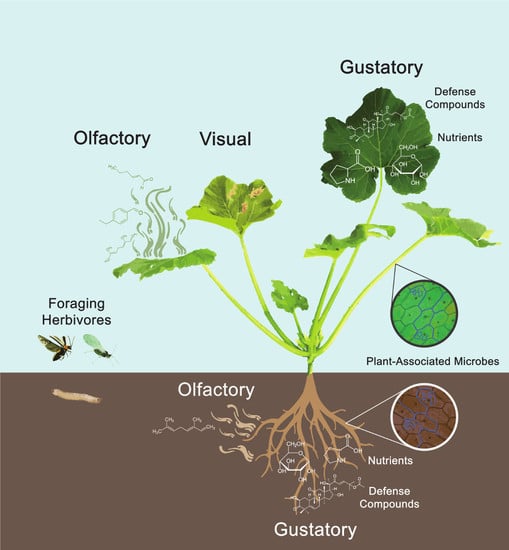
The Role of Plant-Associated Microbes in Mediating Host-Plant Selection by Insect Herbivores
Abstract
:1. Introduction
2. Beneficial Plant-Associated Microbes
3. Pathogenic Plant-Associated Microbes
4. Visual Cues
4.1. Influence of Beneficial Microbes on Plant-Produced Visual Cues
4.2. Influence of Pathogenic Microbes on Plant-Produced Visual Cues
5. Olfactory Cues
5.1. Influence of Beneficial Microbes on Plant-Produced Olfactory Cues
5.2. Influence of Pathogenic Microbes on Plant-Produced Olfactory Cues
6. Gustatory Cues
6.1. Influence of Beneficial Microbes on Plant-Produced Gustatory Cues
6.2. Influence of Pathogenic Microbes on Plant-Produced Gustatory Cues
7. Conclusions and Perspectives for Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Behmer, S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, W.C.; Kharouba, H.M.; Robinson, M.; Holyoak, M.; Karban, R. Variability in plant nutrients reduces insect herbivore performance. Nature 2016, 539, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef]
- Biere, A.; Bennett, A.E. Three-way interactions between plants, microbes and insects. Funct. Ecol. 2013, 27, 567–573. [Google Scholar] [CrossRef] [Green Version]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Mauck, K.E.; Balogh, C.M.; Stephenson, A.G.; Mescher, M.C.; De Moraes, C.M. Inbreeding in horsenettle (Solanum carolinense) alters night-time volatile emissions that guide oviposition by Manduca sexta moths. Proc. R. Soc. B Biol. Sci. 2013, 280. [Google Scholar] [CrossRef] [Green Version]
- Reeves, J.L. Vision should not be overlooked as an important sensory modality for finding host plants. Environ. Entomol. 2011, 40, 855–863. [Google Scholar] [CrossRef]
- Couty, A.; Van Emden, H.; Perry, J.N.; Hardie, J.; Pickett, J.A.; Wadhams, L.J. The roles of olfaction and vision in host-plant finding by the diamondback moth, Plutella xylostella. Physiol. Entomol. 2006, 31, 134–145. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Pickett, J.A. Perception of plant volatile blends by herbivorous insects—Finding the right mix. Phytochemistry 2011, 72, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xiu, C.; Lu, Y. A Combination of olfactory and visual cues enhance the behavioral responses of Apolygus lucorum. J. Insect Behav. 2015, 28, 525–534. [Google Scholar] [CrossRef]
- Jönsson, M.; Rosdahl, K.; Anderson, P. Responses to olfactory and visual cues by over-wintered and summer generations of the pollen beetle, Meligethes aeneus. Physiol. Entomol. 2007, 32, 188–193. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Roles of olfactory cues, visual cues, and mating status in orientation of Diaphorina citri kuwayama (Hemiptera: Psyllidae) to four different host plants. Environ. Entomol. 2009, 38, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, R.; Clarke, A.R. The “sequential cues hypothesis”: A conceptual model to explain host location and ranking by polyphagous herbivores. Insect Sci. 2019, 1–11. [Google Scholar] [CrossRef]
- Turlure, C.; Schtickzelle, N.; Van Dyck, H.; Seymoure, B.; Rutowski, R. Flight morphology, compound eye structure and dispersal in the bog and the cranberry fritillary butterflies: An inter- and intraspecific comparison. PLoS ONE 2016, 11, e0158073. [Google Scholar] [CrossRef]
- Backus, E.A.; Cervantes, F.A.; Guedes, R.N.C.; Li, A.Y.; Wayadande, A.C. AC–DC electropenetrography for in-depth studies of feeding and oviposition behaviors. Ann. Entomol. Soc. Am. 2019, 112, 236–248. [Google Scholar] [CrossRef] [Green Version]
- Hassell, M.P.; Southwood, T.R.E. Foraging strategies of insects. Annu. Rev. Ecol. Syst. 1978, 9, 75–98. [Google Scholar] [CrossRef]
- Schumann, M.; Ladin, Z.S.; Beatens, J.M.; Hiltpold, I. Navigating on a chemical radar: Usage of root exudates by foraging Diabrotica virgifera virgifera larvae. J. Appl. Entomol. 2018, 142, 911–920. [Google Scholar] [CrossRef]
- Shiojiri, K.; Ozawa, R.; Takabayashi, J. Plant volatiles, rather than light, determine the nocturnal behavior of a caterpillar. PLoS Biol. 2006, 4, e164. [Google Scholar] [CrossRef] [Green Version]
- Shikano, I.; Rosa, C.; Tan, C.-W.; Felton, G.W. Tritrophic interactions: Microbe-mediated plant effects on insect herbivores. Annu. Rev. Phytopathol. 2017, 55, 313–331. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.S.; Bantay, R.; Friel, C.A.; Garoutte, A.; Gdanetz, K.; Ibarreta, K.; Moore, B.M.; Shetty, P.; Siler, E.; Friesen, M.L. Beneficial microbes ameliorate abiotic and biotic sources of stress on plants. Funct. Ecol. 2019, 1365–2435, 13499. [Google Scholar] [CrossRef] [Green Version]
- Pineda, A.; Dicke, M.; Pieterse, C.M.J.; Pozo, M.J. Beneficial microbes in a changing environment: Are they always helping plants to deal with insects? Funct. Ecol. 2013, 27, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Partida-Martínez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant Sci. 2011, 2, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, F.P.; Moura, D.S.; Vivanco, J.M.; Silva-Filho, M.C. Plant–insect–pathogen interactions: A naturally complex ménage à trois. Curr. Opin. Microbiol. 2017, 37, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-borne plant pathogens and their vectors: Ecology, evolution, and complex interactions. Annu. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Effects of pathogens on sensory-mediated interactions between plants and insect vectors. Curr. Opin. Plant Biol. 2016, 32, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Gibert, A.; Tozer, W.; Westoby, M. Plant performance response to eight different types of symbiosis. New Phytol. 2019, 222, 526–542. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Elias, J.D.; Balkan, M.A.; Fordyce, R.F.; Kennedy, P.G. Colonization by nitrogen-fixing Frankia bacteria causes short-term increases in herbivore susceptibility in red alder (Alnus rubra) seedlings. Oecologia 2017, 184, 497–506. [Google Scholar] [CrossRef]
