Polyphenols Content in Capsicum chinense Fruits at Different Harvest Times and Their Correlation with the Antioxidant Activity
Abstract
:1. Introduction
2. Results
2.1. Quantification of Polyphenols
2.2. Statistical Analysis of Polyphenols
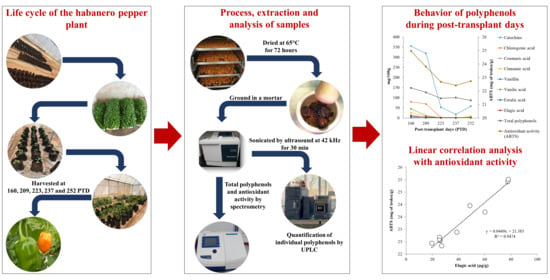
2.3. Linear Correlation Analysis
2.4. Polyphenols Change through Harvests
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions
4.2. Sample Collection and Processing
4.3. Extraction of Habanero Pepper Powder
4.4. Antioxidant Activity by DPPH Radical Scavenging
4.5. Antioxidant Activity by ABTS Assay
4.6. Analysis of Total Polyphenols
4.7. Quantification of Polyphenols by UPLC-DAD
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| PTD | 160 | 209 | 223 | 237 | 252 |
|---|---|---|---|---|---|
| Temperature (°C) | 42.7 ± 3.5 | 42.0 ± 3.0 | 41.7 ± 5.1 | 41.3 ± 4.7 | 40.0 ± 3.6 |
| Humidity (%) | 85.3 ± 3.2 | 86.0 ± 3.6 | 85.7 ± 4.5 | 88.0 ± 3.6 | 90.3 ± 2.6 |
| Light (lum/ft2) | 1336.7 ± 209.8 | 1343.4 ± 168.6 | 1330.0 ± 72.1 | 1293.3 ± 253.2 | 1450.0 ± 50.0 |
Appendix B
| Physical Characteristic | Maturity | PTD | ||||
|---|---|---|---|---|---|---|
| 160 | 209 | 223 | 237 | 252 | ||
| Weight (g) | Green | 4.1 ± 1.1 | 4.7 ± 1.4 | 4.8 ± 1.5 | 4.1 ± 0.6 | 4.1 ± 1.1 |
| Orange | 4.2 ± 1.2 | 4.4 ± 0.7 | 5.6 ± 1.9 | 4.4 ± 0.5 | 5.8 ± 1.0 | |
| Long (cm) | Green | 3.3 ± 0.4 | 3.2 ± 0.3 | 3.4 ± 0.5 | 3.1 ± 0.3 | 3.1 ± 0.5 |
| Orange | 3.3 ± 0.3 | 3.0 ± 0.4 | 3.3 ± 0.5 | 3.1 ± 0.3 | 3.4 ± 0.5 | |
| Width (cm) | Green | 2.3 ± 0.3 | 2.4 ± 0.4 | 2.4 ± 0.3 | 2.3 ± 0.2 | 2.2 ± 0.2 |
| Orange | 2.2 ± 0.2 | 2.3 ± 0.1 | 2.4 ± 0.2 | 2.3 ± 0.1 | 2.3 ± 0.2 | |
References
- Gaytán, D.; Benita, F. On the competitiveness of Mexico’s dry chili production. Ekon. Poljopr. 2014, 61, 307–317. [Google Scholar] [CrossRef]
- Medina-Lara, F.; Echevarría-Machado, I.; Pacheco-Arjona, R.; Ruiz-Lau, N.; Guzmán-Antonio, A.; Martinez-Estevez, M. Influence of Nitrogen and Potassium Fertilization on Fruiting and Capsaicin Content in Habanero Pepper (Capsicum chinense Jacq.). HortScience 2008, 43, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Meneses-Lazo, R.E.; René Garruña, R. The Habanero pepper (Capsicum chinense Jacq.) as a stud plant model in Mexico. Trop. Subtrop. Agroecosystems 2020, 23, 21. [Google Scholar]
- Canto-Flick, A.; Balam-Uc, E.; Bello-Bello, J.J.; Lecona-Guzmán, C.; Solís-Marroquín, D.; Avilés-Viñas, S.; Gómez-Uc, E.; López-Puc, G.; Santana-Buzzy, N.; Iglesias-Andreu, L.G. Capsaicinoids content in Habanero pepper (Capsicum chinense Jacq.): Hottest known cultivars. HortScience 2008, 43, 1344–1349. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Ramírez, L.S.; Peña-Yam, L.P.; Avilés-Viñas, S.A.; Canto-Flick, A.; Guzmán-Antonio, A.A.; Santana-Buzzy, N. Behavior of the hottest chili peppers in the world cultivated in Yucatan, Mexico. HortScience 2018, 53, 1772–1775. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Uribe, J.A.; Novelo-Pérez, J.I.; Ruiz-Mercado, C. Cost estimation for CO2 supercritical extraction systems and manufacturing cost for Habanero chili. J. Supercrit. Fluids 2014, 93, 38–41. [Google Scholar] [CrossRef]
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Secondary metabolites of Capsicum species and their importance in the human diet. J. Nat. Prod. 2013, 76, 783–793. [Google Scholar] [CrossRef]
- Tun, D.C. Características y tecnología de producción del chile habanero. In Centro de Investigación Regional del Sureste; INIFAP SAGARPA; Mocochá: Yucatán, México, 2001; pp. 5–74. Available online: http://biblioteca.inifap.gob.mx (accessed on 28 May 2020).
