induced hemolysis | Human whole blood | MeOH extract (0.5–5.0 mg/mL) possess anti-inflammatory activity (41.6–32.0%) in a dose dependent manner, comparable to the standard drug (indomethacin). | [109] |
| V. coloratum | β-hexosaminidase assay, enzyme-linked immunosorbent assay for TNF-α and IL-4, enzyme immunoassay for PGD2 and LTC4 | Mast cell line from rat basophilic leukemia RBL-2H3 cells | EtOH extract inhibited degranulation (IC50 93.04 μg/mL), production of IL-4 (IC50 73.28 μg/mL), TNF-α (IC50 50.59 μg/mL), PGD2 and LTC4 and the activation of the FcεRI signalling cascade in IgE/Ag-activated RBL-2H3 cells. | [14] |
| V. coloratum | Mast cell-mediated colitis | Mast cell-derived inflammatory mediator (MDIM)-activated Caco-2 cells | EtOH extract (100–200 µg/mL) suggested multiple targets, such as mast cells, macrophages, neutrophils, MMP-2, MMP-9, Jak2 and STAT3 for anticolitic activity. | [119] |
| Species | Assay | Material | Main Results | Authors |
|---|---|---|---|---|
| V. album | Anti-glycation and superoxides assays | MeOH extract (IC50 199.8 µM) exhibited significant (72.5%) antiglycation activity, as well as six isolated compounds, inhibiting advanced glycation end products formation | [45] | |
| V. album | α-amylase and α-glucosidase type IV inhibitory activities | Porcine pancreatic α-amylase, type IV α-glucosidase enzyme from B. stearothermophilus | EtOH extract (100–3000 µg/mL) of subsp. album presented 1.8–8.7% of α-amylase inhibition, while spp. austriacum (300–3000 µg/mL) presented 2.6–44.3% of inhibition. It possessed dose-dependent activity on α-glucosidase, with IC50 (mg/mL) of 0.7962 and 0.6653, respectively. Thus, it can ameliorate hyperglycaemia in type 2 diabetics | [46] |
| V. album | Pancreatic lipase and phosphodiesterase (PDE) inhibitory activities | EtOH extract presented inhibitory activity on lipase (IC50 33.32 µg/mL) and on phosphodiesterase (IC50 35.15 µg/mL) | [47] | |
| V. articulatum | α-Amylase inhibitory activity | EtOH extract (0.2–1 mg/mL) inhibited α-amylase from 25 to 83% (IC50 53.79 µg/mL). Thus, it showed anti-diabetic activity | [110] | |
| V. schimperi | Glycation of albumin and its endproducts, protein aggregation using thioflavin T | MeOH extract (3–330 mg/mL) decreased advanced glycation endproducts (AGE) and protein aggregation (PA). Fractions from MeOH extract showed different inhibitory activity on AGE formation and PA | [137] |
| Species | Assay | Main Results | Authors |
|---|---|---|---|
| V. album | Hydroxyl scavenging activity (HRSA) using deoxyribose method, superoxide radicals (SRSA) using xanthine oxidase, lipid oxidation using thiobarbituric acid reactive substance (TBARS) | EtOH extract (0.5 mg/mL) presented 40.19% of inhibitory activity for HRSA and 30.05% for SRSA. The extract reduced TBARS after 14 days of storage. | [31] |
| V. album | SOD, CAT, glutathione-Stransferase (GST), GR | MeOH extract (50–1000 mg/L) presented IC50 of 12.75 mg/L. SOD, Catalase, Glutathione S-Transferase, Glutathione Reductase activities increased in the presence of the extract, reducing superoxide and hydrogen peroxide radicals accumulated in the MDA-MB-231 | [13] |
| V. album | Oxidative stress and intracellular ROS lever by H2O2 induction and DCFH-DA | Extracts from Robinia and Tilia host trees completely prevented nuclear and mitochondrial DNA damage under stress conditions, while extract from Acer was completely effective for nuclear DNA damage but only half-effective for mitochondrial DNA damage. | [37] |
| V. album | Lipid peroxidation using malondialdehyde (MDA) | EtOH extract (0.5 µg/mL) had protective effect against lipid peroxidation and DNA repairing. Thus, it is promising as antioxidant, anti-mutagenic and DNA repair-inducing properties | [42] |