- Wilkinson, T.D.J.; Ferrari, J.; Hartley, S.E.; Hodge, A. Aphids can acquire the nitrogen delivered to plants by arbuscular mycorrhizal fungi. Funct. Ecol. 2019, 33, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Pineda, A.; Zheng, S.-J.; van Loon, J.J.A.; Pieterse, C.M.J.; Dicke, M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Schoenherr, A.P.; Rizzo, E.; Jackson, N.; Manosalva, P.; Gomez, S.K. Mycorrhiza-induced resistance in potato involves priming of defense responses against cabbage looper (Noctuidae: Lepidoptera). Environ. Entomol. 2019, 48, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Hunter, M.D.; de Roode, J.C. Microbial root mutualists affect the predators and pathogens of herbivores above ground: Mechanisms, magnitudes, and missing links. Front. Ecol. Evol. 2017, 5, 160. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.M.; Mescher, M.C.; De Moraes, C.M. Plant dependence on rhizobia for nitrogen influences induced plant defenses and herbivore performance. Int. J. Mol. Sci. 2014, 15, 1466–1480. [Google Scholar] [CrossRef] [Green Version]
- Kempel, A.; Brandl, R.; Schädler, M. Symbiotic soil microorganisms as players in aboveground plant-herbivore interactions—The role of rhizobia. Oikos 2009, 118, 634–640. [Google Scholar] [CrossRef]
- Zehnder, G.; Kloepper, J.; Tuzun, S.; Yao, C.; Wei, G.; Chambliss, O.; Shelby, R. Insect feeding on cucumber mediated by rhizobacteria-induced plant resistance. Entomol. Exp. Appl. 1997, 83, 81–85. [Google Scholar] [CrossRef]
- Crawford, K.M.; Land, J.M.; Rudgers, J.A. Fungal endophytes of native grasses decrease insect herbivore preference and performance. Oecologia 2010, 164, 431–444. [Google Scholar] [CrossRef]
- Babikova, Z.; Gilbert, L.; Bruce, T.; Dewhirst, S.Y.; Pickett, J.A.; Johnson, D. Arbuscular mycorrhizal fungi and aphids interact by changing host plant quality and volatile emission. Funct. Ecol. 2014, 28, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Babikova, Z.; Gilbert, L.; Randall, K.C.; Bruce, T.J.; Pickett, J.A.; Johnson, D. Increasing phosphorus supply is not the mechanism by which arbuscular mycorrhiza increase attractiveness of bean (Vicia faba) to aphids. J. Exp. Bot. 2014, 65, 5231–5241. [Google Scholar] [CrossRef] [Green Version]
- Balog, A.; Loxdale, H.D.; Bálint, J.; Benedek, K.; Szabó, K.A.; Jánosi-Rancz, K.T.; Domokos, E. The arbuscular mycorrhizal fungus Rhizophagus irregularis affects arthropod colonization on sweet pepper in both the field and greenhouse. J. Pest Sci. 2017, 90, 935–946. [Google Scholar] [CrossRef]
- Cosme, M.; Stout, M.J.; Wurst, S. Effect of arbuscular mycorrhizal fungi (Glomus intraradices) on the oviposition of rice water weevil (Lissorhoptrus oryzophilus). Mycorrhiza 2011, 21, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Wurst, S.; Forstreuter, M. Colonization of Tanacetum vulgare by aphids is reduced by earthworms. Entomol. Exp. Appl. 2010, 137, 86–92. [Google Scholar] [CrossRef]
- Roger, A.; Gétaz, M.; Rasmann, S.; Sanders, I.R. Identity and combinations of arbuscular mycorrhizal fungal isolates influence plant resistance and insect preference. Ecol. Entomol. 2013, 38, 330–338. [Google Scholar] [CrossRef]
- Jallow, M.F.A.; Dugassa-Gobena, D.; Vidal, S. Influence of an endophytic fungus on host plant selection by a polyphagous moth via volatile spectrum changes. Arthropod Plant Interact. 2008, 2, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Van Bael, S.A.; Valencia, M.C.; Rojas, E.I.; Gómez, N.; Windsor, D.M.; Herre, E.A. Effects of foliar endophytic fungi on the preference and performance of the leaf beetle Chelymorpha alternans in Panama. Biotropica 2009, 41, 221–225. [Google Scholar] [CrossRef]
- Bultman, T.L.; Pulas, C.; Grant, L.; Bell, G.; Sullivan, T.J. Effects of fungal endophyte isolate on performance and preference of bird cherry-oat aphid. Environ. Entomol. 2006, 35, 1690–1695. [Google Scholar] [CrossRef]
- Latch, G.C.M.; Hunt, W.F.; Musgrave, D.R. Endophytic fungi affect growth of perennial ryegrass. N. Z. J. Agric. Res. 1985, 28, 165–168. [Google Scholar] [CrossRef]
- Clement, S.L.; Hu, J.; Stewart, A.V.; Wang, B.; Elberson, L.R. Detrimental and neutral effects of a wild grass-fungal endophyte symbiotum on insect preference and performance. J. Insect Sci. 2011, 11, 1–13. [Google Scholar] [CrossRef]
- Hardy, T.N.; Clay, K.; Hammond, A.M. Fall armyworm (Lepidoptera: Noctuidae): A laboratory bioassay and larval preference study for the fungal endophyte of perennial ryegrass. J. Econ. Entomol. 1985, 78, 571–575. [Google Scholar] [CrossRef]
- Qawasmeh, A.; Raman, A.; Wheatley, W. Volatiles in perennial ryegrass infected with strains of endophytic fungus: Impact on African black beetle host selection. J. Appl. Entomol. 2014, 139, 94–104. [Google Scholar] [CrossRef]
- Rostás, M.; Cripps, M.G.; Silcock, P. Aboveground endophyte affects root volatile emission and host plant selection of a belowground insect. Oecologia 2015, 177, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.C.; Potter, D.A. Turfgrass species and endophyte effects on survival, development, and feeding preference of black cutworms (Lepidoptera: Noctuidae). J. Econ. Entomol. 1997, 90, 1290–1299. [Google Scholar] [CrossRef]
