Progress in Microbial Fertilizer Regulation of Crop Growth and Soil Remediation Research
Abstract
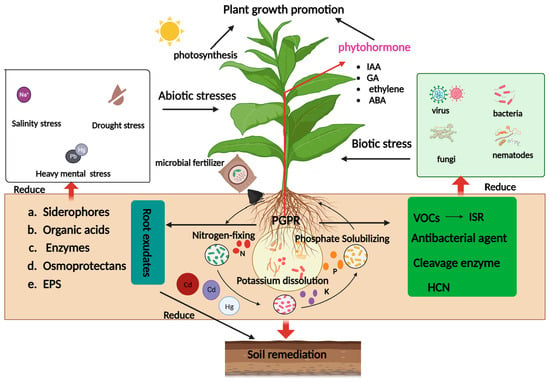
:1. Introduction
2. Microbiological Fertilizer Classification
2.1. Bio-Organic Fertilizer
2.2. Microbial Agents
2.3. Complex Microbial Fertilizer
| Types of Microbial Fertilizers | Crop | Plant Growth Promoting Rhizobacteria | References | |
|---|---|---|---|---|
| 1 | Bio-organic fertilizer | Lettuce | Actinobacteria, Proteobacteria, Chloroflexi, Acidobacteria, Gemmatimonadota Ascomycota, and Basidiomycota | [26] |
| 2 | Bio-organic fertilizer | Tobacco | Actinobacteria, Chloroflexi, Proteobacteria, Acidobacteria, Firmicutes, Gemmatimonadota, StreptomyceBacillus, Arthrobacter, and Paenibacillus | [27] |
| 3 | Bio-organic fertilizer | Beet, potato, winter, wheat | Actinobacteria, Proteobacteria, Acidobacteria, Arthrobacter, and Paenibacillus | [28,29] |
| 4 | Bio-organic fertilizer | Cauliflower | Proteobacteria, Actinobacteria, Acidobacteria, Gemmatimonadetes, Bacteroidetes, and Chloroflexi | [30] |
| 5 | Bio-organic fertilizer | Tomato | Proteobacteria, Actinobacteriota, Bacteroidota, Firmicutes, Firmicutes, and Verrucomicrobiota | [31] |
| 6 | Microbial inoculants | Watermelon | Pseudomonas, flavobacterium Aspergillus, Myceliophthora, Trichoderma, and Humicola and Neocosmospora | [32] |
| 7 | Microbial inoculants | Radish | Proteobacteria, Bacterioidetes, Acidobacteria, Actinobacteria, and Planctomycetes | [33] |
| 8 | Microbial inoculants | Rice | Proteobacteria, Acidobacteria, Bacteroidetes, Gemmatimonadetes, Actinobacteria, Planctomycetes Ascomycota, and Chytridiomycota | [34] |
| 9 | Microbial inoculants | Prunusdavidana | Proteobacteria, Bacteroidetes, Acidobacteria, Gemmatimonadetes, Actinobacteria, Patescibacteria, Chloroflexi, Verrucomicrobia, Nitrospirae, Latescibacteria, and Rokubacteria | [35] |
| 10 | Microbial inoculants | Cucumber | Alphaproteobacteria, Actinobacteria, Acidobacteria, Betaproteobacteria, Gammaproteobacteria, Deltaproteobacteria, Gemmatimonadetes, Bacteroidetes, Chloroflexi, Planctomycetes, Firmicutes, Verrucomicrobia, Nitrospirae, Armatimonadetes, Cyanobacteria, TM7, Fibrobacteres, and Chlorobi | [36] |
| 11 | Compound microbial fertilizer | Soybean | nitrogen-fixing bacteria, phosphorus-solubilizing bacteria | [37] |
| 12 | Compound microbial fertilizer | Sugarcane | Trichoderma harzianum, Gluconcetobacter diazotrophicus, and Pseudomonas fluorescents | [38] |
3. Microbial Fertilizers Regulate Crop Growth and Resistance
3.1. Microbial Fertilizers Regulate Crop Growth
3.1.1. Nitrogen Fixation
3.1.2. Phosphate Solubilizing
3.1.3. Potassium Dissolution
3.1.4. Regulating Phytohormone Levels
3.1.5. Iron Carrier Production
| Microorganisms | Crop | Phytohormones | Mechanism of Action | References |
|---|---|---|---|---|
| Rhizophila Y1 | Corn | IAA, ABA | Rhizophila Y1 regulates phytohormone levels and alleviates salt stress in maize growth | [83] |
| Bacillus velezensis | Strawberries | IAA | Bacillus velezensis produces large amounts of IAA for growth promotion | [84] |
| Bacillus thuringiensis RZ2MS9 | Corn | IAA | Genetic basis for the induction of IAA biosynthesis by Bacillus thuringiensis RZ2MS9 for maize growth | [85] |
| Leifsonia soli SE134 | Cucumbers, Tomato | GA | GA secretion by L. soli SE134 may favor its ameliorative role in crop growth | [86] |
| Bacillus subtilis | Maize, Brassica pekinensis | GA | Bacillus subtilis secretes gibberellins that promote the growth of rice and cabbage | [87] |
| Bacillus subtilis | Wheat | GA, IAA | Bacillus sp. increases endogenous IAA and GA levels in all genotypes of wheat | [88] |
| B. subtilis CNBG-PGPR-1 | Tomato | Ethylene | B. subtilis CNBG-PGPR-1 regulates the ethylene pathway in tomato, scavenges ROS, and enhances plant salt tolerance | [89] |
| Bacillus subtilis | Corn | Ethylene | Salt-tolerant Bacillus sp. strains reduce stress-inducing ethylene levels in host plants and alleviate salt stress | [90] |
3.2. Increasing Crop Resistance to Environmental Stress
3.2.1. Biotic Stress
- Biological Control of Pest Management
- Plant Pathogen Management
3.2.2. Abiotic Stress
- Drought Stress
- Salt Stress
- Heavy Metal Stress
4. Soil Remediation
5. Future Perspective and Challenges
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerr, R.B.; Madsen, S.; Stüber, M.; Liebert, J.; Enloe, S.; Borghino, N.; Parros, P.; Mutyambai, D.M.; Prudhon, M.; Wezel, A. Can agroecology improve food security and nutrition? A review. Glob. Food Secur. 2021, 29, 100540. [Google Scholar] [CrossRef]
- Akanmu, A.O.; Olowe, O.M.; Phiri, A.T.; Nirere, D.; Odebode, A.J.; Umuhoza, N.J.K.; Asemoloye, M.D.; Babalola, O.O. Bioresources in Organic Farming: Implications for Sustainable Agricultural Systems. Horticulturae 2023, 9, 659. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sindhu, S.S.; Dhanker, R.; Kumari, A. Microbes-mediated sulphur cycling in soil: Impact on soil fertility, crop production and environmental sustainability. Microbiol. Res. 2023, 271, 127340. [Google Scholar] [CrossRef] [PubMed]
- Sabreena; Hassan, S.; Kumar, V.; Bhat, S.A.; Ganai, B.A. Unraveling Microbes as Potential Proxies for Remediation of Heavy Metal and Pesticide Contamination: A State-of-the Art Review. Int. J. Environ. Res. 2023, 17, 55. [Google Scholar] [CrossRef]
- Nosheen, S.; Ajmal, I.; Song, Y. Microbes as Biofertilizers, a Potential Approach for Sustainable Crop Production. Sustainability 2021, 13, 1868. [Google Scholar] [CrossRef]
- Kuppe, C.W.; Schnepf, A.; von Lieres, E.; Watt, M.; Postma, J.A. Rhizosphere models: Their concepts and application to plant-soil ecosystems. Plant Soil 2022, 474, 17–55. [Google Scholar] [CrossRef]
- Hyder, S.; Rizvi, Z.F.; de los Santos-Villalobos, S.; Santoyo, G.; Gondal, A.; Khalid, N.; Fatima, S.N.; Nadeem, M.; Rafique, K.; Rani, A. Applications of plant growth-promoting rhizobacteria for increasing crop production and resilience. J. Plant Nutr. 2023, 46, 2551–2580. [Google Scholar] [CrossRef]
- Larsen, J.; Jaramillo-López, P.; Nájera-Rincon, M.; González-Esquivel, C. Biotic interactions in the rhizosphere in relation to plant and soil nutrient dynamics. J. Soil Sci. Plant Nutr. 2015, 15, 449–463. [Google Scholar] [CrossRef]
