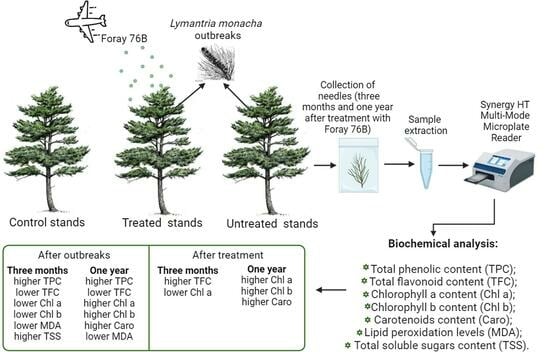
Changes in Biologically Active Compounds in Pinus sylvestris Needles after Lymantria monacha Outbreaks and Treatment with Foray 76B
Abstract
:1. Introduction
2. Results
2.1. Effect of L. monacha Outbreaks on Total Polyphenol (TPC) and Total Flavonoid (TFC) Content
2.2. Effect of L. monacha Outbreaks on Chlorophyll a (Chl a), Chlorophyll b (Chl b), and Carotenoids (Caro)
2.3. Effect of L. monacha Outbreaks on Lipid Peroxidation (MDA) and Total Soluble Sugars (TSS)
2.4. Impact of the Treatment with Foray 76B on Total Polyphenol (TPC) and Total Flavonoid (TFC) Content
2.5. Impact of the Treatment with Foray 76B on the Content of Chlorophyll a (Chl a), Chlorophyll b (Chl b), and Carotenoids (Caro)
2.6. Impact of the Treatment of Foray 76B on Lipid Peroxidation (MDA) and Total Soluble Sugars (TSS)
2.7. Correlation and Principal Component Analysis (PCA) between Biochemical Compounds
3. Discussion
4. Materials and Methods
4.1. Study Sites and Sampling
4.2. Extract Preparation
4.3. Identification of Total Phenolic Content (TPC)
4.4. Identification of Total Flavonoids Content (TFC)
4.5. Quantification of Photosynthetic Pigments
4.6. Identification of Levels of Lipid Peroxidation (MDA)
4.7. Evaluation of Total Soluble Sugars (TSS)
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Caro | carotenoids |
| Chl a | chlorophyll a |
| Chl b | chlorophyll b |
| Btk | Bacillus thuringiensis subspecies kurstaki Strain ABTS-351 |
| MDA | malondialdehyde |
| ROS | reactive oxygen species |
| PSMs | plants secondary metabolites |
| TFC | total flavonoid content |
| TPC | total phenolic content |
| TSS | total soluble sugars |
References
- Mageroy, M.H.; Nagy, N.E.; Steffenrem, A.; Krokene, P.; Hietala, A.M. Conifer Defences against Pathogens and Pests—Mechanisms, Breeding, and Management. Curr. For. Rep. 2023, 9, 429–443. [Google Scholar] [CrossRef]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry Under a Changing Climate. Front. Plant Sci. 2020, 11, 1874. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Lloret, F.; Batllori, E. Climate-Induced Global Forest Shifts due to Heatwave-Drought. In Ecosystem Collapse and Climate Change; Springer: Berlin/Heidelberg, Germany, 2021; pp. 155–186. [Google Scholar] [CrossRef]
- Nahrung, H.F.; Carnegie, A.J. Non-native Forest Insects and Pathogens in Australia: Establishment, Spread, and Impact. Front. For. Glob. Chang. 2020, 3, 37. [Google Scholar] [CrossRef]
- Siliņš, I.; Kārkliņa, A.; Miezīte, O.; Jansons, Ā. Trends in Outbreaks of Defoliating Insects Highlight Growing Threats for Central European Forests, and Implications for Eastern Baltic Region. Forests 2021, 12, 799. [Google Scholar] [CrossRef]
- Goldstein, P.Z. Inscect Biodiversity: Science and Society, Diversity and Significance of Lepidoptera: A Phylogenetic Perspective; Blackwell: Oxford, UK, 2017; Volume 1. [Google Scholar]
- Régnière, J.; Venier, L.; Welsh, D. Avian predation in a declining outbreak population of the spruce budworm, choristoneura fumiferana (Lepidoptera: Tortricidae). Insects 2021, 12, 720. [Google Scholar] [CrossRef]