| V. album | 5-Lipoxygenase and acetylcholinesterase inhibitory activities | EtOH extract inhibited lipoxygenase and acetylcholinesterase with IC50 of 0.236 ± 0.030 and 1.712 ± 0.080 mg/mL, respectively. | [48] |
| V. album | Tryosinase and superoxide dismutase activity | EtOH extract presented 62.88% of inhibitory effect on tyrosinase and 53.55% of superoxide dismutase inhibition | [49] |
| V. album | SOD, (CAT), glutathione-S-transferase (GST), GR | SOD, Catalase, Glutathione S-Transferase and Glutathione Reductase activities varied at 100 mg/L after the periods evaluated (24, 48 and 72 h). | [104] |
| Investigation | Animal Model | Intervention | Main Results | Authors |
|---|---|---|---|---|
| Antidiabetic and hypoglycemic activity in streptozotocin-induced diabetic rats. | Male Wistar rats | 50 mg/kg and 100 mg/kg b.w. of the extract administered for 4 h or 100 mg/kg the extract administered daily for 3 consecutive weeks. | Hypoglycemic effect within 4 h of administration was 41% (50 mg/kg) and 49% (100 mg/kg) at 2 h. Extract at 100 mg/kg for 3 weeks decreased LDL serum level from 1137.0 to 728.4 (IU/L) in streptozotocin-diabetic rats. | [50] |
| Antidiabetic activity in streptozotocin-induced diabetic rats by fasting blood sugar. | New Zealand white albino rats | 250, 500, 750 and 1000 mg/kg b.w. | 750 mg/kg b.w. decrease fasting blood glucose level in normal as well as in streptozotocin-induced diabetic rats similar with the glibenclamide control. | [51] |
| Antidiabetic activity in partial pancreatectomized rats. | Male Sprague-Dawley rats | 0.6% of EtOH extract in diet for 8 weeks. | EtOH extract enhanced glucose-stimulated insulin secretion and β-cell proliferation in diabetic partial pancreactomized rats. | [52] |
| Antidiabetic activity was assessed through fasting blood glucose level, insulin levels and area under the curve in oral glucose tolerance test. | Male Wistar rats | 75 and 150 mg/kg b.w. of the lyophilized extract was administered in an oral dose. | MeOH extract and organic subfractions presented a significant antihyperglycemic activity after 4 weeks of daily doses. Insulin levels increased in a dose dependent manner and all tested fractions produced reduction in AUC of glucose concentration. | [138] |
| Antihyperglycemic and hypolipidemic effect by effect of extract on plasma glucose level, oral glucose tolerance test, plasma insulin level, muscle and liver glycogen and plasma lipid profile. | Male Wistar rats | 500 mg/kg b.w. of the extract given orally by gavage as single daily treatments for 4 weeks. | Antihyperglycemic activity was observed by maximum reduction in blood glucose level of 37%. | [139] |
| Investigation | Animal Model | Intervention | Main Results | Authors |
|---|---|---|---|---|
| Hypocholesterolemic activity through measurement of serum total cholesterol, triglyceride, HDL-C, LDL-C concentrations in mice fed with cholesterol-rich diet. | Male Swiss albino mice | 100 mg/kg b.w. of the extract after suspending in a mixture of distilled H2O and 0.5% sodium carboxymethyl cellulose were used in an orally gastric gavage. | EtOH extract reduced the serum cholesterol concentration in 59.1%, increased the serum HDL in 46.7%, decreased 83.0% the serum LDL-C concentration and decreased the serum triglyceride concentration in 60.7% without inducing any gastric damage. | [54] |