- Soria, F.M.; Tun, S.J.; Trejo, R.A.; Terán, S.R. Paquete Tecnológico Para la Producción de Chile Habanero (Capsicum Chinense JACQ.). SEP. DGTA. ITA-2 Conkal, Yuc, México. 75 (15). Predicción de la Demanda Nutrimental de Potasio Para la Produccion de Capsicum Chinense Jacq. en el Sureste de Mexico. 2020. Available online: https://www.researchgate.net/publication/237042736_Prediccion_de_la_demanda_nutrimental_de_potasio_para_la_produccion_de_capsicum_chinense_jacq_en_el_Sureste_de_Mexico (accessed on 15 August 2020).
- Rodríguez-Maturino, A. Antioxidant activity and bioactive compounds of Chiltepin (Capsicum annuum var. glabriusculum) and Habanero (Capsicum chinense): A comparative study. J. Med. Plants Res. 2012, 6. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Vargas, M.D.L.V.; Tamayo-Cortez, J.A.; López-Sauri, D.A.; Sauri-Duch, E.; Ortiz-Fernández, A.; Betancur-Ancona, D. Solvent Extraction and Measurement of Antioxidant Activity and Total Phenolic Content from Capsicum chinense Jacq. Cv Habanero at Different Maturity Stages. Chiang Mai J. Sci. 2019, 46, 661–671. [Google Scholar]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Dubey, R.K.; Singh, V.; Upadhyay, G.; Pandey, A.K.; Prakash, D. Assessment of phytochemical composition and antioxidant potential in some indigenous chilli genotypes from North East India. Food Chem. 2015, 188, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Troconis-Torres, I.G.; Rojas-López, M.; Hernández-Rodríguez, C.; Villa-Tanaca, L.; Maldonado-Mendoza, I.E.; Dorantes-Álvarez, L.; Tellez-Medina, D.; Jaramillo-Flores, M.E. Biochemical and molecular analysis of some commercial samples of chilli peppers from Mexico. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Butcher, J.D.; Crosby, K.M.; Yoo, K.S.; Patil, B.S.; Ibrahim, A.M.H.; Leskovar, D.I.; Jifon, J.L. Environmental and genotypic variation of capsaicinoid and flavonoid concentrations in Habanero (Capsicum chinense) peppers. HortScience 2012, 47, 574–579. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.W.; Momin, C.M.; Acharya, P.; Kabir, J.; Debnath, M.K.; Dhua, R.S. Dynamics of changes in bioactive molecules and antioxidant potential of Capsicum chinense Jacq. cv. Habanero at nine maturity stages. Acta Physiol. Plant. 2013, 35, 1141–1148. [Google Scholar] [CrossRef]
- Urrea-López, R.; de la Garza, R.I.D.; Valiente-Banuet, J.I. Effects of substrate salinity and nutrient levels on physiological response, yield, and fruit quality of Habanero pepper. HortScience 2014, 49, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Urban, L.; Bourgaud, F.; Léchaudel, M.; Joas, J.; Berti, L.; Gautier, H.; Sallanon, H. The effect of environmental factors on biosynthesis of carotenoids and polyphenolics in fruits and vegetables: A review and prospects. Acta Hortic. 2009, 841, 339–344. [Google Scholar] [CrossRef]