- Stancă-moise, C.; Brereton, T. Monitoring and Control of The Defoliator Population Lymantria monacha (l., 1758) Within The Forestry Field RĂȘinari (Sibiu, Romania). Analele Univ. Oradea Fasc. 2020, 27, 27–31. [Google Scholar]
- Allison, J.D.; Paine, T.D.; Slippers, B.; Wingfield, M.J.A. Forest Entomology and Pathology: Volume 1: Entomology; Springer: Cham, Switzerland, 2023; pp. 237–299. [Google Scholar]
- Žiogas, A. On the survey of nun moth (Lymantria monacha L.). In Methodology of Forest Insect and Disease Survey in Central EUROPE, Proceedings of the Second Workshop of the IUFRO Working Party 7.03.10. 20–23 April 1999, Sion-Châteauneuf, Switzerland; Forster, B., Knížek, M., Grodzki, W., Eds.; Swiss Federal Institute for Forest, Snow and Landscape Research: Birmensdorf, Switzerland, 1999; pp. 261–263. [Google Scholar]
- State Forest Service. Lithuanian Statistical Yearbook of Forestry 2019; Ministry of Environment, State Forest Service: Vilnius, Lithuania, 2020. [Google Scholar]
- Łuców, D.; Lamentowicz, M.; Kołaczek, P.; Łokas, E.; Marcisz, K.; Obremska, M.; Theuerkauf, M.; Tyszkowski, S.; Słowiński, M. Pine Forest Management and Disturbance in Northern Poland: Combining High-Resolution 100-Year-Old Paleoecological and Remote Sensing Data. Front. Ecol. Evol. 2021, 9, 898. [Google Scholar] [CrossRef]
- Šefrová, H.; Lastuvka, Z. Harmful Lepidoptera–in Czechia—Current status, changes and importance. Acta Univ. Agric. Silvic. Mendel. Brun. 2023, 71, 155–162. [Google Scholar] [CrossRef]
- Mastro, V.C.; Munson, A.S.; Wang, B.; Freyman, T.; Humble, L.M. History of the Asian lymantria species program: A unique pathway risk mitigation strategy. J. Integr. Pest Manag. 2021, 12, 31. [Google Scholar] [CrossRef]
- Skrzecz, I.; Ślusarski, S.; Tkaczyk, M. Integration of science and practice for Dendrolimus pini (L.) management—A review with special reference to Central Europe. For. Ecol. Manag. 2020, 455, 117697. [Google Scholar] [CrossRef]
- Wang, K.; Miettinen, I.; Jaber, E.H.; Asiegbu, F. O—Chapter 2—Anatomical, chemical, molecular, and genetic basis for tree defenses. In Forest Microbiology; Asiegbu, F.O., Kovalchuk, A., Eds.; Forest Microbiology; Academic Press: Cambridge, MA, USA, 2023; Volume 3, pp. 33–57. [Google Scholar] [CrossRef]
- Tuladhar, P.; Sasidharan, S.; Saud–gar, P. 17—Role of phenols and polyphenols in plant defense response to biotic and abiotic stresses. In Biocontrol Agents and Secondary Metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 419–441. [Google Scholar] [CrossRef]
- Sperdouli, I.; Andreadis, S.S.; Adamakis, I.D.S.; Moustaka, J.; Koutsogeorgiou, E.I.; Moustakas, M. Reactive Oxygen Species Initiate Defence Responses of Potato Photosystem II to Sap-Sucking Insect Feeding. Insects 2022, 13, 409. [Google Scholar] [CrossRef]
- Kot, I.; Kmieć, K. Poplar tree response to feeding by the petiole gall aphid pemphigus spyrothecae pass. Insects 2020, 11, 282. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, Q.; Jiang, J.; Li, Y. Prediction and Utilization of Malondialdehyde in Exotic Pine Under Drought Stress Using Near-Infrared Spectroscopy. Front. Plant Sci. 2021, 12, 735275. [Google Scholar] [CrossRef]