- Disi, J.O.; Zebelo, S.; Kloepper, J.W.; Fadamiro, H. Seed inoculation with beneficial rhizobacteria affects European corn borer (Lepidoptera: Pyralidae) oviposition on maize plants. Entomol. Sci. 2018, 21, 48–58. [Google Scholar] [CrossRef]
- Disi, J.O.; Kloepper, J.W.; Fadamiro, H.Y. Seed treatment of maize with Bacillus pumilus strain INR-7 affects host location and feeding by Western corn rootworm, Diabrotica virgifera virgifera. J. Pest Sci. 2018, 91, 515–522. [Google Scholar] [CrossRef]
- Zehnder, G.; Kloepper, J.; Yao, C.; Wei, G. Induction of systemic resistance in cucumber against cucumber beetles (Coleoptera: Chrysomelidae) by plant growth-promoting rhizobacteria. J. Econ. Entomol. 1997, 90, 391–396. [Google Scholar] [CrossRef]
- Coy, R.M.; Held, D.W.; Kloepper, J.W. Bacterial inoculant treatment of bermudagrass alters ovipositional behavior, larval and pupal weights of the fall armyworm (Lepidoptera: Noctuidae). Environ. Entomol. 2017, 46, 831–838. [Google Scholar]
- Katayama, N.; Zhang, Z.Q.; Ohgushi, T. Community-wide effects of below-ground rhizobia on above-ground arthropods. Ecol. Entomol. 2011, 36, 43–51. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Schädler, M. Induced plant defense via volatile production is dependent on rhizobial symbiosis. Oecologia 2013, 172, 833–846. [Google Scholar] [CrossRef]
- Burdon, J.J.; Thrall, P.H.; Ericson, A.L. The current and future dynamics of disease in plant communities. Annu. Rev. Phytopathol. 2006, 44, 19–39. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.J.; Liu, X.L.; Liu, X.Q.; Zhang, H.; Yu, Y.J.; Liang, Z.W. Stunted growth caused by blast disease in rice seedlings is associated with changes in phytohormone signaling pathways. Front. Plant Sci. 2017, 8, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chesnais, Q.; Mauck, K.E.; Bogaert, F.; Bamière, A.; Catterou, M.; Spicher, F.; Brault, V.; Tepfer, M.; Ameline, A. Virus effects on plant quality and vector behavior are species specific and do not depend on host physiological phenotype. J. Pest Sci. 2019, 92, 791–804. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cui, H.; Cui, X.; Wang, A. The altered photosynthetic machinery during compatible virus infection. Curr. Opin. Virol. 2016, 17, 19–24. [Google Scholar] [CrossRef]
- Moericke, V. Hostplant specific colour behaviour by Hyalopterus pruni (Aphididae). Entomol. Exp. Appl. 1969, 12, 524–534. [Google Scholar] [CrossRef]
- Navas, M.L.; Friess, N.; Maillet, J. Influence of cucumber mosaic virus infection on the growth response of Portulaca oleracea (purslane) and Stellaria media (chickweed) to nitrogen availability. New Phytol. 1998, 139, 301–309. [Google Scholar] [CrossRef]
- Mann, R.S.; Ali, J.G.; Hermann, S.L.; Tiwari, S.; Pelz-Stelinski, K.S.; Alborn, H.T.; Stelinski, L.L. Induced release of a plant-defense volatile “deceptively” attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog. 2012, 8, e1002610. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Conradi, P.; Jactel, H.; Robin, C.; Tack, A.J.M.; Castagneyrol, B. Fungi reduce preference and performance of insect herbivores on challenged plants. Ecology 2018, 99, 300–311. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef]
- Biggs, A.R. Occurrence and location of suberin in wound reaction zones in xylem of 17 tree species. Phytopathology 1987, 77, 718–725. [Google Scholar] [CrossRef]
- Pearce, R.B. Occurrence of decay-associated xylem suberization in a range of woody species. Eur. J. For. Pathol. 1990, 20, 275–289. [Google Scholar] [CrossRef]
- Dangl, J.L.; Jones, J.D.G. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Harth, J.E.; Ferrari, M.J.; Helms, A.M.; Tooker, J.F.; Stephenson, A.G. Corrigendum: Zucchini yellow mosaic virus infection limits establishment and severity of powdery mildew in wild populations of Cucurbita pepo. Front. Plant Sci. 2018, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: Turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Perilla-Henao, L.M.; Casteel, C.L. Vector-borne bacterial plant pathogens: Interactions with hemipteran insects and plants. Front. Plant Sci. 2016, 7, 1163. [Google Scholar] [CrossRef]
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host-vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Mauck, K.E. Variation in virus effects on host plant phenotypes and insect vector behavior: What can it teach us about virus evolution? Curr. Opin. Virol. 2016, 21, 114–123. [Google Scholar] [CrossRef]
- Pelz-Stelinski, K.S.; Killiny, N. Better together: Association with ‘Candidatus liberibacter asiaticus’ increases the reproductive fitness of its insect vector, Diaphorina citri (Hemiptera: Liviidae). Ann. Entomol. Soc. Am. 2016, 109, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Killiny, N.; Hijaz, F.; Ebert, T.A.; Rogers, M.E. A plant bacterial pathogen manipulates its insect vector’s energy metabolism. Appl. Environ. Microbiol. 2017, 83, e03005-16. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.Z.; Li, Y.; Yang, B. The inhibitory effects of rose powdery mildew infection on the oviposition behaviour and performance of beet armyworms. Entomol. Exp. Appl. 2013, 148, 39–47. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Lait, C.G.; Schmelz, E.A.; Huang, J.; Tumlinson, J.H. Fungus-induced biochemical changes in peanut plants and their effect on development of beet armyworm, Spodoptera exigua Hübner (Lepidoptera: Noctuidae) larvae. Environ. Entomol. 2003, 32, 220–228. [Google Scholar] [CrossRef]
- Cardoza, Y.J.; Teal, P.E.A.; Tumlinson, J.H. Effect of peanut plant fungal infection on oviposition preference by Spodoptera exigua and on host-searching behavior by Cotesia marginiventris. Environ. Entomol. 2003, 32, 970–976. [Google Scholar] [CrossRef]