- Chamkhi, I.; El Omari, N.; Balahbib, A.; El Menyiy, N.; Benali, T.; Ghoulam, C. Is the rhizosphere a source of applicable multi-beneficial microorganisms for plant enhancement? Saudi J. Biol. Sci. 2022, 29, 1246–1259. [Google Scholar] [CrossRef]
- Goyal, S.; Dhanker, R.; Hussain, T.; Ferreira, A.; Gouveia, L.; Kumar, K.; Mohamed, H.I. Modern Advancement in Biotechnological Applications for Wastewater Treatment through Microalgae: A Review. Water Air Soil Pollut. 2023, 234, 417. [Google Scholar] [CrossRef]
- Bamdad, H.; Papari, S.; Lazarovits, G.; Berruti, F. Soil amendments for sustainable agriculture: Microbial organic fertilizers. Soil Use Manag. 2021, 38, 94–120. [Google Scholar] [CrossRef]
- Yap, C.K.; Al-Mutairi, K.A. Effective Microorganisms as Halal-Based Sources for Biofertilizer Production and Some Socio-Economic Insights: A Review. Foods 2023, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Kiruba, N.J.M.; Saeid, A. An Insight into Microbial Inoculants for Bioconversion of Waste Biomass into Sustainable “Bio-Organic” Fertilizers: A Bibliometric Analysis and Systematic Literature Review. Int. J. Mol. Sci. 2022, 23, 13049. [Google Scholar] [CrossRef]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking Crop Nutrition in Times of Modern Microbiology: Innovative Biofertilizer Technologies. Front. Sustain. Food Syst. 2021, 5, 606815. [Google Scholar] [CrossRef]
- Daniel, A.I.; Fadaka, A.O.; Gokul, A.; Bakare, O.O.; Aina, O.; Fisher, S.; Burt, A.F.; Mavumengwana, V.; Keyster, M.; Klein, A. Biofertilizer: The Future of Food Security and Food Safety. Microorganisms 2022, 10, 1220. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Shahwar, D.; Mushtaq, Z.; Mushtaq, H.; Alqarawi, A.A.; Park, Y.; Alshahrani, T.S.; Faizan, S. Role of microbial inoculants as bio fertilizers for improving crop productivity: A review. Heliyon 2023, 9, e16134. [Google Scholar] [CrossRef]
- Jack, C.N.; Petipas, R.H.; Cheeke, T.E.; Rowland, J.L.; Friesen, M.L. Microbial Inoculants: Silver Bullet or Microbial Jurassic Park? Trends Microbiol. 2021, 29, 299–308. [Google Scholar] [CrossRef]
- Comeau, D.; Balthazar, C.; Novinscak, A.; Bouhamdani, N.; Joly, D.L.; Filion, M. Interactions Between Bacillus Spp., Pseudomonas Spp. and Cannabis sativa Promote Plant Growth. Front. Microbiol. 2021, 12, 715758. [Google Scholar] [CrossRef]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next generation of microbial inoculants for agriculture and bioremediation. Microb. Biotechnol. 2016, 10, 19–21. [Google Scholar] [CrossRef]
- Yang, W.; Gong, T.; Wang, J.; Li, G.; Liu, Y.; Zhen, J.; Ning, M.; Yue, D.; Du, Z.; Chen, G. Effects of Compound Microbial Fertilizer on Soil Characteristics and Yield of Wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2020, 20, 2740–2748. [Google Scholar] [CrossRef]
- Kaari, M.; Manikkam, R.; Annamalai, K.K.; Joseph, J. Actinobacteria as a source of biofertilizer/biocontrol agents for bio-organic agriculture. J. Appl. Microbiol. 2022, 134, lxac047. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xiao, C.; Yang, S.; Yin, H.; Yang, Z.; Chi, R. Life cycle assessment and life cycle cost analysis of compound microbial fertilizer production in China. Sustain. Prod. Consum. 2021, 28, 1622–1634. [Google Scholar] [CrossRef]
- Ding, M.J.; Shang, N.J.; Huang, Y.; Liu, L.; Fan, W.; Peng, L.J.; Zhang, Y.; Zhou, J.H.; Zhou, Z.F. Isolation of Plant Growth Promoting Rhizobacteria and Selection of Microbial Organic Fertilizer Carriers. Int. J. Agric. Biol. 2019, 21, 77–84. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Jin, N.; Jin, L.; Wang, S.; Li, J.; Liu, F.; Liu, Z.; Luo, S.; Wu, Y.; Lyu, J.; Yu, J. Reduced Chemical Fertilizer Combined With Bio-Organic Fertilizer Affects the Soil Microbial Community and Yield and Quality of Lettuce. Front. Microbiol. 2022, 13, 863325. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, H.-Y.; Zhou, X.-K.; Yang, C.-G.; Zheng, S.-C.; Duo, J.-L.; Mo, M.-H. Biological control tobacco bacterial wilt and black shank and root colonization by bio-organic fertilizer containing bacterium Pseudomonas aeruginosa NXHG29. Appl. Soil Ecol. 2018, 129, 136–144. [Google Scholar] [CrossRef]
- Jakienė, E.; Spruogis, V.; Romaneckas, K.; Dautartė, A.; Avižienytė, D. The bio-organic nano fertilizer improves sugar beet photosynthesis process and productivity. Zemdirb. Agric. 2015, 102, 141–146. [Google Scholar] [CrossRef]
- Li, W.; Zhang, F.; Cui, G.; Wang, Y.; Yang, J.; Cheng, H.; Liu, H.; Zhang, L. Effects of bio-organic fertilizer on soil fertility, microbial community composition, and potato growth. Scienceasia 2021, 47, 347. [Google Scholar] [CrossRef]
- Xiao, X.; Li, J.; Lyu, J.; Feng, Z.; Zhang, G.; Yang, H.; Gao, C.; Jin, L.; Yu, J. Chemical fertilizer reduction combined with bio-organic fertilizers increases cauliflower yield via regulation of soil biochemical properties and bacterial communities in Northwest China. Front. Microbiol. 2022, 13, 922149. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; Gao, B.; Wan, Y.; Li, Y.C.; Xie, J.; Tang, Y. Slow-released bio-organic–chemical fertilizer improved tomato growth: Synthesis and pot evaluations. J. Soils Sediments 2021, 21, 319–327. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.; Liang, H.; Huang, J.; Chen, Z.; Nie, Y. The rhizosphere microbial community response to a bio-organic fertilizer: Finding the mechanisms behind the suppression of watermelon Fusarium wilt disease. Acta Physiol. Plant. 2018, 40, 17. [Google Scholar] [CrossRef]
- Jatav, M.K.; Kumar, M.; Trehan, S.P.; Dua, V.K.; Lal, S.S. Effect of microbial inoculation on nutritional economics of potato-radish crop sequence in north-west Himalayan region. Int. J. Agric. Stat. Sci. 2011, 7, 309–316. [Google Scholar]
- Xie, H.; Wu, K.; Iqbal, A.; Ali, I.; He, L.; Ullah, S.; Wei, S.; Zhao, Q.; Wu, X.; Huang, Q.; et al. Synthetic nitrogen coupled with seaweed extract and microbial inoculants improves rice (Oryza sativa L.) production under a dual cropping system. Ital. J. Agron. 2021, 16, 1800. [Google Scholar] [CrossRef]
- Shi, H.; Lu, L.; Ye, J.; Shi, L. Effects of Two Bacillus Velezensis Microbial Inoculants on the Growth and Rhizosphere Soil Environment of Prunus davidiana. Int. J. Mol. Sci. 2022, 23, 13639. [Google Scholar] [CrossRef] [PubMed]