- Lu, Y.; Zeng, F.J.; Li, X.Y.; Zhang, B. Physiological changes of three woody plants exposed to progressive salt stress. Photosynthetica 2021, 59, 171–184. [Google Scholar] [CrossRef]
- Malod, K.; du Rand, E.E.; Archer, C.R.; Nicolson, S.W.; Weldon, C.W. Oxidative Damage Is Influenced by Diet But Unaffected by Selection for Early Age of Oviposition in the Marula Fly, Ceratitis cosyra (Diptera: Tephritidae). Front. Physiol. 2022, 13, 794979. [Google Scholar] [CrossRef]
- Riaz, M.; Zhao, S.; Kamran, M.; Rehman, N.U.; Mora-Poblete, F.; Maldonado, C.; Saleem, M.H.; Parveen, A.; Al-Ghamdi, A.A.; Al-Hemaid, F.M.; et al. Effect of nano-silicon on the regulation of ascorbate-glutathione contents, antioxidant defense system and growth of copper stressed wheat (Triticum aestivum L.) seedlings. Front. Plant Sci. 2022, 13, 986991. [Google Scholar] [CrossRef]
- Schiestl-Aalto, P.; Ryhti, K.; Mäkelä, A.; Peltoniemi, M.; Bäck, J.; Kulmala, L. Analysis of the NSC Storage Dynamics in Tree Organs Reveals the Allocation to Belowground Symbionts in the Framework of Whole Tree Carbon Balance. Front. For. Glob. Chang. 2019, 2, 17. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N.; Kaur, H. Molecular cues of sugar signaling in plants. Physiologia Plantarum 2022, 174, e13630. [Google Scholar] [CrossRef]
- Mishra, B.S.; Sharma, M.; Laxmi, A. Role of sugar and auxin crosstalk in plant growth and development. Physiologia Plantarum 2022, 174, e13546. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, C.; Parola, R.; Lando, A.P.; Rodriguez, M.; Martínez-Noël, G.M.A. High Sugar Concentration Inhibits TOR Signaling Pathway in Arabidopsis thaliana. J. Plant Growth Regul. 2023, 42, 4309–4323. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Prade, P.; Coyle, D. R—Chapter 9—Insect pests of forest trees. In Forest Microbiology; Asiegbu, F.O., Kovalchuk, A., Eds.; Forest Microbiology; Academic Press: Cambridge, MA, USA, 2023; Volume 3, pp. 195–211. [Google Scholar] [CrossRef]
- Davis, J.S.; Glover, A.N.; Everson, K.M.; Coyle, D.R.; Linnen, C.R. Identification, biology, and management of conifer sawflies (Hymenoptera: Diprioninae) in eastern North America. J. Integr. Pest Manag. 2023, 14, 13. [Google Scholar] [CrossRef]
- Olivieri, M.; Mannu, R.; Ruiu, L.; Ruiu, P.A.; Lentini, A. Comparative efficacy trials with two different Bacillus thuringiensis serovar kurstaki strains against gypsy moth in mediterranean cork oak forests. Forests 2021, 12, 602. [Google Scholar] [CrossRef]
- Skrzecz, I.; Sierpińska, A.; Tumialis, D. Entomopathogens in the integrated management of forest insects: From science to practice. Pest Manag. Sci. 2023, in press. [CrossRef]
- Nybakken, L.; Lee, Y.; Brede, D.A.; Mageroy, M.H.; Lind, O.C.; Salbu, B.; Kashparov, V.; Olsen, J.E. Long termeustria of ionising radiation in the Chernobyl Exclusion zone on DNA integrity and chemical defence systems of Scots pine (Pinus sylvestris). Sci. Total Environ. 2023, 904, 166844. [Google Scholar] [CrossRef]
- Schowalter, T.D. Insect Ecology: An Ecosystem Approach, 5th ed.; Academic Press: Cambridge, MA, USA, 2022; ISBN 978-0323856737. [Google Scholar]