- Dötterl, S.; Jürgens, A.; Wolfe, L.; Biere, A. Disease status and population origin effects on floral scent: Potential consequences for oviposition and fruit predation in a complex interaction between a plant, fungus, and noctuid moth. J. Chem. Ecol. 2009, 35, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Hilker, M. Does rust infection of willow affect feeding and oviposition behavior of willow leaf beetles? J. Insect Behav. 2005, 18, 115–129. [Google Scholar] [CrossRef]
- Jagiełło, R.; Łakomy, P.; Łukowski, A.; Giertych, M.J. Spreading-the-risk hypothesis may explain Cameraria ohridella oviposition in relation to leaf blotch disease. Arthropod. Plant. Interact. 2019, 13, 787–795. [Google Scholar] [CrossRef] [Green Version]
- Tasin, M.; Knudsen, G.K.; Pertot, I. Smelling a diseased host: Grapevine moth responses to healthy and fungus-infected grapes. Anim. Behav. 2012, 83, 555–562. [Google Scholar] [CrossRef]
- Rizvi, S.Z.M.; Raman, A.; Wheatley, W.; Cook, G.; Nicol, H. Influence of Botrytis cinerea (Helotiales: Sclerotiniaceae) infected leaves of Vitis vinifera (Vitales: Vitaceae) on the preference of Epiphyas postvittana (Lepidoptera: Tortricidae). Austral Entomol. 2015, 54, 60–70. [Google Scholar] [CrossRef]
- Ako, M.; Schulthess, F.; Gumedzoe, M.Y.D.; Cardwell, K.F. The effect of Fusarium verticillioides on oviposition behaviour and bionomics of lepidopteran and coleopteran pests attacking the stem and cobs of maize in West Africa. Entomol. Exp. Appl. 2003, 106, 201–210. [Google Scholar] [CrossRef]
- Friedli, J.; Bacher, S. Direct and indirect effects of a shoot-base boring weevil and plant competition on the performance of creeping thistle, Cirsium arvense. Biol. Control 2001, 22, 219–226. [Google Scholar] [CrossRef]
- McLeod, G.; Gries, R.; Von Reuß, S.H.; Rahe, J.E.; McIntosh, R.; König, W.A.; Gries, G. The pathogen causing Dutch elm disease makes host trees attract insect vectors. Proc. R. Soc. B Biol. Sci. 2005, 272, 2499–2503. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z.; Zhou, W.; Jin, H.; Liu, H.; Zhou, A.; Zhang, A.; Wang, M.-Q. Temporal interactions of plant-insect-predator after infection of bacterial pathogen on rice plants. Sci. Rep. 2016, 6, 26043. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; De Moraes, C.M.; Stephenson, A.G.; Mescher, M.C. Pathogen effects on vegetative and floral odours mediate vector attraction and host exposure in a complex pathosystem. Ecol. Lett. 2012, 15, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S.; Horton, D.R.; Munyaneza, J.E.; Landolt, P.J. Experimental infection of plants with an herbivore-associated bacterial endosymbiont influences herbivore host selection behavior. PLoS ONE 2012, 7, e49330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marucci, R.C.; Lopes, J.R.S.; Vendramim, J.D.; Corrente, J.E. Influence of Xylella fastidiosa infection of citrus on host selection by leafhopper vectors. Entomol. Exp. Appl. 2005, 117, 95–103. [Google Scholar] [CrossRef]
- Mayer, C.J.; Vilcinskas, A.; Gross, J. Phytopathogen lures its insect vector by altering host plant odor. J. Chem. Ecol. 2008, 34, 1045–1049. [Google Scholar] [CrossRef]
- Mayer, C.J.; Vilcinskas, A.; Gross, J. Pathogen-induced release of plant allomone manipulates vector insect behavior. J. Chem. Ecol. 2008, 34, 1518–1522. [Google Scholar] [CrossRef]
- Chuche, J.; Boudon-Padieu, E.; Thiéry, D. Host preferences of the leafhopper Scaphoideus titanus, vector of “flavescence dorée” phytoplasma. Phytopathog. Mollicutes 2016, 6, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Chuche, J.; Boursault, A.; Thiéry, D. Preliminary study of the aggregative behaviour of Scaphoideus titanus larvae. IOBC/WPRS Bull 2011, 67, 239–244. [Google Scholar]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [Green Version]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Effects of Cucumber mosaic virus infection on vector and non-vector herbivores of squash. Commun. Integr. Biol. 2010, 3, 579–582. [Google Scholar] [CrossRef]
- Khan, Z.R.; Saxena, R.C. Behavior and biology of Nephotettix virescens (Homoptera: Cicadellidae) on tungro virus-infected rice plants: Epidemiology implications. Environ. Entomol. 1985, 14, 297–304. [Google Scholar] [CrossRef]
- McMenemy, L.S.; Hartley, S.E.; MacFarlane, S.A.; Karley, A.J.; Shepherd, T.; Johnson, S.N. Raspberry viruses manipulate the behaviour of their insect vectors. Entomol. Exp. Appl. 2012, 144, 56–68. [Google Scholar] [CrossRef]
- Hodge, S.; Powell, G. Conditional facilitation of an aphid vector, Acyrthosiphon pisum, by the plant pathogen, pea enation mosaic virus. J. Insect Sci. 2010, 10, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chisholm, P.J.; Sertsuvalkul, N.; Casteel, C.L.; Crowder, D.W. Reciprocal plant-mediated interactions between a virus and a non-vector herbivore. Ecology 2018, 99, 2139–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musser, R.O.; Hum-Musser, S.M.; Felton, G.W.; Gergerich, R.C. Increased larval growth and preference for virus-infected leaves by the Mexican bean beetle, Epilachna varivestis Mulsant, a plant virus vector. J. Insect Behav. 2003, 16, 247–256. [Google Scholar] [CrossRef]
- Rajabaskar, D.; Ding, H.; Wu, Y.; Eigenbrode, S.D. Behavioral responses of green peach aphid, Myzus persicae (Sulzer), to the volatile organic compound emissions from four potato varieties. Am. J. Potato Res. 2013, 90, 171–178. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Garzo, E.; Verbeek, M.; Vosman, B.; Dicke, M.; Tjallingii, W.F. Infection of potato plants with potato leafroll virus changes attraction and feeding behaviour of Myzus persicae. Entomol. Exp. Appl. 2007, 125, 135–144. [Google Scholar] [CrossRef]