- Simranjit, K.; Kanchan, A.; Prasanna, R.; Ranjan, K.; Ramakrishnan, B.; Singh, A.K.; Shivay, Y.S. Microbial inoculants as plant growth stimulating and soil nutrient availability enhancing options for cucumber under protected cultivation. World J. Microbiol. Biotechnol. 2019, 35, 51. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Ma, W.; Qiang, B.; Jin, X.; Zhang, Y.; Wang, M. Effect of Chemical Fertilizer with Compound Microbial Fertilizer on Soil Physical Properties and Soybean Yield. Agronomy 2023, 13, 2488. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, L.; Jaiswal, V.P.; Pathak, A.D.; Tiwari, R.; Awasthi, S.K.; Gaur, A. Soil quality parameters vis-a-vis growth and yield attributes of sugarcane as influenced by integration of microbial consortium with NPK fertilizers. Sci. Rep. 2020, 10, 19180. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Zehra, A.; Aamir, M.; Dubey, M.K.; Goutam, J.; Upadhyay, R.S. Beneficial Microbes for Disease Suppression and Plant Growth Promotion. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; pp. 395–432. [Google Scholar] [CrossRef]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial fertilizers: A comprehensive review of current findings and future perspectives. Span. J. Agric. Res. 2018, 16, e09R01. [Google Scholar] [CrossRef]
- Kannan, D.; Kumari, V.J.; Prathiba, B. Degradation of Cyanide in Cassava (Manihot esculenta) Plant Leaves by Pseudomonas sp. J. Pure Appl. Microbiol. 2012, 6, 913–916. [Google Scholar]
- Travaglia, C.; Masciarelli, O.; Fortuna, J.; Marchetti, G.; Cardozo, P.; Lucero, M.; Zorza, E.; Luna, V.; Reinoso, H. Towards sustainable maize production: Glyphosate detoxification by Azospirillum sp. and Pseudomonas sp. Crop. Prot. 2015, 77, 102–109. [Google Scholar] [CrossRef]
- Kang, S.-M.; Radhakrishnan, R.; Lee, K.-E.; You, Y.-H.; Ko, J.-H.; Kim, J.-H.; Lee, I.-J. Mechanism of plant growth promotion elicited by Bacillus sp. LKE15 in oriental melon. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 637–647. [Google Scholar] [CrossRef]
- Saxena, J.; Rana, G.; Pandey, M. Impact of addition of biochar along with Bacillus sp. on growth and yield of French beans. Sci. Hortic. 2013, 162, 351–356. [Google Scholar] [CrossRef]
- Ekin, Z.; Oguz, F.; Erman, M.; Ogun, E. The effect of Bacillus sp. OSU-142 inoculation at various levels of nitrogen fertilization on growth, tuber distribution and yield of potato (Solanum tuberosum L.). Afr. J. Biotechnol. 2009, 8, 4418–4424. [Google Scholar]
- Akhtar, N.; Arshad, I.; Shakir, M.A.; Qureshi, M.A.; Sehrish, J.; Ali, L. Co-inoculation with rhizobium and Bacillus sp. to improve the phosphorus availability and yield of wheat (Triticum aestivum L.). J. Anim. Plant Sci. JAPS 2013, 23, 190–197. [Google Scholar]
- Drew, E.A.; Denton, M.D.; Sadras, V.O.; Ballard, R.A. Agronomic and environmental drivers of population size and symbiotic performance of Rhizobium leguminosarum bv. viciae in Mediterranean-type environments. Crop Pasture Sci. 2012, 63, 467–477. [Google Scholar] [CrossRef]
- Ge, H. Characteristics of Azotobacter sp. strain AC11 and their effects on the growth of tomato seedlings under salt stress. Emir. J. Food Agric. 2019, 31, 520–525. [Google Scholar] [CrossRef]
- Banik, A.; Dash, G.K.; Swain, P.; Kumar, U.; Mukhopadhyay, S.K.; Dangar, T.K. Application of rice (Oryza sativa L.) root endophytic diazotrophic Azotobacter sp. strain Avi2 (MCC 3432) can increase rice yield under green house and field condition. Microbiol. Res. 2019, 219, 56–65. [Google Scholar] [CrossRef]
- Puca, M.; Gonzales, E.Y.; Jayos, E.E.; Llanos, K.N. Comparative study of the growth parameters of cowpea bean plants inoculated with Azotobacter sp. and urea. Afinidad 2022, 79, 70–78. [Google Scholar]
- Pereyra, C.M.; Lago, C.C.D.; Creus, C.M.; Pereyra, M.A. Azospirillum baldaniorum Sp 245 inoculation affects cell wall and polyamines metabolisms in cucumber seedling roots. FEMS Microbiol. Lett. 2023, 370, fnad005. [Google Scholar] [CrossRef]
- Abraham-Juarez, M.D.; Espitia-Vazquez, I.; Guzman-Mendoza, R.; Olalde-Portugal, V.; Ruiz-Aguilar, G.M.D.; Garcia-Hernandez, J.L.; Herrera-Isidron, L.; Nunez-Palenius, H.G. Development, Yield, and quality of melon fruit(Cucumis melo L.) inoculated with Mexican native strains of Bacillus subtilis (Ehrenberg). Agrociencia 2018, 52, 91–102. [Google Scholar]
- Cao, Y.-Y.; NI, H.-T.; Li, T.; Lay, K.-D.; Liu, D.-S.; He, X.-Y.; Ou, K.; Tang, X.-Y.; Wang, X.-B.; Qiu, L.-J. Pseudomonas sp. TK35-L enhances tobacco root development and growth by inducing HRGPnt3 expression in plant lateral root formation. J. Integr. Agric. 2020, 19, 2549–2560. [Google Scholar] [CrossRef]
- Gholami, A.; Biyari, A.; Gholipoor, M.; Rahmani, H.A. Growth Promotion of Maize (Zea mays L.) by Plant-Growth-Promoting Rhizobacteria under Field Conditions. Commun. Soil Sci. Plant Anal. 2012, 43, 1263–1272. [Google Scholar] [CrossRef]
- Goyal, R.K.; Schmidt, M.A.; Hynes, M.F. Molecular Biology in the Improvement of Biological Nitrogen Fixation by Rhizobia and Extending the Scope to Cereals. Microorganisms 2021, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.C.; Jiang, X.; Wang, Q.F.; Guan, D.W.; Li, L.; Ongena, M.; Li, J. Isolation and Identification of PGPR Strain and its Effect on Soybean Growth and Soil Bacterial Community Composition. Int. J. Agric. Biol. 2018, 20, 1289–1297. [Google Scholar] [CrossRef]
- Xavier, G.R.; Jesus, E.d.C.; Dias, A.; Coelho, M.R.R.; Molina, Y.C.; Rumjanek, N.G. Contribution of Biofertilizers to Pulse Crops: From Single-Strain Inoculants to New Technologies Based on Microbiomes Strategies. Plants 2023, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Xu, Q.; Wang, G.J.; Shi, K.X. Mixed Enterobacter and Klebsiella bacteria enhance soybean biological nitrogen fixation ability when combined with rhizobia inoculation. Soil Biol. Biochem. 2023, 184, 109100. [Google Scholar] [CrossRef]
- Fahde, S.; Boughribil, S.; Sijilmassi, B.; Amri, A. Rhizobia: A Promising Source of Plant Growth-Promoting Molecules and Their Non-Legume Interactions: Examining Applications and Mechanisms. Agriculture 2023, 13, 1279. [Google Scholar] [CrossRef]