- Szwajkowska-Michałek, L.; Przybylska-Balcerek, A.; Rogoziński, T.; Stuper-Szablewska, K. Phenolic Compounds in Trees and Shrubs of Central Europe. Appl. Sci. 2020, 10, 6907. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Jiang, H.; Yan, S.; Meng, Z.; Zhao, S.; Jiang, D.; Li, P. Effects of the Larch–Ashtree Mixed Forest on Contents of Secondary Metabolites in Larix olgensis. Forests 2023, 14, 871. [Google Scholar] [CrossRef]
- Jiang, S.; Han, S.; He, D.; Cao, G.; Fang, K.; Xiao, X.; Yi, J.; Wan, X. The accumulation of phenolic compounds and increased activities of related enzymes contribute to early defense against walnut blight. Physiol. Mol. Plant Pathol. 2019, 108, 101433. [Google Scholar] [CrossRef]
- Vaca-Sánchez, M.S.; Maldonado-López, Y.; Oyama, K.; Delgado, G.; Aguilar-Peralta, J.S.; Borges, M.A.Z.; de Faria, M.L.; Fagundes, M.; López-Maldonado, M.C.; Cuevas-Reyes, P. Changes in herbivory patterns and insect herbivore assemblages associated to canopy of Quercus laurina: Importance of oak species diversity and foliar chemical defense. Trees 2023, 37, 699–715. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Bouket, A.C.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The threat of pests and pathogens and the potential for biological control in forest ecosystems. Forests 2021, 12, 1579. [Google Scholar] [CrossRef]
- Šigutová, H.; Pyszko, P.; Šigut, M.; Czajová, K.; Kostovčík, M.; Kolařík, M.; Hařovská, D.; Drozd, P. Concentration-dependent effect of plant secondary metabolites on bacterial and fungal microbiomes in caterpillar guts. Microbiol. Spectr. 2024, 12, e02994-23. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, L.; Wang, Q.; Ding, J. Plant Chemistry Determines Host Preference and Performance of an Invasive Insect. Front. Plant Sci. 2020, 11, 594663. [Google Scholar] [CrossRef]
- Grüning, M.M.; Simon, J.; Rennenberg, H.; L-M-Arnold, A. Defoliating insect mass outbreak affects soil N fluxes and tree N nutrition in scots pine forests. Front. Plant Sci. 2017, 8, 954. [Google Scholar] [CrossRef]
- Wallis, C.M.; Galarneau, E.R.A. Phenolic Compound Induction in Plant-Microbe and Plant-Insect Interactions: A Meta-Analysis. Front. Plant Sci. 2020, 11, 580753. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Mwamatope, B.; Tembo, D.; Chikowe, I.; Kampira, E.; Nyirenda, C. Total phenolic contents and antioxidant activity of Senna singueana, Melia azedarach, Moringa oleifera and Lannea discolor herbal plants. Sci. Afr. 2020, 9, e00481. [Google Scholar] [CrossRef]
- Ramaroson, M.-L.; Koutouan, C.; Helesbeux, J.-J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef] [PubMed]
- Čėsnienė, I.; Miškelytė, D.; Novickij, V.; Mildažienė, V.; Sirgedaitė-Šėžienė, V. Seed Treatment with Electromagnetic Field Induces Different Effects on Emergence, Growth and Profiles of Biochemical Compounds in Seven Half-Sib Families of Silver Birch. Plants 2023, 12, 3048. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Beniušytė, E.; Čėsnienė, I.; Sirgedaitė-Šėžienė, V.; Vaitiekūnaitė, D. Genotype-Dependent Jasmonic Acid Effect on Pinus sylvestris L. Growth and Induced Systemic Resistance Indicators. Plants 2023, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, A.M.; Gallardo, P. Relationship between insect damage and chlorophyll content in mediterranean oak species. Appl. Ecol. Environ. Res. 2016, 14, 477–491. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Moustakas, M. Early-Stage Detection of Biotic and Abiotic Stress on Plants by Chlorophyll Fluorescence Imaging Analysis. Biosensors 2023, 13, 796. [Google Scholar] [CrossRef] [PubMed]