| Hypolipemic activity in atherogenic diet induced hyperlipidaemic model in mice was performed by cholesterol, triglyceride, low-density lipoprotein and high-density lipoprotein in blood serum of albino mice. | Swiss albino mice | Extract at 200 mg/kg/day in oral suspension 0.2% w/v of the gum acacia powder in distilled water. | EtOH extract did not present activity on reduction of total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein and atherogenic index when compared with the control. | [111] |
| Antihyperglycemic and hypolipidemic effect by effect of extract on plasma glucose level, oral glucose tolerance test, plasma insulin level, muscle and liver glycogen and plasma lipid profile. | Male Wistar rats | 500 mg/kg b.w. of the extract given orally by gavage as single daily treatments for 4 weeks. | Hypolipemic effect was demonstrated by significant reductions in plasma total cholesterol (32.6%), in triglyceride (32.2%) and in low-density lipoprotein cholesterol (27.2%) and an increase in high-density lipoprotein-cholesterol of 171.5%. | [139] |
| Investigation | Animal Model | Intervention | Main Results | Authors |
|---|---|---|---|---|
| Antimetastatic activity in Wistar rats. | Female Wistar rats | Mixture of V. album L. + Abies alba (136 mg/kg b.w. of extract in the first week, 271 mg/kg b.w. of extract for the 2 week and 406 mg/kg b.w. of extract for the last 3 weeks intraperitoneally. | The V. album L. + Abies alba extract reduced the metastatic locations almost by 77%. | [41] |
| Anticancer activity in Ehrlich ascitic carcinoma model. | White Swiss female mice | EtOH extract at 50 mg dry substance/kg b.w. in the days 1, 3 and 6 intraperitoneally. | Doxorrubicin and V. album association provided anti proliferative effect when compared to doxorubicin alone reducing difference in body weight, Ehrlich ascitic volume and cellular concentration. | [55] |
| Anticancer effect evaluated by Ehrlich ascites carcinoma in mice. | White Swiss female mice | 50 mg/kg b.w. of the extract restored with sterile saline solution, equivalent to 18 µL of EtOH tincture, i.p. | Extract at 50 mg/kg + doxorubicin reduced the Ehrlich ascite carcinoma volume from 8.43 (control) to 0 when compared to untreated group, the catalase activity from 3.5 (control) to 1.5 mU/mL and the xanthine oxidase activity from 0 to approximately 4 mU/mL. | [56] |
| Investigation | Animal Model | Intervention | Main Results | Authors |
|---|---|---|---|---|
| Hypotensive activity on values of arterial blood pressure by direct method in the left carotid artery. | Male and female Wistar rats | 3.33 × 10−5, 1 × 10−4, 3.33 × 10−4, 1 × 10−3 mg/kg of the extract were administered through right outer jugular vein. | EtOH extracts presented dose dependent response and the maximal reduction was observed at 1 × 10−3 mg/kg with arterial blood pressure reduction of the 23.56 mmHg with effect via muscarine receptors. | [15] |
| Diuretic, saluretic and natriuretic effects by Na+ and Cl− excretions. | Male Wistar rats and Swiss albino mice | 100, 200 and 400 mg/kg b.w. of the MeOH extract dissolved in water and administered orally. | The extract at 400 mg/kg had a diuretic index (volume in test group/volume in control group) in 24 h of 2.76 and increased saluretic (Na+ + Cl−) at 168 and natriuretic index (Na+/K+) at 2.20. | [105] |
| Diuretic, saluretic and natriuretic effects by Na+ and Cl− excretions. | Male Wistar rats and Swiss albino mice | 100, 200 and 400 mg/kg b.w. of the MeOH extract dissolved in water and administered orally. | 400 mg/kg of the extract had a diuretic index (volume in test group/volume in control group) at 24 h of 3.00 and increased saluretic (Na+ + Cl−) at 272 and natriuretic index (Na+/K+) at 2.16. | [112] |