- Amalfitano, C.; Vacchio, L.; Del Somma, S.; Cuciniello, A.; Caruso, G. Effects of cultural cycle and nutrient solution electrical conductivity on plant growth, yield and fruit quality of “Friariello” pepper grown in hydroponics. Hortic. Sci. 2017, 44, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Noh-Medina, J.; Borges-Gómez, L.; Soria-Fregoso, M. Composición nutrimental de biomasa y tejidos conductores en chile Habanero. Trop. Subtripical Agroecosystems 2010, 12, 219–228. [Google Scholar]
- Bhandari, S.R.; Bashyal, U.; Lee, Y.S. Variations in proximate nutrients, phytochemicals, and antioxidant activity of field-cultivated red pepper fruits at different harvest times. Hortic. Environ. Biotechnol. 2016, 57, 493–503. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, A.; Wyzgolik, G.; Klepacz-Baniak, J.; Czekońska, K. Antioxidative properties of bee pollen in selected plant species. Food Chem. 2007, 100, 237–240. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherova, G.; Pavlov, A.; Georgiev, V. Polyphenols profiles and antioxidant activities of extracts from Capsicum chinense in vitro plants and callus cultures. Food Sci. Appl. Biotechnol. 2019, 2, 30. [Google Scholar] [CrossRef]
- Howard, L.R.; Talcott, S.T.; Brenes, C.H.; Villalon, B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000, 48, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Oney-Montalvo, J.; Uc-Varguez, A.; Ramírez-Rivera, E.; Ramírez-Sucre, M.; Rodríguez-Buenfil, I. Influence of Soil Composition on the Profile and Content of Polyphenols in Habanero Peppers (Capsicum chinense Jacq). Agronomy 2020, 10, 1234. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Sherafati, M.; Payvast, G.A. Variation in phenolic compounds, ascorbic acid and antioxidant activity of five coloured bell pepper (Capsicum annum) fruits at two different harvest times. J. Funct. Foods 2011, 3, 44–49. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P.J.; Menichini, F. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Inui, T.; Okumura, K.; Matsui, H.; Hosoya, T.; Kumazawa, S. Effect of harvest time on some in vitro functional properties of hop polyphenols. Food Chem. 2017, 225, 69–76. [Google Scholar] [CrossRef]
- Gao, C.Y.; Lu, Y.H.; Tian, C.R.; Xu, J.G.; Guo, X.P.; Zhou, R.; Hao, G. Main nutrients, phenolics, antioxidant activity, DNA damage protective effect and microstructure of Sphallerocarpus gracilis root at different harvest time. Food Chem. 2011, 127, 615–622. [Google Scholar] [CrossRef]
- Mihai, C.M.; Marghitas, L.; Dezmirean, D.S.; Barnutiu, L. Correlation between Polyphenolic Profile and Antioxidant Activity of Propolis from Transylvania. Anim. Sci. Biotechnol. 2011, 44, 100–103. [Google Scholar]
- Reddivari, L.; Hale, A.L.; Miller, J.C. Determination of phenolic content, composition and their contribution to antioxidant activity in specialty potato selections. Am. J. Potato Res. 2007, 84, 275. [Google Scholar] [CrossRef]