- Sunitha, N.; Reddy, G.K.; Reddy, S.T.; Reddy, M.R.; Nagamadhuri, K. Performance of Groundnut (Arachis hypogaea L.) as Influenced by Integrated Nutrient Management Under Fertigation. Legum. Res. Int. J. 2023, 46, 876–881. [Google Scholar] [CrossRef]
- Motamedi, M.; Zahedi, M.; Karimmojeni, H.; Baldwin, T.C.; Motamedi, H. Rhizosphere-Associated Bacteria as Biofertilizers in Herbicide-Treated Alfalfa (Medicago sativa). J. Soil Sci. Plant Nutr. 2023, 23, 2585–2598. [Google Scholar] [CrossRef]
- Gupta, R.; Kumari, A.; Sharma, S.; Alzahrani, O.M.; Noureldeen, A.; Darwish, H. Identification, characterization and optimization of phosphate solubilizing rhizobacteria (PSRB) from rice rhizosphere. Saudi J. Biol. Sci. 2022, 29, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Cai, B. Phosphate-Solubilizing Bacteria: Advances in Their Physiology, Molecular Mechanisms and Microbial Community Effects. Microorganisms 2023, 11, 2904. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Shao, W.; Zhang, K.; Huo, Y.; Li, M. Characterization of phosphate solubilizing bacteria isolated from heavy metal contaminated soils and their potential for lead immobilization. J. Environ. Manag. 2019, 231, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Ahmad, A.; Moin, S.F.; Liaqat, I.; Saleem, S.; Muhammad, F.; Mujahid, T.; Zafar, U. Isolation, Solubilization of Inorganic Phosphate, and Production of Organic Acids by Individual and Co-inoculated Microorganisms. Geomicrobiol. J. 2023, 40, 111–121. [Google Scholar] [CrossRef]
- Kalayu, G. Phosphate Solubilizing Microorganisms: Promising Approach as Biofertilizers. Int. J. Agron. 2019, 2019, 4917256. [Google Scholar] [CrossRef]
- Rawat, P.; Das, S.; Shankhdhar, D.; Shankhdhar, S.C. Phosphate-Solubilizing Microorganisms: Mechanism and Their Role in Phosphate Solubilization and Uptake. J. Soil Sci. Plant Nutr. 2021, 21, 49–68. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Negi, R.; Kour, D.; Chaubey, K.K.; Yadav, A.N. Indigenous plant growth-promoting rhizospheric and endophytic bacteria as liquid bioinoculants for growth of sweet pepper (Capsicum annuum L.). Biologia 2023, 78, 2623–2633. [Google Scholar] [CrossRef]
- Khanghahi, M.Y.; Pirdashti, H.; Rahimian, H.; Nematzadeh, G.; Sepanlou, M.G. Potassium solubilising bacteria (KSB) isolated from rice paddy soil: From isolation, identification to K use efficiency. Symbiosis 2018, 76, 13–23. [Google Scholar] [CrossRef]
- Pantoja-Guerra, M.; Valero-Valero, N.; Ramírez, C.A. Total auxin level in the soil–plant system as a modulating factor for the effectiveness of PGPR inocula: A review. Chem. Biol. Technol. Agric. 2023, 10, 6. [Google Scholar] [CrossRef]
- Dashti, N.H.; Al-Sarraf, N.Y.A.; Cherian, V.M.; Montasser, M.S. Isolation and characterization of novel plant growth-promoting rhizobacteria (PGPR) isolates from tomato (Solanum lycopersicum L.) rhizospherical soil: A novel IAA producing bacteria. Kuwait J. Sci. 2021, 48. [Google Scholar] [CrossRef]
- Hassan, T.U.; Bano, A. The stimulatory effects of L-tryptophan and plant growth promoting rhizobacteria (PGPR) on soil health and physiology of wheat. J. Soil Sci. Plant Nutr. 2015, 15, 190–201. [Google Scholar] [CrossRef]
- Carrillo-Flores, E.; Arreola-Rivera, J.; Pazos-Solis, D.M.; Bocanegra-Mondragon, M.; Fierro-Romero, G.; Mellado-Rojas, M.E.; Beltran-Pena, E. Participation of Auxin Transport in the Early Response of the Arabidopsis Root System to Inoculation with Azospirillum Brasilense. Phyton-Int. J. Exp. Bot. 2022, 91, 2383–2401. [Google Scholar] [CrossRef]
- Tsukanova, K.A.; Сhеbоtаr, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Ellermann, M.; Arthur, J.C. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free. Radic. Biol. Med. 2017, 105, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Dehghani, S.; Karimi, B.; Yousefi, M.; Taghdisi, S.M.; Abnous, K.; Alibolandi, M.; Ramezani, M. Siderophore-based biosensors and nanosensors; new approach on the development of diagnostic systems. Biosens. Bioelectron. 2018, 117, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-Producing Rhizobacteria as a Promising Tool for Empowering Plants to Cope with Iron Limitation in Saline Soils: A Review. Pedosphere 2019, 29, 409–420. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bera, T.; Chakrabarty, A.M. Microbial siderophore—A boon to agricultural sciences. Biol. Control. 2020, 144, 104214. [Google Scholar] [CrossRef]
- Rehan, M.; Al-Turki, A.; Abdelmageed, A.H.A.; Abdelhameid, N.M.; Omar, A.F. Performance of Plant-Growth-Promoting Rhizobacteria (PGPR) Isolated from Sandy Soil on Growth of Tomato (Solanum lycopersicum L.). Plants 2023, 12, 1588. [Google Scholar] [CrossRef]
- Han, L.Z.; Zhang, H.; Bai, X.; Jiang, B. The peanut root exudate increases the transport and metabolism of nutrients and enhances the plant growth-promoting effects of burkholderia pyrrocinia strain P10. BMC Microbiol. 2023, 23, 85. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174, 104023. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Chizhevskaya, E.P.; Vorobyov, N.I.; Bobkova, V.V.; Pomyaksheva, L.V.; Khomyakov, Y.V.; Konovalov, S.N. The Quality and Productivity of Strawberry (Fragaria × ananassa Duch.) Improved by the Inoculation of PGPR Bacillus velezensis BS89 in Field Experiments. Agronomy 2022, 12, 2600. [Google Scholar] [CrossRef]
- Figueredo, E.F.; da Cruz, T.A.; de Almeida, J.R.; Batista, B.D.; Marcon, J.; de Andrade, P.A.M.; Hayashibara, C.A.d.A.; Rosa, M.S.; Azevedo, J.L.; Quecine, M.C. The key role of indole-3-acetic acid biosynthesis by Bacillus thuringiensis RZ2MS9 in promoting maize growth revealed by the ipdC gene knockout mediated by the CRISPR-Cas9 system. Microbiol. Res. 2023, 266, 127218. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Khan, A.L.; You, Y.-H.; Kim, J.-G.; Kamran, M.; Lee, I.-J. Gibberellin Production by Newly Isolated Strain Leifsonia soli SE134 and Its Potential to Promote Plant Growth. J. Microbiol. Biotechnol. 2014, 24, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Hamayun, M.; Khan, M.A.; Iqbal, A.; Lee, I.J. Bacillus subtilis JW1 enhances plant growth and nutrient uptake of Chinese cabbage through gibberellins secretion. J. Appl. Bot. Food Qual. 2019, 92, 172–178. [Google Scholar] [CrossRef]