| Antihypertensive activity against Nω-nitro-L-arginine methyl ester induced hypertension by blood pressure and heart rate; urine volume and urine sodium/potassium; serum creatinine; serum lipid estimation. | Male Wistar rats | 200 or 400 mg/kg/day of the extract orally. | MeOH extract prevented progression of hypertension in rats produced by chronic administration of Nω-nitro-L-arginine methyl ester, which may be due to its diuretic, nephroprotective, hypolipemic and antioxidant effects. | [113] |
| Investigation | Animal Model | Intervention | Main Results | Authors |
|---|---|---|---|---|
| Anticolitic effect in induced colitis for 8 days by Dextran Sodium Sulfate (DSS). | Male C57BL/6 mice | 0–200 mg/kg −100 μL orally once a day. | Extract attenuated the body weight loss, reduced the scores of Disease Activity Index (DAI), suppressed enterorrhagia and colonic oedema in DSS-treated mice. | [119] |
| Analgesic activity by tail immersion method and anti-diarrhoeal activity by Castor oil-induced diarrhoea method. | Male and female Swiss-albino mice | For the analgesic activity, the tail immersion test in hot water was performed after treating the animals via a gastric tube. For the anti-diarrhoea activity, the mice were treated orally after being induced to diarrhoea. | EtOH extract of V. monoicum exhibited analgesic activity through central nervous system in dose dependent manner. EtOH extract of V. monoicum showed anti-diarrheal activity decreasing defecation in a dose dependent manner. | [132] |
| Anti-inflammatory activity by Carrageenan-induced oedema and liver-protective effects in CCl4-induced hepatotoxicity. | Male Wistar albino rats | 100 and 300 mg/kg b.w. of the extract subcutaneously. | EtOH extract promoted anti-inflammatory activity reducing the paw oedema to indomethacin. Extract was not effective in protecting the liver against CCI4-induced damage. | [133] |
| Investigation | Animal Model | Intervention | Main Results | Authors |
|---|---|---|---|---|
| Antianxiety, anti-depressant, hypnotic, anti-stress and analgesic activities were performed using elevated plus maze (EPM), forced swim test (FST), thiopentone sodium-induced sleeping assay, cold swim test and tail immersion test, respectively. Behavior activity was preformed using open field test. | Male and female Laca mice | 50–400 mg/kg b.w. of the extract orally. | 50–400 mg/kg of MeOH extract exhibited significant antianxiety activity, increasing the number of entries and time spent in open arms of EPM; reduced duration of immobility in antidepressant activity by despair swim and the rearing and crossings in open field test. Higher doses increased duration of sleeping mice and reduced time spent by mice in immobile state activity and analgesic activity. | [57] |
| Monoamine oxidase A and monoamine oxidase B activity | Galleria mellonella larvae | 1.0%, 2.5% and 5.0% EtOH extract in diet | All concentrations evaluated inhibited both MAO-A and MAO-B. | [96] |
| Anticonvulsant activity induced in mice with pentylenetetrazole, bicuculline and N methyl-DL-aspartic acid. | Male and female albino mice | Intraperitoneal injection at 50–100 mg/kg b.w. of the extract in a physiological saline solution. | MeOH extract protected the mice against pentylenetetrazole- and bicuculline-induced tonic seizures but did not significantly alter N-methyl-DL-aspartic acid-induced tonic seizures, suggesting its antiepileptic effect. | [115] |
| Behavior and antianxiety by open field exploratory, hypnotic/sedative effect by pentobarbitone-induced sleep. | Swiss albino mice | 100, 200 or 400 mg/kg b.w. of extract orally. | Animals treated with a dose of 400 mg/kg of MeOH extract decreased the number of total crossings. The extract demonstrated a dose dependent increase in pentobarbitone induced sleep. | [117] |