- Srinivasan, R.; Alvarez, J.M.; Eigenbrode, S.D.; Bosque-pérez, N.A. Influence of hairy nightshade Solanum sarrachoides (Sendtner) and Potato leafroll virus (Luteoviridae: Polerovirus) on the host preference of Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ. Entomol. 2006, 35, 546–553. [Google Scholar] [CrossRef]
- Jiménez-Martínez, E.S.; Bosque-Pérez, N.A.; Berger, P.H.; Zemetra, R.S.; Ding, H.; Eigenbrode, S.D. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to barley yellow dwarf virus–infected transgenic and untransformed wheat. Environ. Entomol. 2004, 33, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Peñaflor, M.F.G.V.; Mauck, K.E.; Alves, K.J.; De Moraes, C.M.; Mescher, M.C. Effects of single and mixed infections of Bean pod mottle virus and Soybean mosaic virus on host-plant chemistry and host–vector interactions. Funct. Ecol. 2016, 30, 1648–1659. [Google Scholar] [CrossRef]
- Thaler, J.S.; Agrawal, A.A.; Halitschke, R. Salicylate-mediated interactions between pathogens and herbivores. Ecology 2010, 91, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Fereres, A.; Peñaflor, M.; Favaro, C.; Azevedo, K.; Landi, C.; Maluta, N.; Bento, J.; Lopes, J. Tomato infection by whitefly-transmitted circulative and non-circulative viruses induce contrasting changes in plant volatiles and vector behaviour. Viruses 2016, 8, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bak, A.; Patton, M.K.F.; Perilla-Henao, L.M.; Aegerter, B.J.; Casteel, C.L. Ethylene signaling mediates potyvirus spread by aphid vectors. Oecologia 2019, 190, 139–148. [Google Scholar] [CrossRef]
- Adhab, M.; Finke, D.; Schoelz, J. Turnip aphids (Lipaphis erysimi) discriminate host plants based on the strain of Cauliflower mosaic virus infection. Emir. J. Food Agric. 2019, 31, 69–75. [Google Scholar]
- Ajayi, O.; Dewar, A.M. The effect of barley yellow dwarf virus on field populations of the cereal aphids, Sitobion avenae and Metopolophium dirhodum. Ann. Appl. Biol. 1983, 103, 1–11. [Google Scholar] [CrossRef]
- Fereres, A.; Kampmeier, G.E.; Irwin, M.E. Aphid attraction and preference for soybean and pepper plants infected with Potyviridae. Ann. Entomol. Soc. Am. 1999, 92, 542–548. [Google Scholar] [CrossRef]
- Prokopy, R.; Owens, E. Visual detection of plants by herbivorous insects. Annu. Rev. Entomol 1983, 28, 337–364. [Google Scholar] [CrossRef]
- Sétamou, M.; Sanchez, A.; Patt, J.M.; Nelson, S.D.; Jifon, J.; Louzada, E.S. Diurnal patterns of flight activity and effects of light on host finding behavior of the Asian citrus psyllid. J. Insect Behav. 2012, 25, 264–276. [Google Scholar] [CrossRef]
- Irwin, R.E.; Strauss, S.Y.; Storz, S.; Emerson, A.; Guibert, G. The role of herbivores in the maintenance of a flower color polymorphism in wild radish. Ecology 2003, 84, 1733–1743. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zverev, V.; Zvereva, E.L. Do defoliating insects distinguish between symmetric and asymmetric leaves within a plant? Ecol. Entomol. 2018, 43, 656–664. [Google Scholar] [CrossRef]
- Karageorgou, P.; Manetas, Y. The importance of being red when young: Anthocyanins and the protection of young leaves of Quercus coccifera from insect herbivory and excess light. Tree Physiol. 2006, 26, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016, 18, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempel, A.; Schmidt, A.K.; Brandl, R.; Schädler, M. Support from the underground: Induced plant resistance depends on arbuscular mycorrhizal fungi. Funct. Ecol. 2010, 24, 293–300. [Google Scholar] [CrossRef]
- Kariyat, R.R.; Hardison, S.B.; Ryan, A.B.; Stephenson, A.G.; De Moraes, C.M.; Mescher, M.C. Leaf trichomes affect caterpillar feeding in an instar-specific manner. Commun. Integr. Biol. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Malik, R.J.; Ali, J.G.; Bever, J.D. Mycorrhizal composition influences plant anatomical defense and impacts herbivore growth and survival in a life-stage dependent manner. Pedobiologia 2018, 66, 29–35. [Google Scholar] [CrossRef]
- Mazzoni, V.; Trona, F.; Ioriatti, C.; Lucchi, A.; Eriksson, A.; Anfora, G. Attractiveness of different colours to Scaphoideus titanus Ball (Hemiptera: Cicadellidae) adults. 2011, 67, 281–284. IOBC/wprs Bull 2011, 67, 281–284. [Google Scholar]
- Bosque-Pérez, N.A.; Eigenbrode, S.D. The influence of virus-induced changes in plants on aphid vectors: Insights from luteovirus pathosystems. Virus Res. 2011, 159, 201–205. [Google Scholar] [CrossRef]
- Hodge, S.; Powell, G. Do plant viruses facilitate their aphid vectors by inducing symptoms that alter behavior and performance? Environ. Entomol. 2008, 37, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Touhara, K.; Vosshall, L.B. Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 2009, 71, 307–332. [Google Scholar] [CrossRef] [PubMed]
- Szendrei, Z.; Rodriguez-Saona, C. A meta-analysis of insect pest behavioral manipulation with plant volatiles. Entomol. Exp. Appl. 2010, 134, 201–210. [Google Scholar] [CrossRef]
- Egonyu, J.P.; Ekesi, S.; Kabaru, J.; Irungu, L.; Torto, B. Cashew volatiles mediate short-range location responses in Pseudotheraptus wayi (Heteroptera: Coreidae). Environ. Entomol. 2013, 42, 1400–1407. [Google Scholar] [CrossRef]
- Ballhorn, D.J.; Kautz, S.; Heil, M. Distance and sex determine host plant choice by herbivorous beetles. PLoS ONE 2013, 8, e55602. [Google Scholar] [CrossRef] [Green Version]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef]