- Zapata-Bustamante, S.; Tamayo-Tenorio, A.; Alberto-Rojano, B. Effect of roasting on the secondary metabolites and antioxidant activity of Colombian cocoa clones. Revista Facultad Nacional Agronomía Medellín 2015, 68, 7497–7507. [Google Scholar] [CrossRef]
- Festa, F.; Aglitti, T.; Duranti, G.; Ricordy, R.; Perticone, P.; Cozzi, R. Strong antioxidant activity of ellagic acid in mammalian cells in vitro revealed by the comet assay. Anticancer Res. 2001, 21, 3903. [Google Scholar] [PubMed]
- Christensen, K.B.; Kaemper, M.; Loges, R.; Fretté, X.C.; Christensen, L.P.; Grevsen, K. Effects of nitrogen fertilization, harvest time, and species on the concentration of polyphenols in aerial parts and seeds of normal and tartary buckwheat (Fagopyrum sp.). Eur. J. Hortic. Sci. 2010, 75, 153–164. [Google Scholar]
- Muscolo, A.; Sidari, M.; Settineri, G.; Papalia, T.; Mallamaci, C.; Attinà, E. Influence of Soil Properties on Bioactive Compounds and Antioxidant Capacity of Brassica rupestris Raf. J. Soil Sci. Plant Nutr. 2019, 19, 808–815. [Google Scholar] [CrossRef]
- André, C.M.; Schafleitner, R.; Legay, S.; Lefèvre, I.; Aliaga, C.A.A.; Nomberto, G.; Hoffmann, L.; Hausman, J.F.; Larondelle, Y.; Evers, D. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress. Phytochemistry 2009, 70, 1107–1116. [Google Scholar] [CrossRef]
- Medina-Lara, F.; Souza-Perera, R.; Martínez-Estévez, M.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M.; MacHado, I.E. Red and brown soils increase the development and content of nutrients in Habanero pepper subjected to irrigation water with high electrical conductivity. HortScience 2019, 54, 2039–2049. [Google Scholar] [CrossRef] [Green Version]
- Zamacona-Ruiz, M.; Ramírez-Sucre, M.; Rodríguez-Buenfil, I. Comparación de dos métodos de extracción y secado para la cuantificación de carotenoides en chile Habanero. Revista del Centro de Graduados e Investigación. Instituto Tecnológico de Mérida 2018, 33, 65–68. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; pp. 152–178. [Google Scholar] [CrossRef]


| Polyphenol (mg/100 g of Dry Pepper) | 160 PTD | 209 PTD | 223 PTD | 237 PTD | 252 PTD | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Green | Orange | Green | Orange | Green | Orange | Green | Orange | Green | Orange | |
| Gallic acid | 0.56 ± 0.2 c | 1.73 ± 0.45 B | 0.00 ± 0.00 d | 0.00 ± 0.00 C | 0.00 ± 0.00 d | 0.00 ± 0.00 C | 6.93 ± 1.09 b | 0.00 ± 0.00 C | 29.45 ± 10.11 a | 48.40 ± 12.31 A |
| Protocatechuic acid | 15.91 ± 0.19 b | 85.75 ± 10.45 C | 41.94 ± 0.53 a | 102.85 ± 14.57 C | 6.04 ± 0.02 c | 122.73 ± 3.31 B | 5.67 ± 0.23 d | 56.22 ± 9.89 D | 39.84 ± 12.44 a | 162.21 ± 0.55 A |
| Catechine | 182.52 ± 2.74 a | 355.30 ± 5.81 A | 117.42 ± 1.91 b | 319.73 ± 3.19 B | 3.79 ± 0.17 d | 53.36 ± 4.72 D | 3.46 ± 0.14 d | 17.99 ± 0.27 E | 5.11 ± 0.03 c | 58.33 ± 0.02 C |