- Mirskaya, G.V.; Khomyakov, Y.V.; Rushina, N.A.; Vertebny, V.E.; Chizhevskaya, E.P.; Chebotar, V.K.; Chesnokov, Y.V.; Pishchik, V.N. Plant Development of Early-Maturing Spring Wheat (Triticum aestivum L.) under Inoculation with Bacillus sp. V2026. Plants-Basel 2022, 11, 1817. [Google Scholar] [CrossRef]
- Feng, L.; Li, Q.; Zhou, D.; Jia, M.; Liu, Z.; Hou, Z.; Ren, Q.; Ji, S.; Sang, S.; Lu, S.; et al. B. subtilis CNBG-PGPR-1 induces methionine to regulate ethylene pathway and ROS scavenging for improving salt tolerance of tomato. Plant J. 2023, 117, 193–211. [Google Scholar] [CrossRef]
- Misra, S.; Chauhan, P.S. ACC deaminase-producing rhizosphere competent Bacillus spp. mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 2020, 10, 119. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Borah, P.; Gogoi, N.; Asad, S.A.; Rabha, A.J.; Farooq, M. An Insight into Plant Growth-Promoting Rhizobacteria-Mediated Mitigation of Stresses in Plant. J. Plant Growth Regul. 2023, 42, 3229–3256. [Google Scholar] [CrossRef]
- Aioub, A.A.A.; Elesawy, A.E.; Ammar, E.E. Plant growth promoting rhizobacteria (PGPR) and their role in plant-parasitic nematodes control: A fresh look at an old issue. J. Plant Dis. Prot. 2022, 129, 1305–1321. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Shameer, S.; Prasad, T.N.V.K.V. Plant growth promoting rhizobacteria for sustainable agricultural practices with special reference to biotic and abiotic stresses. Plant Growth Regul. 2018, 84, 603–615. [Google Scholar] [CrossRef]
- Bhat, B.A.; Tariq, L.; Nissar, S.; Islam, S.T.; Islam, S.U.; Mangral, Z.; Ilyas, N.; Sayyed, R.Z.; Muthusamy, G.; Kim, W.; et al. The role of plant-associated rhizobacteria in plant growth, biocontrol and abiotic stress management. J. Appl. Microbiol. 2022, 133, 2717–2741. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; Das Mohapatra, P.K.; Panneerselvam, P.; Boutaj, H.; Alizadeh, M.; et al. Current scenario and future prospects of plant growth-promoting rhizobacteria: An economic valuable resource for the agriculture revival under stressful conditions. J. Plant Nutr. 2020, 43, 3062–3092. [Google Scholar] [CrossRef]
- Chandran, H.; Meena, M.; Swapnil, P. Plant Growth-Promoting Rhizobacteria as a Green Alternative for Sustainable Agriculture. Sustainability 2021, 13, 10986. [Google Scholar] [CrossRef]
- Khan, A.R.; Mustafa, A.; Hyder, S.; Valipour, M.; Rizvi, Z.F.; Gondal, A.S.; Yousuf, Z.; Iqbal, R.; Daraz, U. Bacillus spp. as Bioagents: Uses and Application for Sustainable Agriculture. Biology 2022, 11, 1763. [Google Scholar] [CrossRef]
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711. [Google Scholar] [CrossRef]
- Melo, A.L.d.A.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2014, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Aslam, Z.; Khaliq, A.; Ahmed, J.N.; Nawaz, A.; Hussain, M. Plant growth promoting rhizobacteria reduce aphid population and enhance the productivity of bread wheat. Braz. J. Microbiol. 2018, 49, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Katoch, V.; Shavnam; Sharma, S.; Negi, M. Utilization of Plant Growth-Promoting Rhizobacteria (PGPR) for Managing Recently Reported Potato Cyst Nematodes, Globodera spp. in North Himalayan Regions of India. Potato Res. 2023, 1–16. [Google Scholar] [CrossRef]
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Khoshru, B.; Mitra, D.; Joshi, K.; Adhikari, P.; Rion, S.I.; Fadiji, A.E.; Alizadeh, M.; Priyadarshini, A.; Senapati, A.; Sarikhani, M.R.; et al. Decrypting the multi-functional biological activators and inducers of defense responses against biotic stresses in plants. Heliyon 2023, 9, e13825. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, H.G.; Singh, S.B.; Shilpa, N.; Aiyaz, M.; Nataraj, K.; Udayashankar, A.C.; Amruthesh, K.N.; Murali, M.; Poczai, P.; Gafur, A.; et al. Insight into Recent Progress and Perspectives in Improvement of Antioxidant Machinery upon PGPR Augmentation in Plants under Drought Stress: A Review. Antioxidants 2022, 11, 1763. [Google Scholar] [CrossRef]
- Dutta, P.; Muthukrishnan, G.; Gopalasubramaiam, S.K.; Dharmaraj, R.; Karuppaiah, A.; Loganathan, K.; Periyasamy, K.; Pillai, M.A.; Upamanya, G.; Boruah, S.; et al. Plant growth-promoting rhizobacteria (PGPR) and its mechanisms against plant diseases for sustainable agriculture and better productivity. Biocell 2022, 46, 1843–1859. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Lucas, J.A.; Velasco, A.G.-V.; Ramos, B.; Gutierrez-Mañero, F.J. Changes of enzyme activities related to oxidative stress in rice plants inoculated with random mutants of a Pseudomonas fluorescens strain able to improve plant fitness upon biotic and abiotic conditions. Funct. Plant Biol. 2017, 44, 1063–1074. [Google Scholar] [CrossRef]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Kour, D.; Yadav, A.N. Bacterial Mitigation of Drought Stress in Plants: Current Perspectives and Future Challenges. Curr. Microbiol. 2022, 79, 248. [Google Scholar] [CrossRef] [PubMed]
- Sati, D.; Pande, V.; Pandey, S.C.; Samant, M. Recent Advances in PGPR and Molecular Mechanisms Involved in Drought Stress Resistance. J. Soil Sci. Plant Nutr. 2023, 23, 106–124. [Google Scholar] [CrossRef]
- Bouremani, N.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Baranov, O.; Belbahri, L. Plant Growth-Promoting Rhizobacteria (PGPR): A Rampart against the Adverse Effects of Drought Stress. Water 2023, 15, 418. [Google Scholar] [CrossRef]
- Rosa, A.P.; Dias, T.; Mouazen, A.M.; Cruz, C.; Santana, M.M. Finding optimal microorganisms to increase crop productivity and sustainability under drought—A structured reflection. J. Plant Interact. 2023, 18, 2178680. [Google Scholar] [CrossRef]
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Shahid, M.A.; Yang, J. Impact of Salicylic Acid and PGPR on the Drought Tolerance and Phytoremediation Potential of Helianthus annus. Front. Microbiol. 2018, 9, 2507. [Google Scholar] [CrossRef]