| Anti-nociceptive and central nervous system activity through acetic acid and formalin-induced pain models, respectively, and cross and open field test behavior profiles. | Male and female Swiss albino mice | 300 or 500 mg/kg b.w. of the extract orally. | In the acetic acid induced writhing test, extract produced 88.8% of writhing inhibition at 500 mg/kg of body weight. Extract has both peripheral and neurogenic anti-nociceptive and CNS depressant activities. | [135] |
| Investigation | Animal Model | Intervention | Main Results | Authors |
|---|---|---|---|---|
| Protective effects against cyclophosphamide-induced cardiotoxicity, urotoxicity and genotoxicity through anti-oxidative stress and -inflammation in the heart and bladder and chromosomal damage in the bone marrow. | Male Swiss albino mice | 250 mg/kg b.w./day of the extract were administered orally by gastric gavage. | Antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase, Glutathione-S-transferases and mitotic index were restored to near normalcy as compared to the control group. Lipid peroxidation in heart and bladder were reduced by extract when compared to the cyclophosphamide group. | [58] |
| Anti-cytogenotoxic effects of pre-treatment with V. album extract on methotrexate-induced chromosomal aberrations. | Male Swiss albino mice | Extract by oral gavage at 250 mg/kg b.w./day for 10 days. | MeOH extract had a protective effect against methotrexate-induced cyto-genotoxicity in mouse bone marrow decreasing chromosomal aberrations of 96.40 ± 8.25 to 59.20 ± 1.65 in relation to the control. | [59] |
| Acute toxicity testing according to the oral administration method. | Male Balb/c mice | EtOH extract up to 5000 mg/kg body weight orally. | No animal deaths were observed in the study. | [94] |
| Protective effect against chlorpyrifos-induced hepatotoxicity. | Male Wistar albino rats | EtOH extracts 350 mg/kg b.w. intraperitoneally. | The extract recovered the antioxidative system parameters and alleviated some histopathological changes caused by chlorpyrifos. | [97] |
| Protective effect against tetrachloride (CCl4)-induced acute/chronic liver injury. | Male Wistar rats | MeOH extract 300 mg/kg b.w. orally in single dose | The extract decreased ALT and AST enzymes increased by CCl4. | [101] |
| Acute toxicity testing according to the oral administration method. | Swiss albino mice | MeOH extract at 5–2000 mg/kg b.w. suspended in 0.5% carboxymethyl cellulose | The extract was safe up to 2000 mg/kg body weight. | [117] |
| Acute toxicity testing according to the intragastric administration method. | Kunming mice of both sexes | Ethanolic extract. | EtOH extract presented an acute toxicity of the LD50 7.67 g/kg. | [118] |
| Investigation | Animal Model | Intervention | Main Results | Authors |
|---|---|---|---|---|
| Bleeding time test by tail transection. | Rats | 125, 250 and 500 mg/kg of the extract b.w. given orally once a day for 12 days. | 500 mg/kg of the Korean and European MeOH extract prolonged the bleeding time 185.6% and 175.7% respectively compared to the control. | [60] |
| Effects of V. album on cardiac function through the nitric oxide pathway in isoproterenol-induced heart failure rats evaluated by echocardiographic and biochemical evaluation. | Male Wistar albino rats | Concentrated extract reconstituted in 0.9% NaCl and administered 250 mg/kg/day orally. | MeOH extract improved: left ventricular diameters, ejection fraction, serum N-terminal pro b-type natriuretic peptide) levels and histopathological changes. Attenuation of increased levels of nitric oxide and nitric oxide synthase. The levels of high-sensitivity C-reactive protein were lower in the V. album group compared to the controls. | [61] |