| Chlorogenic acid | 6.86 ± 0.20 b | 79.97 ± 2.02 A | 28.36 ± 0.29 a | 69.5 ± 0.1 B | 0.33 ± 0.01 d | 1.41 ± 0.38 C | 0.74 ± 0.05 c | 0.40 ± 0.11 D | 0.00 ± 0.00 e | 0.00 ± 0.00 E |
| Coumaric acid | 0.78 ± 0.15 a | 2.31 ± 0.54 A | 0.05 ± 0.01 b | 0.58 ± 1.2 B | 0.00 ± 0.00 c | 0.45 ± 0.21 B | 0.00 ± 0.00 c | 0.00 ± 0.00 | 0.67 ± 0.05 a | 0.38 ± 0.09 B |
| Cinnamic acid | 7.81 ± 0.16 a | 25.78 ± 5.14 A | 1.04 ± 0.01 c | 8.07 ± 2.71 B | 5.92 ± 0.87 b | 0.42 ± 0.06 C | 0.48 ± 0.17 d | 0.24 ± 0.04 D | 0.35 ± 0.02 d | 6.73 ± 0.97 B |
| Rutin | 19.19 ± 8.52 a | 46.05 ± 5.57 A | 6.41 ± 0.69 c | 35.11 ± 15.73 A | 17.25 ± 6.59 ab | 3.64 ± 0.04 C | 1.45 ± 0.04 d | 10.95 ± 0.09 B | 17.06 ± 0.75 b | 43.41 ± 4.86 A |
| Luteolin + quercetin | 0.65 ± 0.17 a | 1.38 ± 0.09 B | 0.00 ± 0.00 c | 0.00 ± 0.00 D | 0.00 ± 0.00 c | 18.99 ± 4.62 A | 0.41 ± 0.18 b | 17.19 ± 8.41 A | 0.00 ± 0.00 c | 1.07 ± 0.06 C |
| Kaempferol | 0.44 ± 0.09 a | 1.87 ± 0.01 A | 0.00 ± 0.00 c | 0.00 ± 0.00 B | 0.06 ± 0.04 b | 0.00 ± 0.00 B | 0.00 ± 0.00 c | 0.00 ± 0.00 B | 0.49 ± 0.06 a | 1.49 ± 0.91 A |
| Vanillin | 5.86 ± 0.05 a | 2.07 ± 0.09 A | 1.36 ± 0.04 b | 1.32 ± 0.02 B | 0.16 ± 0.01 c | 0.19 ± 0.02 D | 0.19 ± 0.07 c | 0.20 ± 0.09 D | 0.82 ± 0.75 bc | 1.09 ± 0.02 C |
| Diosmin + hesperidin | 6.53 ± 0.09 a | 14.28 ± 0.07 A | 1.29 ± 0.01 c | 2.67 ± 0.29 C | 4.98 ± 0.74 b | 0.85 ± 0.01 D | 0.72 ± 0.02 d | 0.82 ± 0.11 D | 0.60 ± 0.01 e | 5.44 ± 0.05 B |
| Neohesperidin | 0.22 ± 0.01 c | 2.65 ± 0.03 A | 0.21 ± 0.01 c | 0.49 ± 0.36 D | 0.00 ± 0.00 d | 2.03 ± 0.55 B | 0.29 ± 0.01 b | 2.44 ± 0.03 B | 0.79 ± 0.02 a | 0.85 ± 0.06 C |
| Naringenin | 0.38 ± 0.11 ab | 0.49 ± 0.23 D | 0.34 ± 0.03 b | 1.89 ± 0.01 C | 0.39 ± 0.02 a | 5.51 ± 2.46 B | 0.34 ± 0.01 b | 12.32 ± 2.22 A | 0.35 ± 0.03 b | 0.69 ± 0.26 D |
| Apigenin | 0.49 ± 0.04 a | 2.05 ± 0.08 A | 0.41 ± 0.01 b | 1.11 ± 0.01 B | 0.31 ± 0.01 c | 0.91 ± 0.26 BC | 0.19 ± 0.09 d | 1.21 ± 0.20 B | 0.34 ± 0.04 c | 0.79 ± 0.03 C |
| Diosmetin | 0.59 ± 0.13 b | 1.19 ± 0.85 BC | 0.64 ± 0.09 b | 2.53 ± 0.02 A | 0.76 ± 0.02 a | 0.68 ± 0.01 BC | 0.57 ± 0.09 b | 0.61 ± 0.01 BD | 0.81 ± 0.31 ab | 0.60 ± 0.01 BD |
| Vanillic acid | 25.24 ± 0.13 a | 45.48 ± 0.55 A | 22.41 ± 0.23 b | 11.81 ± 9.86 B | 0.39 ± 0.02 d | 1.06 ± 0.05 C | 0.51 ± 0.02 c | 0.35 ± 0.26 E | 0.31 ± 0.01 e | 0.65 ± 0.02 D |
| Ferulic acid | 2.52 ± 0.16 b | 12.12 ± 0.05 A | 1.92 ± 0.15 c | 3.60 ± 0.77 B | 0.05 ± 0.04 e | 0.25 ± 0.03 C | 1.46 ± 0.04 d | 0.45 ± 0.39 C | 4.09 ± 0.18 a | 4.17 ± 0.17 B |
| Ellagic acid | 3.67 ± 0.02 a | 7.78 ± 0.03 A | 1.79 ± 0.36 b | 5.44 ± 0.81 B | 1.99 ± 0.17 b | 2.58 ± 0.02 C | 1.69 ± 0.03 b | 2.34 ± 0.53 C | 3.34 ± 0.39 a | 3.22 ± 0.93 C |
| Total polyphenols | 79.34 ± 0.42 a | 148.77 ± 2.57 A | 68.38 ± 1.39 b | 127.00 ± 0.46 B | 50.38 ± 0.26 c | 97.44 ± 0.31 C | 42.07 ± 0.46 d | 100.84 ± 0.52 D | 39.61 ± 0.26 e | 87.08 ± 0.31 E |