- Santacruz-García, A.C.; Bravo, S.; del Corro, F.; García, E.M.; Molina-Terrén, D.M.; Nazareno, M.A. How Do Plants Respond Biochemically to Fire? The Role of Photosynthetic Pigments and Secondary Metabolites in the Post-Fire Resprouting Response. Forests 2021, 12, 56. [Google Scholar] [CrossRef]
- Azizoglu, U. Bacillus thuringiensis as a Biofertilizer and Biostimulator: A Mini-Review of the Little-Known Plant Growth-Promoting Properties of Bt. Curr. Microbiol. 2019, 76, 1379–1385. [Google Scholar] [CrossRef]
- Belousova, M.E.; Malovichko, Y.V.; Shikov, A.E.; Nizhnikov, A.A.; Antonets, K.S. Dissecting the environmental consequences of bacillus thuringiensis application for natural ecosystems. Toxins 2021, 13, 355. [Google Scholar] [CrossRef]
- Cheeke, T.E.; Darby, H.; Rosenstiel, T.N.; Bever, J.D.; Cruzan, M.B. Effect of Bacillus thuringiensis (Bt) maize cultivation history on arbuscular mycorrhizal fungal colonization, spore abundance and diversity, and plant growth. Agric. Ecosyst. Environ. 2014, 195, 29–35. [Google Scholar] [CrossRef]
- Bandopadhyay, S. Application of plant growth promoting bacillus thuringiensis as biofertilizer on abelmoschus esculentus plants under field condition. J. Pure Appl. Microbiol. 2020, 14, 1287–1294. [Google Scholar] [CrossRef]
- Bhusal, B.; Mmbaga, M.T. Biological control of Phytophthora blight and growth promotion in sweet pepper by Bacillus species. Biol. Control 2020, 150, 104373. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- He, L.; Magney, T.; Dutta, D.; Yin, Y.; Köhler, P.; Grossmann, K.; Stutz, J.; Dold, C.; Hatfield, J.; Guan, K.; et al. From the Ground to Space: Using Solar-Induced Chlorophyll Fluorescence to Estimate Crop Productivity. Geophys. Res. Lett. 2020, 47, e2020GL087474. [Google Scholar] [CrossRef]
- Aono, Y.; Asikin, Y.; Wang, N.; Tieman, D.; Klee, H.; Kusano, M. High-Throughput Chlorophyll and Carotenoid Profiling Reveals Positive Associations with Sugar and Apocarotenoid Volatile Content in Fruits of Tomato Varieties in Modern and Wild Accessions. Metabolites 2021, 11, 398. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The Role of Sugars in Plant Responses to Stress and Their Regulatory Function during Development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Rodziewicz, P.; Swarcewicz, B.; Chmielewska, K.; Wojakowska, A.; Stobiecki, M. Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiologiae Plantarum 2014, 36, 1–19. [Google Scholar] [CrossRef]
- Behrooz, A.; Vahdati, K.; Rejali, F.; Lotfi, M.; Sarikhani, S.; Leslie, C. Arbuscular mycorrhiza and plant growth-promoting bacteria alleviate drought stress in walnut. HortScience 2019, 54, 1087–1092. [Google Scholar] [CrossRef]