- del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Gowtham, H.G.; Singh, B.; Murali, M.; Shilpa, N.; Prasad, M.; Aiyaz, M.; Amruthesh, K.N.; Niranjana, S.R. Induction of drought tolerance in tomato upon the application of ACC deaminase producing plant growth promoting rhizobacterium Bacillus subtilis Rhizo SF 48. Microbiol. Res. 2020, 234, 126422. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Pandey, S.S.; Pandey, A.; Chanotiya, C.S.; Kalra, A. Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol. Plant. 2017, 161, 502–514. [Google Scholar] [CrossRef]
- Kálmán, C.D.; Nagy, Z.; Berényi, A.; Kiss, E.; Posta, K. Investigating PGPR bacteria for their competence to protect hybrid maize from the factor drought stress. Cereal Res. Commun. 2023, 1–22. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ahmad, I.; Pichtel, J. Synergistic effects of biofilm-producing PGPR strains on wheat plant colonization, growth and soil resilience under drought stress. Saudi J. Biol. Sci. 2023, 30, 103664. [Google Scholar] [CrossRef]
- Carlson, R.; Tugizimana, F.; Steenkamp, P.A.; Dubery, I.A.; Hassen, A.I.; Labuschagne, N. Rhizobacteria-induced systemic tolerance against drought stress in Sorghum bicolor (L.) Moench. Microbiol. Res. 2020, 232, 126388. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Rahman, M.A.; Guo, J.; Kang, Z.Y.; Babar, A. Comparative Physiological and Metabolic Analysis Reveals a Complex Mechanism Involved in Drought Tolerance in Chickpea (Cicer arietinum L.) Induced by PGPR and PGRs. Sci. Rep. 2019, 9, 2097. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- Saghafi, D.; Delangiz, N.; Lajayer, B.A.; Ghorbanpour, M. An overview on improvement of crop productivity in saline soils by halotolerant and halophilic PGPRs. 3 Biotech 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many Unanswered Questions Remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Bhat, M.A.; Kumar, V.; Bhat, M.A.; Wani, I.A.; Dar, F.L.; Farooq, I.; Bhatti, F.; Koser, R.; Rahman, S.; Jan, A.T. Mechanistic Insights of the Interaction of Plant Growth-Promoting Rhizobacteria (PGPR) With Plant Roots Toward Enhancing Plant Productivity by Alleviating Salinity Stress. Front. Microbiol. 2020, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Hoque, N.; Hannan, A.; Imran, S.; Paul, N.C.; Mondal, F.; Sadhin, M.R.; Bristi, J.M.; Dola, F.S.; Hanif, A.; Ye, W.; et al. Plant Growth-Promoting Rhizobacteria-Mediated Adaptive Responses of Plants Under Salinity Stress. J. Plant Growth Regul. 2023, 42, 1307–1326. [Google Scholar] [CrossRef]
- Khumairah, F.H.; Setiawati, M.R.; Fitriatin, B.N.; Simarmata, T.; Alfaraj, S.; Ansari, M.J.; El Enshasy, H.A.; Sayyed, R.Z.; Najafi, S. Halotolerant Plant Growth-Promoting Rhizobacteria Isolated From Saline Soil Improve Nitrogen Fixation and Alleviate Salt Stress in Rice Plants. Front. Microbiol. 2022, 13, 905210. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, H.S.; Sang, M.K.; Song, J.; Weon, H.-Y. Effect of Bacillus mesonae H20-5 Treatment on Rhizospheric Bacterial Community of Tomato Plants under Salinity Stress. Plant Pathol. J. 2021, 37, 662–672. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wu, G.; Zhang, N.; Shen, Q.; Zhang, R. Beneficial Rhizobacterium Bacillus amyloliquefaciens SQR9 Induces Plant Salt Tolerance through Spermidine Production. Mol. Plant-Microbe Interact. 2017, 30, 423–432. [Google Scholar] [CrossRef]
- Asad, S.A.; Farooq, M.; Afzal, A.; West, H. Integrated phytobial heavy metal remediation strategies for a sustainable clean environment—A review. Chemosphere 2019, 217, 925–941. [Google Scholar] [CrossRef] [PubMed]
- Zainab, N.; Amna; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Munis, M.F.H.; Hashem, M.; et al. PGPR-Mediated Plant Growth Attributes and Metal Extraction Ability of Sesbania sesban L. in Industrially Contaminated Soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Ullah, A.; Bano, A.; Javed, H. PGPR assisted bioremediation of heavy metals and nutrient accumulation in Zea mays under saline sodic soil. Pak. J. Bot. 2021, 53, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, Y.-Y.; Cho, K.-S. Inoculation effect of heavy metal tolerant and plant growth promoting rhizobacteria for rhizoremediation. Int. J. Environ. Sci. Technol. 2023, 21, 1419–1434. [Google Scholar] [CrossRef]
- Lal, S.; Ratna, S.; Ben Said, O.; Kumar, R. Biosurfactant and exopolysaccharide-assisted rhizobacterial technique for the remediation of heavy metal contaminated soil: An advancement in metal phytoremediation technology. Environ. Technol. Innov. 2018, 10, 243–263. [Google Scholar] [CrossRef]
- Ghorbel, S.; Aldilami, M.; Zouari-Mechichi, H.; Mechichi, T.; AlSherif, E.A. Isolation and characterization of a plant growth-promoting rhizobacterium strain MD36 that promotes barley seedlings and growth under heavy metals stress. 3 Biotech 2023, 13, 145. [Google Scholar] [CrossRef]
- Desoky, E.-S.M.; Merwad, A.-R.M.; Semida, W.M.; Ibrahim, S.A.; El-Saadony, M.T.; Rady, M.M. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020, 198, 110685. [Google Scholar] [CrossRef]
- Husna; Hussain, A.; Shah, M.; Hamayun, M.; Iqbal, A.; Qadir, M.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O.; Lee, I.-J. Phytohormones producing rhizobacteria alleviate heavy metals stress in soybean through multilayered response. Microbiol. Res. 2023, 266, 127237. [Google Scholar] [CrossRef]
- Lim, J.-H.; Kim, S.-D. Induction of Drought Stress Resistance by Multi-Functional PGPR Bacillus licheniformis K11 in Pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Bresson, J.; Varoquaux, F.; Bontpart, T.; Touraine, B.; Vile, D. The PGPR strain Phyllobacterium brassicacearum STM196 induces a reproductive delay and physiological changes that result in improved drought tolerance in Arabidopsis. N. Phytol. 2013, 200, 558–569. [Google Scholar] [CrossRef]
- Akbar, A.; Han, B.; Khan, A.H.; Feng, C.; Ullah, A.; Khan, A.S.; He, L.; Yang, X. A transcriptomic study reveals salt stress alleviation in cotton plants upon salt tolerant PGPR inoculation. Environ. Exp. Bot. 2022, 200, 104928. [Google Scholar] [CrossRef]
- Khan, V.; Umar, S.; Iqbal, N. Palliating Salt Stress in Mustard through Plant-Growth-Promoting Rhizobacteria: Regulation of Secondary Metabolites, Osmolytes, Antioxidative Enzymes and Stress Ethylene. Plants 2023, 12, 705. [Google Scholar] [CrossRef] [PubMed]