- Helms, A.M.; Ray, S.; Matulis, N.L.; Kuzemchak, M.C.; Grisales, W.; Tooker, J.F.; Ali, J.G. Chemical cues linked to risk: Cues from below-ground natural enemies enhance plant defences and influence herbivore behaviour and performance. Funct. Ecol. 2019, 33, 798–808. [Google Scholar] [CrossRef]
- Low, P.A.; McArthur, C.; Fisher, K.; Hochuli, D.F. Elevated volatile concentrations in high-nutrient plants: Do insect herbivores pay a high price for good food? Ecol. Entomol. 2014, 39, 480–491. [Google Scholar] [CrossRef]
- Maffei, M.E. Sites of synthesis, biochemistry and functional role of plant volatiles. S. Afr. J. Bot. 2010, 76, 612–631. [Google Scholar] [CrossRef] [Green Version]
- Brzozowski, L.J.; Mazourek, M.; Agrawal, A.A. Mechanisms of resistance to insect herbivores in isolated breeding lineages of Cucurbita pepo. J. Chem. Ecol. 2019, 45, 313–325. [Google Scholar] [CrossRef]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.C.; Unsicker, S.B.; Gershenzon, J. The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci. 2012, 17, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Bohm, K.; Gerards, S.; Hundscheid, M.; Melenhorst, J.; De Boer, W.; Garbeva, P. Calling from distance: Attraction of soil bacteria by plant root volatiles. ISME J. 2018, 12, 1252–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, A.; Reichelt, M.; Hempel, S.; Gershenzon, J.; Unsicker, S.B. The effects of arbuscular mycorrhizal fungi on direct and indirect defense metabolites of Plantago lanceolata L. J. Chem. Ecol. 2009, 35, 833–843. [Google Scholar] [CrossRef] [Green Version]
- Leitner, M.; Kaiser, R.; Hause, B.; Boland, W.; Mithöfer, A. Does mycorrhization influence herbivore-induced volatile emission in Medicago truncatula? Mycorrhiza 2010, 20, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Blande, J.D.; Gundel, P.E.; Helander, M.; Saikkonen, K. Epichloë endophytes alter inducible indirect defences in host grasses. PLoS ONE 2014, 9, e101331. [Google Scholar] [CrossRef] [Green Version]
- Meier, A.R.; Hunter, M.D. Mycorrhizae alter constitutive and herbivore-induced volatile emissions by milkweeds. J. Chem. Ecol. 2019, 45, 610–625. [Google Scholar] [CrossRef]
- Johnson, S.N.; Nielsen, U.N. Foraging in the dark—Chemically mediated host plant location by belowground insect herbivores. J. Chem. Ecol. 2012, 38, 604–614. [Google Scholar] [CrossRef]
- Chiriboga, X.; Guo, H.; Campos-Herrera, R.; Röder, G.; Imperiali, N.; Keel, C.; Maurhofer, M.; Turlings, T.C.J. Root-colonizing bacteria enhance the levels of (E)-β-caryophyllene produced by maize roots in response to rootworm feeding. Oecologia 2018, 187, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Disi, J.O.; Mohammad, H.K.; Lawrence, K.; Kloepper, J.; Fadamiro, H. A soil bacterium can shape belowground interactions between maize, herbivores and entomopathogenic nematodes. Plant Soil 2019, 437, 83–92. [Google Scholar] [CrossRef]
- Robert, C.A.M.; Erb, M.; Duployer, M.; Zwahlen, C.; Doyen, G.R.; Turlings, T.C.J. Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytol. 2012, 194, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.J.; Mowry, T.M.; Bosque-Pérez, N.A.; Ding, H.; Eigenbrode, S.D. Changes in green peach aphid responses to Potato leafroll virus-induced volatiles emitted during disease progression. Environ. Entomol. 2009, 38, 1429–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabaskar, D.; Wu, Y.; Bosque-Pérez, N.A.; Eigenbrode, S.D. Dynamics of Myzus persicae arrestment by volatiles from Potato leafroll virus-infected potato plants during disease progression. Entomol. Exp. Appl. 2013, 148, 172–181. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. B Biol. Sci. 2002, 269, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, B.K.; Itagaki, H.; Rivet, M.P. Peripheral and central structures involved in insect gustation. Microsc. Res. Tech. 1999, 47, 401–415. [Google Scholar] [CrossRef]
- Sisterson, M.S. Effects of insect-vector preference for healthy or infected plants on pathogen spread: Insights from a model. J. Econ. Entomol. 2008, 101, 1–8. [Google Scholar] [CrossRef]
- Machado, R.A.R.; Arce, C.C.M.; Ferrieri, A.P.; Baldwin, I.T.; Erb, M. Jasmonate-dependent depletion of soluble sugars compromises plant resistance to Manduca sexta. New Phytol. 2015, 207, 91–105. [Google Scholar] [CrossRef]
- Nishida, R. Chemical ecology of insect-plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Walling, L.L. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008, 125, 1074–1085. [Google Scholar] [CrossRef] [Green Version]
- Acevedo, F.E.; Rivera-Vega, L.J.; Chung, S.H.; Ray, S.; Felton, G.W. Cues from chewing insects—The intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 2015, 26, 80–86. [Google Scholar] [CrossRef]
- Tao, L.; Ahmad, A.; de Roode, J.C.; Hunter, M.D. Arbuscular mycorrhizal fungi affect plant tolerance and chemical defences to herbivory through different mechanisms. J. Ecol. 2016, 104, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.L.; Agrawal, A.A. Beyond preference and performance: Host plant selection by monarch butterflies, Danaus plexippus. Oikos 2019, 128, 1092–1102. [Google Scholar] [CrossRef]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria activates (+)-δ-cadinene synthase genes and induces systemic resistance in cotton against beet armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, A.E.; Bever, J.D.; Deane Bowers, M. Arbuscular mycorrhizal fungal species suppress inducible plant responses and alter defensive strategies following herbivory. Oecologia 2009, 160, 771–779. [Google Scholar] [CrossRef]
- Orlob, G.B.; Arny, D.C. Some metabolic changes accompanying infection by barley yellow dwarf virus. Phytopathology 1961, 51, 768–775. [Google Scholar]