- Havaux, M. Review of Lipid Biomarkers and Signals of Photooxidative Stress in Plants. In Plant Abiotic Stress Signaling; Couée, I., Ed.; Springer: New York, NY, USA, 2023; pp. 111–128. [Google Scholar] [CrossRef]
- Homayoonzadeh, M.; Moeini, P.; Talebi, K.; Roessner, U.; Hosseininaveh, V. Antioxidant system status of cucumber plants under pesticides treatment. Acta Physiologiae Plantarum 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Pięt, M.; Gąsecka, M.; Stroińska, A.; Niedzielski, P.; Mleczek, M.; Rzymski, P.; Wilczak, M. Relation between polyphenols, malondialdehyde, antioxidant capacity, lactate dehydrogenase and toxic elements in human colostrum milk. Chemosphere 2018, 191, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.S.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Vaičys, M. Miško dirvožemiu klasifikacija. In Lietuvos Dirvožemiai; Mokslas: Vilnius, Lithuania, 2001; pp. 1040–1043. (In Lithuanian) [Google Scholar]
- Karazija, S. Miško Tipologija. In Miško Ekologija; Padaiga, V., Stravinskienė, V., Eds.; Enciklopedija: Vilnius, Lithuania, 2008; pp. 220–254. (In Lithuanian) [Google Scholar]
- Mensah, P.K.; Palmer, C.G.; Muller, W.J. Lipid peroxidation in the freshwater shrimp Caridina nilotica as a biomarker of Roundup® herbicide pollution of freshwater systems in South Africa. Water Sci. Technol. 2012, 65, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Murshed, R.; Lopez-Lauri, F.; Keller, C.; Monnet, F.; Sallanon, H. Acclimation to drought stress enhances oxidative stress tolerance in Solanum lycopersicum L. fruits. Plant Stress 2008, 2, 145–151. [Google Scholar]
- Murshed, R.; Lopez-Lauri, F.; Sallanon, H. Effect of water stress on antioxidant systems and oxidative parameters in fruits of tomato (Solanum lycopersicon L., cv. Micro-tom). Physiol. Mol. Biol. Plants 2013, 19, 363–378. [Google Scholar] [CrossRef]
- Leyva, A.; Quintana, A.; Sánchez, M.; Rodríguez, E.N.; Cremata, J.; Sánchez, J.C. Rapid and sensitive anthrone–sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef]










| Importance of Components (2020) | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 |
|---|---|---|---|---|---|---|---|
| Standard deviation | 1.739 | 1.172 | 0.911 | 0.826 | 0.776 | 0.621 | 0.247 |
| Proportion of Variance | 0.435 | 0.197 | 0.119 | 0.098 | 0.087 | 0.055 | 0.009 |
| Cumulative Proportion | 0.435 | 0.632 | 0.751 | 0.849 | 0.936 | 0.991 | 1.000 |
| Importance of Components (2021) | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 |
| Standard deviation | 1.448 | 1.311 | 0.954 | 0.933 | 0.840 | 0.702 | 0.402 |
| Proportion of Variance | 0.301 | 0.247 | 0.131 | 0.125 | 0.101 | 0.071 | 0.023 |
| Cumulative Proportion | 0.301 | 0.548 | 0.679 | 0.805 | 0.906 | 0.977 | 1.000 |
| Trait | 2020 | 2021 | ||
|---|---|---|---|---|
| 1st Component | 2nd Component | 1st Component | 2nd Component | |
| TPC | 0.06144 | 0.49082 | 0.28465 | 0.01474 |
| TFC | 0.16538 | −0.48788 | 0.13065 | 0.67010 |
| Chl a | −0.64602 | −0.07751 | 0.53737 | −0.13954 |
| Chl b | −0.59924 | −0.14574 | 0.50337 | −0.08715 |
| Caro | −0.39843 | 0.40333 | 0.47682 | −0.07655 |
| MDA | −0.03230 | −0.47597 | 0.03444 | 0.71146 |
| TSS | 0.18071 | 0.32341 | 0.36223 | 0.10796 |