- Becze, A.; Vincze, E.B.; Varga, H.M.; Gyöngyvér, M. Effect of plant growth-promoting spirulina on Zea mays development and growth of under heavy metal and salt stress condition. Environ. Eng. Manag. J. 2021, 20, 547–557. [Google Scholar]
- Naqqash, T.; Aziz, A.; Gohar, M.; Khan, J.; Ali, S.; Radicetti, E.; Babar, M.; Siddiqui, M.H.; Haider, G. Heavy metal-resistant rhizobacteria fosters to alleviate the cadmium toxicity in Arabidopsis by upregulating the plant physiological responses. Int. J. Phytoremediat. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, M.A.; Zhao, H.; Cheng, J.; Xie, J.; Jiang, D.; Fu, Y. Sclerotia of a phytopathogenic fungus restrict microbial diversity and improve soil health by suppressing other pathogens and enriching beneficial microorganisms. J. Environ. Manag. 2020, 259, 109857. [Google Scholar] [CrossRef]
- Teng, Y.; Chen, W. Soil Microbiomes—A Promising Strategy for Contaminated Soil Remediation: A Review. Pedosphere 2019, 29, 283–297. [Google Scholar] [CrossRef]
- Liu, H.; Tan, X.; Guo, J.; Liang, X.; Xie, Q.; Chen, S. Bioremediation of oil-contaminated soil by combination of soil conditioner and microorganism. J. Soils Sediments 2020, 20, 2121–2129. [Google Scholar] [CrossRef]
- Zheng, W.; Cui, T.; Li, H. Combined technologies for the remediation of soils contaminated by organic pollutants. A review. Environ. Chem. Lett. 2022, 20, 2043–2062. [Google Scholar] [CrossRef]
- Batista, B.D.; Singh, B.K. Realities and hopes in the application of microbial tools in agriculture. Microb. Biotechnol. 2021, 14, 1258–1268. [Google Scholar] [CrossRef]
- Bhanse, P.; Kumar, M.; Singh, L.; Awasthi, M.K.; Qureshi, A. Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: Opportunities, challenges, and prospects. Chemosphere 2022, 303, 134954. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Opeolu, B.O.; Jackson, V.A. Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manag. 2017, 60, 758–783. [Google Scholar] [CrossRef] [PubMed]
- Santillan, J.Y.; Muzlera, A.; Molina, M.; Lewkowicz, E.S.; Iribarren, A.M. Microbial degradation of organophosphorus pesticides using whole cells and enzyme extracts. Biodegradation 2020, 31, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Dynamism of PGPR in bioremediation and plant growth promotion in heavy metal contaminated soil. Indian J. Exp. Biol. 2016, 54, 286–290. [Google Scholar] [PubMed]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of Heavy Metal Stress in Plants and Remediation of Soil by Rhizosphere Microorganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Cui, Y.; Zhang, Q.; Yin, N.; Cai, X.; Yuan, X.; Senadheera, S.; Cho, Y.; Ok, Y.S. A critical review of the interactions between rhizosphere and biochar during the remediation of metal(loid) contaminated soils. Biochar 2023, 5, 87. [Google Scholar] [CrossRef]
- Morcillo, R.J.L.; Manzanera, M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Vibhandik, R.; Agrahari, R.; Daverey, A.; Rani, R. Role of Root Exudates on the Soil Microbial Diversity and Biogeochemistry of Heavy Metals. Appl. Biochem. Biotechnol. 2023, 1–21. [Google Scholar] [CrossRef]
- Ren, Z.; Cheng, R.; Chen, P.; Xue, Y.; Xu, H.; Yin, Y.; Huang, G.; Zhang, W.; Zhang, L. Plant-associated Microbe System in Treatment of Heavy Metals–contaminated Soil: Mechanisms and Applications. Water Air Soil Pollut. 2023, 234, 39. [Google Scholar] [CrossRef]
- Cicatelli, A.; Ferrol, N.; Rozpadek, P.; Castiglione, S. Editorial: Effects of Plant-Microbiome Interactions on Phyto- and Bio-Remediation Capacity. Front. Plant Sci. 2019, 10, 533. [Google Scholar] [CrossRef]
- Hassen, W.; Neifar, M.; Cherif, H.; Najjari, A.; Chouchane, H.; Driouich, R.C.; Salah, A.; Naili, F.; Mosbah, A.; Souissi, Y.; et al. Pseudomonas rhizophila S211, a New Plant Growth-Promoting Rhizobacterium with Potential in Pesticide-Bioremediation. Front. Microbiol. 2018, 9, 34. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M.S.; Singh, U.B. Pesticide-tolerant microbial consortia: Potential candidates for remediation/clean-up of pesticide-contaminated agricultural soil. Environ. Res. 2023, 236, 116724. [Google Scholar] [CrossRef]
- Fu, R.M.; Chang, H.P.; Zhu, M.F.; Chen, W.L. Research, Application Demonstration of Key Technology for Microbial Remediation of Saline-Alkali Soil. Int. J. Agric. Biol. 2018, 20, 2556–2560. [Google Scholar] [CrossRef]
- Gao, M.; Gao, B.; Zhang, X.; Fan, J.; Liu, X.; Wang, C. Effects of Plant Growth–Promoting Rhizobacteria (PGPR) on the Phytoremediation of Pyrene-Nickel-Contaminated Soil by Juncus effusus. Water Air Soil Pollut. 2022, 233, 458. [Google Scholar] [CrossRef]
- Jiang, H.-Y.; Wu, H.-K.; Yuan, P.-P.; Guo, J.-J.; Wang, L.; Dai, Y.-J. Biodegradation of sulfoxaflor by Pseudomonas stutzeri CGMCC 22915 and characterization of the nitrile hydratase involved. Int. Biodeterior. Biodegrad. 2022, 170, 105403. [Google Scholar] [CrossRef]
- Esringü, A.; Turan, M.; Güneş, A.; Karaman, M.R. Roles of Bacillus megateriumin Remediation of Boron, Lead, and Cadmium from Contaminated Soil. Commun. Soil Sci. Plant Anal. 2014, 45, 1741–1759. [Google Scholar] [CrossRef]
- Muratova, A.; Golubev, S.; Romanova, V.; Sungurtseva, I.; Nurzhanova, A. Effect of Heavy-Metal-Resistant PGPR Inoculants on Growth, Rhizosphere Microbiome and Remediation Potential of Miscanthus × giganteus in Zinc-Contaminated Soil. Microorganisms 2023, 11, 1516. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, F.; Munis, M.F.H.; Arif, S.; Haroon, U.; Shengquan, C.; Qunlu, L. Cd-tolerant SY-2 strain of Stenotrophomonas maltophilia: A potential PGPR, isolated from the Nanjing mining area in China. 3 Biotech 2020, 10, 519. [Google Scholar] [CrossRef]
- Wang, P.; Wei, H.; Ke, T.; Fu, Y.; Zeng, Y.; Chen, C.; Chen, L. Characterization and genome analysis of Acinetobacter oleivorans S4 as an efficient hydrocarbon-degrading and plant-growth-promoting rhizobacterium. Chemosphere 2023, 331, 138732. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Kumar, N.; Shandilya, C.; Mohapatra, S.; Bhayana, S.; Varma, A. Revisiting Plant–Microbe Interactions and Microbial Consortia Application for Enhancing Sustainable Agriculture: A Review. Front. Microbiol. 2020, 11, 560406. [Google Scholar] [CrossRef]