- Jensen, S.G. Metabolism and carbohydrate composition in barley yellow dwarf virus-infected wheat. Phytopathology 1972, 62, 587–592. [Google Scholar] [CrossRef]
- Carmo-Sousa, M.; Moreno, A.; Garzo, E.; Fereres, A. A non-persistently transmitted-virus induces a pull-push strategy in its aphid vector to optimize transmission and spread. Virus Res. 2014, 186, 38–46. [Google Scholar] [CrossRef]
- Whitehead, S.R.; Turcotte, M.M.; Poveda, K. Domestication impacts on plant-herbivore interactions: A meta-analysis. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Vannette, R.L.; Hunter, M.D. Plant defence theory re-examined: Nonlinear expectations based on the costs and benefits of resource mutualisms. J. Ecol. 2011, 99, 66–76. [Google Scholar] [CrossRef]
- De Bobadilla, M.F.; Friman, J.; Pangesti, N.; Dicke, M.; van Loon, J.J.A.; Pineda, A. Does drought stress modify the effects of plant-growth promoting rhizobacteria on an aboveground chewing herbivore? Insect Sci. 2017, 24, 1034–1044. [Google Scholar] [CrossRef]
- Gershenzon, J. Changes in the levels of plant secondary metabolites under water and nutrient stress. In Phytochemical Adaptations to Stress; Springer: Boston, MA, USA, 1984; pp. 273–320. [Google Scholar]
- Dillon, F.M.; Chludil, H.D.; Reichelt, M.; Mithöfer, A.; Zavala, J.A. Field-grown soybean induces jasmonates and defensive compounds in response to thrips feeding and solar UV-B radiation. Environ. Exp. Bot. 2018, 156, 1–7. [Google Scholar] [CrossRef]
- Hahn, P.G.; Agrawal, A.A.; Sussman, K.I.; Maron, J.L. Population variation, environmental gradients, and the evolutionary ecology of plant defense against herbivory. Am. Nat. 2019, 193, 20–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 2008, 135, 575–586. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive fly larvae to overcome host defences. R. Soc. Open Sci. 2015, 2, 150170. [Google Scholar] [CrossRef] [Green Version]
- Body, M.; Kaiser, W.; Dubreuil, G.; Casas, J.; Giron, D. Leaf-miners co-opt microorganisms to enhance their nutritional environment. J. Chem. Ecol. 2013, 39, 969–977. [Google Scholar] [CrossRef]
- Hansen, A.K.; Moran, N.A. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol. Ecol. 2014, 23, 1473–1496. [Google Scholar] [CrossRef]

| Beneficial Microbe | Plant Species | Insect Species | Cue | Effect on Insect Preference | Reference |
|---|---|---|---|---|---|
| AMF | |||||
| Glomus spp., Rhizophagus irregularis, Gigaspora margarita, Paraglomus brasilianum | Fava bean (Vicia faba) | Pea aphid (Acyrthosiphon pisum) | Olfactory, Gustatory | Attractive | [39,40] |
| Rhizophagus irregularis | Sweet pepper (Capsicum annuum) | Green peach aphid (Myzus persicae), western flower thrips (Frankliniella occidentalis) | Gustatory | Repellent, No Effect | [41] |
| Glomus intraradices | Rice (Oryza sativa) | Rice water weevil (Lissorhoptrus oryzophilus) | Visual, Gustatory | Attractive | [42] |
| Glomus spp. | Tansy (Tanacetum vulgare) | Green peach aphid | Visual, Gustatory | No Effect | [43] |
| Rhizophagus irregularis isolates | Strawberry (Fragaria vesca) | African cotton bollworm (Spodoptera littoralis) | Visual | Variable | [44] |
| Root Endophyte | |||||
| Acremonium strictum | Tomato (Lycopersicon esculentum) | Cotton bollworm (Helicoverpa armigera) | Olfactory | Attractive | [45] |
| Foliar Endophyte | |||||
| Glomerella cingulate | Tropical vine (Merremia umbellata) | Leaf beetle (Chelymorpha alternans) | Unknown | No Effect | [46] |
| Neotyphodium coenophialum | Tall fescue (Lolium arundinaceum) | Bird cherry-oat aphid (Rhopalosiphum padi) | Unknown | Repellent | [47,48] |
| Neotyphodium spp. | Alpine timothy hay (Phleum alpinum) | Bird cherry-oat aphid, Cereal leaf beetle (Oulema melanopus) | Unknown | Repellent, No Effect | [49] |
| Epichloë spp. Neotyphodium spp. | Multiple native grasses | Fall armyworm (Spodoptera frugiperda), American grasshopper (Schistocerca americana), Bird cherry-oat aphid | Unknown | Variable | [38] |
| Acremonium loliae | Perennial ryegrass (Lolium perenne) | Fall armyworm | Unknown | Repellent | [50] |
| Neotyphodium lolii | Perennial ryegrass | African black beetle (Heteronychus arator) | Olfactory | Repellent | [51] |
| Neotyphodium uncinatum | Grass hybrid (Festuca pratensis X Lolium perenne) | Root herbivore (Costelytra zealandica) | Olfactory | Repellent | [52] |
| Neotyphodium spp. | Numerous grass species | Black cutworm (Agrotis ipsilon) | Unknown | Repellent | [53] |
| PGPR | |||||
| Bacillus spp., Fictibacillus spp. | Maize (Zea mays) | European corn borer (Ostrinia nubilalis) | Olfactory | Repellent | [54] |
| Bacillus spp., Fictibacillus spp. | Maize | Western corn rootworm (Diabrotica virgifera virgifera) | Unknown | Variable | [55] |
| Bacillus pumilus | Cucumber (Cucumis sativus) | Striped cucumber beetle (Acalymma vittatum), Spotted cucumber beetle (Diabrotica undecimpunctata) | Visual | Repellent | [56] |
| Paenibacillus spp., Bacillus spp., Brevibacillus spp. | Bermudagrass (Cynodon dactylon) | Fall armyworm | Unknown | Repellent | [57] |
| Rhizobia | |||||
| Bradyrhizobium spp., Rhizobium spp. | Soybean (Glycine max) | Chewing and piercing-sucking herbivores | Unknown | Attractive | [58] |
| Rhizobia spp. | Lima bean (Phaseolus lunatus) | Mexican bean beetle (Epilachna varivestis) | Olfactory | No Effect | [59] |
| Pathogenic Microbe | Plant Species | Insect Species | Vector Status | Cue | Effect on Insect Preference | Reference |
|---|---|---|---|---|---|---|
| Fungi | ||||||
| Podosphaera pannosa | Rose (Rosa chinensis) | Beet armyworm (Spodoptera exigua) | Non-Vector | Olfactory | Repellent | [80] |