| Pharmacokinetic studies | Male and female Wistar rats | Injection of 13.2 mg·kg−1 of the homoeriodictyol-7-O-b-D-glucopyranoside isolated from V. coloratum via the tail vein. | The method was successfully applied to the pre-clinical pharmacokinetic study of homoeriodictyol-7-O-b-D-glucopyranoside with AUC of e 16.04 ± 3.19 µg.h.mL−1. T1/2α and t1/2β were 0.06 ± 01 h and 1.27 ± 0.31 h, respectively. Homoeriodictyol-7-O-b-D-glucopyranoside was cleared from the blood and mainly distributed to the liver and small intestine. | [63,120] |
| Growth performance | Rainbow trout (Oncorhynchus mykiss) | T1 (0.5%); T2 (1.5%); T3 (2.5%); and T4 (4%) MeOH supplemented in diet. | Here, 1.5 and 2.5% of extract in diet increased protease activity and serum antioxidant enzyme activities. These concentrations decreased serum activities of hepatic enzymes. The highest serum lysozyme and total Ig values were observed in the 2.5% of the extract in diet. | [100] |
| Investigation | Main Results | Authors |
|---|---|---|
| Vascular effects in noradrenaline-contracted rat aortic rings. | n-BuOH fraction produced a contractile response in noradrenaline-contracted rat aortic rings. | [63] |
| Vascular effects on isolated noradrenaline-contracted rat aortic segments. | EtOH extract contained marked vasodilator activity especially from cherry, quince and acacia host trees. | [62] |
| Gut inhibitory and stimulatory effects by in rabbit jejunum and guinea-pig ileum respectively. | Spasmogenic effect was observed with a concentration-dependent contractile effect in guinea-pig ileum at 5–10 mg/mL of the extract. Spasmolytic effect was demonstrated to relax the spontaneous and K+ (80 mM)-induced contractions of isolated rabbit jejunum, with EC50 values of 0.66 and 0.55 mg/mL, respectively. | [129] |
| Antispasmodic and relaxant activity in smooth muscle evaluated in isolated rabbit jejunum and in rabbit aortic rings. | Crude MeOH extract inhibited spontaneous and high K+-induced contractions in rabbit jejunum. The extract showed a partial relaxation against high K+ (80 Mm) and phenylephrine (1 μM)-induced contractions in isolated rabbit aorta rings. | [65] |
| Cardioprotective activity in myocardial ischemia and reperfusion injury in rats. | 5 mg/L of the MeOH extract reduced 53.2% in mean infarct size compared to control hearts. | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, M.N.d.O.; Batista, J.V.d.C.; Peñaloza, E.M.C.; Oliveira, A.P.; Garrett, R.; Baumgartner, S.; Holandino, C. A Scoping Review of Genus Viscum: Biological and Chemical Aspects of Alcoholic Extracts. Plants 2023, 12, 1811. https://doi.org/10.3390/plants12091811
Melo MNdO, Batista JVdC, Peñaloza EMC, Oliveira AP, Garrett R, Baumgartner S, Holandino C. A Scoping Review of Genus Viscum: Biological and Chemical Aspects of Alcoholic Extracts. Plants. 2023; 12(9):1811. https://doi.org/10.3390/plants12091811
Chicago/Turabian StyleMelo, Michelle Nonato de Oliveira, João Vitor da Costa Batista, Evelyn Maribel Condori Peñaloza, Adriana Passos Oliveira, Rafael Garrett, Stephan Baumgartner, and Carla Holandino. 2023. "A Scoping Review of Genus Viscum: Biological and Chemical Aspects of Alcoholic Extracts" Plants 12, no. 9: 1811. https://doi.org/10.3390/plants12091811
APA StyleMelo, M. N. d. O., Batista, J. V. d. C., Peñaloza, E. M. C., Oliveira, A. P., Garrett, R., Baumgartner, S., & Holandino, C. (2023). A Scoping Review of Genus Viscum: Biological and Chemical Aspects of Alcoholic Extracts. Plants, 12(9), 1811. https://doi.org/10.3390/plants12091811