- Wang, K.; Hou, J.; Zhang, S.; Hu, W.; Yi, G.; Chen, W.; Cheng, L.; Zhang, Q. Preparation of a new biochar-based microbial fertilizer: Nutrient release patterns and synergistic mechanisms to improve soil fertility. Sci. Total. Environ. 2023, 860, 160478. [Google Scholar] [CrossRef]
- Parveen, N.; Mishra, R.; Singh, D.V.; Kumar, P.; Singh, R.P. Assessment of different carrier materials for the preparation of microbial formulations to enhance the shelf life and its efficacy on the growth of spinach (Spinacia oleracea L.). World J. Microbiol. Biotechnol. 2023, 39, 180. [Google Scholar] [CrossRef] [PubMed]

| Microorganisms | Crop | Mechanism of Action | References |
|---|---|---|---|
| Pseudomonas sp. | Maize, cassava, spring wheat, tomato, Arabidopsisthaliana | Promotion of nutrient uptake, regulation of hormone levels, ISR, ACC-deaminase activity, siderophore, nitrogen fixation, solubilization of phosphorus | [41,42] |
| Bacillus sp. | Soya, oriental melons, potatoes, barley, maize | Vocs, antibacterial compound, organic acids, exopolysaccharides, different enzymes, ISR | [43,44,45] |
| Rhizobium sp. | Soya, peanuts, | Nitrogenfixation, exopolysaccharides, phosphate solubilization | [46,47] |
| Azotobacter sp. | Rice, tomato, cowpea bean, | Nitrogenfixation, dissolved phosphorus and potassium, generation of IAA, siderophore | [48,49,50] |
| Azospirillum sp. | Cucumber | Siderophore, indole-3-aceticacid, ISR | [51] |
| Pseudomonasputida | Melon | Different enzymes, solubilization of phosphorus, siderophore | [52] |
| Pseudomonasaeruginosa | Tobacco | Dissolved phosphorus and potassium, growth hormone | [53] |
| Bacillusaryabhattai | Tomato, maize, bean | Growth hormone, solubilization of phosphorus | [54] |
| Microorganisms | Crop | Type of Abiotic Stress | Mechanism of Action | References |
|---|---|---|---|---|
| Bacillus licheniformis K11 | Pepper | Drought stress | Auxin and ACC deaminase producing PGPR B. licheniformis K11 could reduce drought stress in drought-affected regions | [140] |
| Bacillus subtilis-FAB1, Pseudomonas azotoformans-FAP3 | Wheat | Drought stress | FAB1 and FAP3 strains show unique multifunctional plant growth-stimulating properties and effective root and rhizosphere colonization to promote wheat growth during drought | [121] |
| Phyllobacterium brassicacearum | Arabidopsis thaliana | Drought stress | Bacteria induce growth and development and coordinate to improve water use efficiency in plants | [141] |
| Bacillus subtilis, Bacillus pumilus | Cotton | Salt stress | B. subtilis and B. pumilus significantly enhance salt stress tolerance in cotton plants during salt stress conditions. | [142] |
| B. subtilis CNBG-PGPR-1 | Tomato | Salt stress | CNBG-PGPR-1 significantly improved the cellular homeostasis and photosynthetic efficiency of leaves and reduced ion toxicity and osmotic stress caused by salt in tomato | [89] |
| Pseudomonas fluorescens | Mustard | Salt stress | Two strains increase cell viability and reduce leaf damage and superoxide production | [143] |
| Viridibacillus sp. | Maize | Heavy mental stress | Inoculation with the strain promoted plant growth and development and alleviated the effects of stress on the plant | [144] |
| Morganella morganii strains | Arabidopsis thaliana | Heavy mental stress | PGPR can protect plants from Cd toxicity, and Cd-tolerant rhizobacterial strains can remediate heavy metal-polluted sites and improve plant growth | [145] |
| Acinetobacter beijerinckii, Raoultella planticola | Soybean | Heavy mental stress | PGPR strain promotes host antioxidant production and alters physiological and metabolic responses in soybean, enabling it to better cope with chromate and arsenic toxicity and grow well under stress | [139] |
| Microorganisms | Main Pollutants | Repair Results | References |
|---|---|---|---|
| Pseudomonas spp., Bacillus substilis, B. megaterium | saline–alkaline soil0 | These complex microbial agents could not only reduce the salt content and pH but also increase the organic content of the saline soil | [163] |
| Klebsiella sp. | Pyrene–nickel-contaminated soil | The pyrene degradation rate was 97.3% and 97.1% in pyrene-contaminated soil and pyrene–Ni-contaminated soil, respectively | [164] |
| Pseudomonas | pesticide-contaminated agricultural soil | Pseudomonas stutzeri CGMCC 22915 rapidly degraded sulfoxaflor to sulfoxaflor-amide via hydration. | [165] |
| Bacillus megaterium | Boron (B)- lead (Pb)- and cadmium (Cd)-contaminated soil | Reduced boron (B), lead (Pb) and cadmium (Cd) in soil and remediation of soil environment | [166] |
| Mycolicibacterium sp. Pb113 and Chitinophaga sp. Zn19 | Heavy metal-contaminated soil | Two inoculants promote manzanita growth and improve soil zinc pollution remediation efficiency | [167] |
| plant growth-promoting rhizobacterium strain MD36 | Heavy metal-contaminated soil | Strain MD36 effectively improves growth and yield of heavy metal-contaminated soils and bioremediation of HM-contaminated saline soils and water | [137] |
| Stenotrophomonas maltophilia | Cadmium (Cd)-contaminated soil | The SY-2 strain of S. maltophilia possesses significant metal tolerance and bioremediation potential against cadmium | [168] |
| Acinetobacter oleivorans | Soil contamination by hydrocarbons | Acinetobacter oleivorans S4 promoted plant growth and degraded total oil hydrocarbons in soil | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Xu, J.; Chen, J.; Liu, P.; Hou, X.; Yang, L.; Zhang, L. Progress in Microbial Fertilizer Regulation of Crop Growth and Soil Remediation Research. Plants 2024, 13, 346. https://doi.org/10.3390/plants13030346
Wang T, Xu J, Chen J, Liu P, Hou X, Yang L, Zhang L. Progress in Microbial Fertilizer Regulation of Crop Growth and Soil Remediation Research. Plants. 2024; 13(3):346. https://doi.org/10.3390/plants13030346
Chicago/Turabian StyleWang, Tingting, Jiaxin Xu, Jian Chen, Peng Liu, Xin Hou, Long Yang, and Li Zhang. 2024. "Progress in Microbial Fertilizer Regulation of Crop Growth and Soil Remediation Research" Plants 13, no. 3: 346. https://doi.org/10.3390/plants13030346
APA StyleWang, T., Xu, J., Chen, J., Liu, P., Hou, X., Yang, L., & Zhang, L. (2024). Progress in Microbial Fertilizer Regulation of Crop Growth and Soil Remediation Research. Plants, 13(3), 346. https://doi.org/10.3390/plants13030346