| Location | Forest Stand Code | Plot Code | Group | Geographical Position | Stand Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | E | Age (y) | Mean Height (m) | Mean Diameter (cm) | Stocking Level | Forest Site Type * | Forest Vegetation Type ** | Tree Species Composition, % *** | ||||
| Neringa | 1 | N1C | Control | 55.62489 | 21.112491 | 50 | 17.1 | 20.5 | 0.89 | Nal | cl | 100% P |
| N1T | Damaged/Treated | 55.69654 | 21.106127 | 120 | 16.5 | 23.0 | 1.00 | Nal | cl | 100% P | ||
| N1U | Damaged/Untreated | 55.67744 | 21.106809 | 65 | 16.7 | 18.0 | 0.84 | Nal | cl | 100% P | ||
| 2 | N2C | Control | 55.53548 | 21.10357 | 92 | 19.2 | 30.4 | 0.67 | Nal | cl | 100% P | |
| N2T | Damaged/Treated | 55.33166 | 21.041377 | 60 | 18.1 | 19.0 | 1.04 | Nal | cl | 100% P | ||
| N2U | Damaged/Untreated | 55.40839 | 21.078366 | 110 | 11.5 | 13.0 | 1.16 | Nal | cl | 100% P | ||
| Kapčiamiestis | 3 | K1C | Control | 54.01370 | 23.519638 | 94 | 27.1 | 31.8 | 0.92 | Nbl | vm | 100% P |
| K1T | Damaged/Treated | 54.04471 | 23.537946 | 89 | 29.3 | 34.0 | 0.92 | Nbl | vm | 100% P | ||
| K1U | Damaged/Untreated | 54.03606 | 23.535732 | 74 | 23.6 | 28.7 | 0.90 | Nbl | vm | 100% P | ||
| 4 | K2C | Control | 53.97177 | 23.507236 | 99 | 28.0 | 33.8 | 0.70 | Nbl | vm | 100% P | |
| K2T | Damaged/Treated | 54.01868 | 23.525614 | 89 | 25.1 | 29.9 | 0.80 | Nbl | vm | 100% P | ||
| K2U | Damaged/Untreated | 54.00465 | 23.501548 | 76 | 28.2 | 31.3 | 0.92 | Nbl | vm | 100% P | ||
| Marcinkonys | 5 | M1C | Control | 54.09489 | 24.45175 | 90 | 27.0 | 32.0 | 0.81 | Nbl | vm | 100% P |
| M1T | Damaged/Treated | 54.10660 | 24.433008 | 98 | 28.6 | 35.4 | 0.80 | Nbl | vm | 100% P | ||
| M1U | Damaged/Untreated | 54.12597 | 24.454743 | 78 | 25.2 | 25.6 | 0.81 | Nbl | vm | 100% P | ||
| 6 | M2C | Control | 54.05331 | 24.443881 | 85 | 26.1 | 30.2 | 0.74 | Nbl | vm | 100% P | |
| M2T | Damaged/Treated | 54.03104 | 24.435073 | 125 | 25.3 | 25.7 | 0.71 | Nbl | vm | 100% P | ||
| M2U | Damaged/Untreated | 54.03598 | 24.424077 | 83 | 26.8 | 29.6 | 0.81 | Nal | cl | 100% P | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čėsna, V.; Čėsnienė, I.; Sirgedaitė-Šėžienė, V.; Marčiulynienė, D. Changes in Biologically Active Compounds in Pinus sylvestris Needles after Lymantria monacha Outbreaks and Treatment with Foray 76B. Plants 2024, 13, 328. https://doi.org/10.3390/plants13020328
Čėsna V, Čėsnienė I, Sirgedaitė-Šėžienė V, Marčiulynienė D. Changes in Biologically Active Compounds in Pinus sylvestris Needles after Lymantria monacha Outbreaks and Treatment with Foray 76B. Plants. 2024; 13(2):328. https://doi.org/10.3390/plants13020328
Chicago/Turabian StyleČėsna, Vytautas, Ieva Čėsnienė, Vaida Sirgedaitė-Šėžienė, and Diana Marčiulynienė. 2024. "Changes in Biologically Active Compounds in Pinus sylvestris Needles after Lymantria monacha Outbreaks and Treatment with Foray 76B" Plants 13, no. 2: 328. https://doi.org/10.3390/plants13020328
APA StyleČėsna, V., Čėsnienė, I., Sirgedaitė-Šėžienė, V., & Marčiulynienė, D. (2024). Changes in Biologically Active Compounds in Pinus sylvestris Needles after Lymantria monacha Outbreaks and Treatment with Foray 76B. Plants, 13(2), 328. https://doi.org/10.3390/plants13020328