| DPPH (%) | 87.50 ± 0.51 a | 89.50 ± 0.30 A | 86.79 ± 0.51 a | 88.29 ± 0.61 B | 85.93 ± 0.10 b | 86.43 ± 0.81 C | 85.57 ± 0.40 bc | 86.21 ± 0.30 C | 84.71 ± 0.81 c | 85.86 ± 0.61 C |
| ABTS (mg of trolox/g) | 24.47 ± 0.18 a | 24.96 ± 0.07 A | 23.24 ± 0.38 b | 23.81 ± 0.19 B | 22.51 ± 0.41 bc | 22.68 ± 0.09 C | 21.69 ± 0.30 c | 22.42 ± 0.07 D | 22.54 ± 0.38 bc | 22.73 ± 0.22 C |
| Polyphenol | A: PTD | B: Maturity | AB |
|---|---|---|---|
| Gallic acid | <0.0001 * | 0.2752 | 0.0464 * |
| Protocatechuic acid | <0.0001 * | <0.0001 * | 0.0001 * |
| Catechin | <0.0001 * | <0.0001 * | <0.0001 * |
| Chlorogenic acid | <0.0001 * | <0.0001 * | <0.0001 * |
| Coumaric acid | <0.0001 * | 0.0005 * | 0.0006 * |
| Cinnamic acid | 0.0012 * | 0.0150 | 0.0169 * |
| Rutin | 0.2381 | 0.0853 | 0.4596 |
| Luteolin + quercetin | 0.2415 | 0.0474 * | 0.2404 |
| Kaempferol | 0.0002 * | 0.0045 * | 0.0115 * |
| Vanillin | <0.0001 * | 0.0001 * | <0.0001 * |
| Diosmin + hesperidin | 0.0009 * | 0.0744 | 0.0308 * |
| Neohesperidin | 0.3775 | 0.0034 * | 0.1838 |
| Naringenin | 0.0001 * | <0.0001 * | 0.0001 * |
| Apigenin | <0.0001 * | <0.0001 * | 0.0002 * |
| Diosmetin | 0.0048 * | 0.0062 * | 0.0025 * |
| Vanillic acid | <0.0001 * | 0.1641 | 0.0006 * |
| Ferulic acid | <0.0001 * | <0.0001 * | <0.0001 * |
| Ellagic acid | <0.0001 * | <0.0001 * | 0.0001 * |
| Total polyphenols | <0.0001 * | <0.0001 * | <0.0001 * |
| DPPH | <0.0001 * | <0.0001 * | 0.0286 * |
| ABTS | <0.0001 * | 0.0001 * | 0.2258 |
| Polyphenol | DPPH | ABTS | ||||||
|---|---|---|---|---|---|---|---|---|
| Immature (Green Color) | Mature (Orange Color) | Immature (Green Color) | Mature (Orange Color) | |||||
| r2 | Equation | r2 | Equation | r2 | Equation | r2 | Equation | |
| Gallic acid | 0.6099 | y = −0.068x + 86.605 | 0.1797 | y = −0.032x + 87.573 | 0.1184 | y = −0.029x + 23.105 | 0.0628 | y = −0.012x + 23.425 |
| Protocatechuic acid | 0.0248 | y = −0.010x + 86.317 | 0.1002 | y = −0.013x + 88.607 | 0.0416 | y = 0.012x + 22.620 | 0.0411 | y = −0.005x + 23.878 |
| Catechin | 0.7245 | y = 0.012x + 85.362 | 0.8661 | y = −0.009x + 85.756 | 0.7842 | y = 0.012x + 22.150 | 0.8989 | y = 0.006x + 22.278 |
| Chlorogenic acid | 0.2587 | y = 0.048x + 85.749 | 0.8794 | y = 0.037x + 86.124 | 0.1533 | y = 0.036x + 22.629 | 0.8934 | y = 0.025x + 22.537 |
| Coumaric acid | 0.0176 | y = 0.389x + 85.984 | 0.7168 | y = 1.493x + 86.146 | 0.2638 | y = 1.449x + 22.457 | 0.8172 | y = 1.066x + 22.507 |
| Cinnamic acid | 0.2074 | y = 0.115x + 85.744 | 0.7139 | y = 0.126x + 86.217 | 0.3845 | y = 0.150x + 22.422 | 0.8344 | y = 0.091x + 22.549 |
| Rutin | 0.0004 | y = −0.002x + 86.128 | 0.1803 | y = 0.025x + 86.550 | 0.2362 | y = 0.052x + 22.258 | 0.2301 | y = 0.019x + 22.766 |
| Luteolin + quercetin | 0.2027 | y = 1.412x + 85.802 | 0.1828 | y = −0.055x + 87.683 | 0.1309 | y = 1.095x + 22.659 | 0.2670 | y = −0.045x + 23.645 |