| Sclerotium rolfsii | Peanut (Arachis hypogaea) | Beet armyworm | Non-Vector | Olfactory, Gustatory | Attractive | [81,82] |
| Microbotryum violaceum | White campion (Silene latifolia) | Lychnis moth (Hadena bicruris) | Non-Vector | Olfactory | Repellent | [83] |
| Melampsora allii-fragilis | Willow (Salix x cuspidata) | Willow leaf beetle (Plagiodera versicolora) | Non-Vector | Unknown | Attractive | [84] |
| Phyllosticta paviae | Horse chestnut (Aesculus hippocastanum) | Horse chestnut leaf miner (Cameraria ohridella) | Non-Vector | Visual | No Effect | [85] |
| Botrytis cinerea | Grape (Vitis vinifera) | European grapevine moth (Lobesia botrana) | Vector | Olfactory | Repellent | [86] |
| B. cinerea | Grape | Light brown apple moth (Epiphyas postvittana) | Vector | Visual, Olfactory | Repellent | [87] |
| Fusarium verticillioides | Maize (Zea mays) | African sugar-cane borer (Eladana saccharina) | Vector | Visual, Olfactory | Attractive | [88] |
| Puccinia punctiformis | Creeping thistle (Cirsium arvense) | Weevil (Apion onopordi) | Vector | Unknown | Attractive | [89] |
| Ophiostoma novo-ulmi | American elm (Ulmus americana) | Elm bark beetle (Hylurgopinus rufipes) | Vector | Olfactory | Attractive | [90] |
| Bacteria | ||||||
| Xanthomonas oryzae | Rice (Oryza sativa) | Brown rice planthopper (Nilaparvata lugens) | Non-Vector | Visual, Olfactory | Attractive | [91] |
| Erwinia tracheiphila | Wild gourd (Cucurbita pepo ssp. texana) | Striped cucumber beetle (Acalymma vittatum) | Vector | Olfactory | Attractive | [92] |
| Candidatus Liberibacter asiaticus | Citrus (Citrus spp.) | Asian citrus psyllid (Diaphorina citri) | Vector | Olfactory, Gustatory | Attractive then Repellent | [66] |
| Candidatus Liberibacter solanacearum | Potato (Solanum tuberosum) | Potato psyllid (Bactericera cockerelli) | Vector | Olfactory | Attractive then Repellent | [93] |
| Xylella fastidiosa | Citrus (Citrus sinensis) | Sharpshooters, leafhoppers (Dilobopterus costalimai, Oncometopia facialis) | Vector | Visual | Repellent | [94] |
| Phytoplasmas | ||||||
| Candidatus Phytoplasma mali | Apple (Malus domestica) | Psyllid (Cacopsylla picta) | Vector | Olfactory | Attractive | [95,96] |
| Candidatus Phytoplasma vitis | Grape | Leafhopper (Scaphoideus titanus) | Vector | Visual | Attractive | [97,98] |
| Viruses | ||||||
| Cucomovirus spp. | Squash (Cucurbita pepo) | Green peach aphid (Myzus persicae), Melon aphid (Aphis gossypii) | Vector | Olfactory, Gustatory | Attractive then Repellent | [68,99] |
| Cucumovirus spp. | Squash | Squash bug (Anasa tristis) | Non-Vector | Unknown | Repellent | [100] |
| Tunrgovirus spp., Waikavirus spp. | Rice | Green rice leafhopper (Nephotettix virescens) | Vector | Gustatory | Attractive then Repellent | [101] |
| Sadwavirus spp., Closterovirus spp. | Red raspberry (Rubus idaeus) | Large raspberry aphid (Amphorophora idaei) | Vector | Olfactory, Gustatory | Attractive then No Effect | [102] |
| Enamovirus spp. | Fava bean (Vicia faba) | Pea aphid (Acyrthosiphon pisum) | Vector | Visual | Attractive | [103] |
| Enamovirus spp. | Pea (Pisum sativum) | Weevil (Sitona lineatus) | Non-Vector | Gustatory | Attractive | [104] |
| Sobemovirus spp., Comovirus spp. | Common bean (Phaseolus vulgaris) | Mexican bean beetle (Epilachna varivestis) | Vector | Unknown | Attractive | [105] |
| Polerovirus spp. | Potato | Green peach aphid | Vector | Olfactory | Attractive | [106,107] |
| Polerovirus spp. | Hairy nightshade (Solanum sarrachoides) | Green peach aphid | Vector | Olfactory | Attractive | [108] |
| Luteovirus spp. | Wheat (Triticum aestivum) | Bird cherry-oat aphid | Vector | Olfactory | Attractive | [109] |
| Comovirus spp., Potyvirus spp. | Soybean (Glycine max) | Mexican bean beetle, Soybean aphid (Aphis glycines) | Vector/ Non-Vector | Gustatory, Olfactory | Attractive | [110] |
| Tobamovirus spp | Tomato (Solanum lycopersicum) | Green peach aphid | Non-Vector | Unknown | Repellent | [111] |
| Crinivirus spp., Begomovirus spp. | Tomato | Silverleaf whitefly (Bemisia tabaci) | Vector | Visual, Olfactory | Attractive | [112] |
| Potyvirus spp. | Potato | Green peach aphid | Vector | Olfactory | Attractive | [113] |
| Caulimoviruss spp. | Turnip (Brassica rapa) | Turnip aphid (Lipaphis erysimi) | Vector | Olfactory | Attractive | [114] |
| Luteovirus spp. | Winter oat (Avena spp.), Winter barley (Hordeum spp.) | Rose-grain aphid (Metopolophium dirhodum), English grain aphid (Sitobion avenae) | Vector | Visual | Attractive | [115] |
| Potyvirus spp. | Soybean, Pepper (Capsicum spp.) | Green peach aphid, Corn aphid (Rhopalosiphum maidis) | Vector | Visual | No Effect | [116] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grunseich, J.M.; Thompson, M.N.; Aguirre, N.M.; Helms, A.M. The Role of Plant-Associated Microbes in Mediating Host-Plant Selection by Insect Herbivores. Plants 2020, 9, 6. https://doi.org/10.3390/plants9010006
Grunseich JM, Thompson MN, Aguirre NM, Helms AM. The Role of Plant-Associated Microbes in Mediating Host-Plant Selection by Insect Herbivores. Plants. 2020; 9(1):6. https://doi.org/10.3390/plants9010006
Chicago/Turabian StyleGrunseich, John M., Morgan N. Thompson, Natalie M. Aguirre, and Anjel M. Helms. 2020. "The Role of Plant-Associated Microbes in Mediating Host-Plant Selection by Insect Herbivores" Plants 9, no. 1: 6. https://doi.org/10.3390/plants9010006
APA StyleGrunseich, J. M., Thompson, M. N., Aguirre, N. M., & Helms, A. M. (2020). The Role of Plant-Associated Microbes in Mediating Host-Plant Selection by Insect Herbivores. Plants, 9(1), 6. https://doi.org/10.3390/plants9010006