| Kaempferol | 0.0009 | y = −0.142x + 86.128 | 0.1395 | y = 0.618x + 86.842 | 0.1546 | y = 1.747x + 22.541 | 0.3022 | y = 0.608x + 22.892 |
| Vanillin | 0.4656 | y = 0.328x + 85.551 | 0.6183 | y = 1.605x + 85.690 | 0.7537 | y = 0.402x + 22.216 | 0.8406 | y = 1.252x + 22.079 |
| Diosmin + hesperidin | 0.1993 | y = 0.139x + 85.707 | 0.4842 | y = 0.202x + 86.287 | 0.3772 | y = 0.185x + 22.369 | 0.7147 | y = 0.164x + 22.512 |
| Neohesperidin | 0.3551 | y = −2.317x + 86.805 | 0.0002 | y = −0.017x + 87.286 | 0.0619 | y = −0.933x + 23.174 | 0.0064 | y = 0.065x + 23.190 |
| Naringenin | 0.0870 | y = 12.681x + 81.540 | 0.2513 | y = −0.160x + 87.925 | 0.1122 | y = 13.894x + 17.894 | 0.3793 | y = −0.131x + 23.849 |
| Apigenin | 0.2022 | y = 3.492x + 84.884 | 0.6610 | y = 2.455x + 84.316 | 0.4363 | y = 4.948x + 21.166 | 0.6610 | y = 1.702x + 21.262 |
| Diosmetin | 0.3381 | y = −4.178x + 88.921 | 0.2963 | y = 1.012x + 86.120 | 0.0026 | y = −0.355x + 23.130 | 0.2455 | y = 0.616x + 22.608 |
| Vanillic acid | 0.7061 | y = 0.075x + 85.364 | 0.7226 | y = 0.070x + 86.424 | 0.7017 | y = 0.072x + 22.182 | 0.8980 | y = 0.052x + 22.679 |
| Ferulic acid | 0.0447 | y = −0.165x + 86.431 | 0.6073 | y = 0.263x + 86.173 | 0.0162 | y = 0.096x + 22.698 | 0.8143 | y = 0.204x + 22.461 |
| Ellagic acid | 0.0174 | y = 0.160x + 85.702 | 0.8976 | y = 0.654x + 84.466 | 0.2591 | y = 0.594x + 21.409 | 0.9474 | y = 0.449x + 21.383 |
| Total polyphenols | 0.8348 | y = 0.0160x + 82.648 | 0.8999 | y = 0.0612x + 80.385 | 0.8191 | y = 0.0589x + 19.592 | 0.8922 | y = 0.0407x + 18.726 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oney-Montalvo, J.E.; Avilés-Betanzos, K.A.; Ramírez-Rivera, E.d.J.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Polyphenols Content in Capsicum chinense Fruits at Different Harvest Times and Their Correlation with the Antioxidant Activity. Plants 2020, 9, 1394. https://doi.org/10.3390/plants9101394
Oney-Montalvo JE, Avilés-Betanzos KA, Ramírez-Rivera EdJ, Ramírez-Sucre MO, Rodríguez-Buenfil IM. Polyphenols Content in Capsicum chinense Fruits at Different Harvest Times and Their Correlation with the Antioxidant Activity. Plants. 2020; 9(10):1394. https://doi.org/10.3390/plants9101394
Chicago/Turabian StyleOney-Montalvo, Julio Enrique, Kevin Alejandro Avilés-Betanzos, Emmanuel de Jesús Ramírez-Rivera, Manuel Octavio Ramírez-Sucre, and Ingrid Mayanin Rodríguez-Buenfil. 2020. "Polyphenols Content in Capsicum chinense Fruits at Different Harvest Times and Their Correlation with the Antioxidant Activity" Plants 9, no. 10: 1394. https://doi.org/10.3390/plants9101394
APA StyleOney-Montalvo, J. E., Avilés-Betanzos, K. A., Ramírez-Rivera, E. d. J., Ramírez-Sucre, M. O., & Rodríguez-Buenfil, I. M. (2020). Polyphenols Content in Capsicum chinense Fruits at Different Harvest Times and Their Correlation with the Antioxidant Activity. Plants, 9(10), 1394. https://doi.org/10.3390/plants9101394