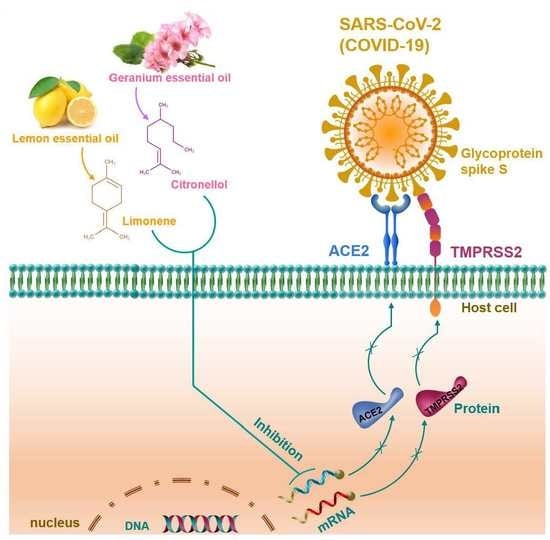
Geranium and Lemon Essential Oils and Their Active Compounds Downregulate Angiotensin-Converting Enzyme 2 (ACE2), a SARS-CoV-2 Spike Receptor-Binding Domain, in Epithelial Cells
Abstract
:1. Introduction
2. Results
2.1. Cytotoxic Effect of Essential Oils on HT-29 Cells
2.2. Essential Oils Downregulate ACE2 Activity in HT-29 Cells
2.3. Chemical Compositions of Geranium and Lemon Essential Oils
2.4. ACE2 Inhibitory Effects of Major Constituents in Geranium and Lemon Essential Oils
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Cell Viability Assay
4.3. Determination of ACE2 Activity
4.4. Determination of ACE2 Protein
4.5. Quantitative Real-Time PCR
4.6. GC–MS Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280e. [Google Scholar] [CrossRef]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Du, Q. Potential natural compounds for preventing 2019-nCoV infection. Preprints 2020, 2020010358. [Google Scholar]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the sars coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in sars coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef] [PubMed]
- Letko, M.; Munster, V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020, 5, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Pastick, K.A.; Okafor, E.C.; Wang, F.; Lofgren, S.M.; Skipper, C.P.; Nicol, M.R.; Pullen, M.F.; Rajasingham, R.; McDonald, E.G.; Lee, T.C.; et al. Review: Hydroxychloroquine and chloroquine for treatment of sars-cov-2 (covid-19). Open Forum Infect. Dis. 2020, 7, ofaa130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, J.M.; Monogue, M.L.; Jodlowski, T.Z.; Cutrell, J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 323, 1824–1836. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Michaelis, M.; Hsu, H.-K.; Tsai, C.-C.; Yang, K.D.; Wu, Y.-C.; Cinatl, J.; Doerr, H.W. Toona sinensis roem tender leaf extract inhibits sars coronavirus replication. J. Ethnopharmacol. 2008, 120, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, H. Liquorice may tackle SARS. Nature 2003. [Google Scholar] [CrossRef]
- Wen, C.C.; Kuo, Y.H.; Jan, J.T.; Liang, P.H.; Wang, S.Y.; Liu, H.G.; Lee, C.K.; Chang, S.T.; Kuo, C.J.; Lee, S.S.; et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.Y.; Jan, J.T.; Ma, S.H.; Kuo, C.J.; Juan, H.F.; Cheng, Y.S.; Hsu, H.H.; Huang, H.C.; Wu, D.; Brik, A.; et al. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. USA 2004, 101, 10012–10017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astani, A.; Reichling, J.; Schnitzler, P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother. Res. 2010, 24, 673–679. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Jackwood, M.W.; Rosenbloom, R.; Petteruti, M.; Hilt, D.A.; McCall, A.W.; Williams, S.M. Avian coronavirus infectious bronchitis virus susceptibility to botanical oleoresins and essential oils in vitro and in vivo. Virus Res. 2010, 149, 86–94. [Google Scholar] [CrossRef]
- Fayed, S.A. Antioxidant and anticancer activities of citrus reticulate (petitgrain mandarin) and pelargonium graveolens (geranium) essential oils. Res. J. Agric. Biol. Sci. 2009, 5, 740–747. [Google Scholar]
- Lis-Balchin, M. A chemotaxonomic study of the pelargonium (geraniaceae) species and their modern cultivars. J. Hort. Sci. 1997, 72, 791–795. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Ramezanloo, F. An overview on phytopharmacology of Pelargonium graveolens L. Ind. J. Trad. Knowl. 2015, 14, 558–563. [Google Scholar]
- Standen, M.D.; Connellan, P.A.; Leach, D.N. Natural killer cell activity and lymphocyte activation: Investigating the effects of a selection of essential oils and components in vitro. Int. J. Aromather. 2006, 16, 133–139. [Google Scholar] [CrossRef]
- Peterson, A.; Machmudah, S.; Roy, B.C.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of essential oil from geranium (Pelargonium graveolens) with supercritical carbon dioxide. J. Chem. Technol. Biotechnol. 2006, 81, 167–172. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Complement. Med. Res. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadjib, B.M. Effective antiviral activity of essential oils and their characteristic terpenes against coronaviruses: An update. J. Pharmacol. Clin. Toxicol. 2020, 8, 1138. [Google Scholar]
- Zhu, J.D.; Meng, W.; Wang, X.J.; Wang, H.C.R. Broad-spectrum antiviral agents. Front. Microbiol. 2015, 6, 517. [Google Scholar] [CrossRef] [Green Version]
- Warner, F.J.; Lew, R.A.; Smith, A.I.; Lambert, D.W.; Hooper, N.M.; Turner, A.J. Angiotensin-converting enzyme 2 (ACE2), but not ace, is preferentially localized to the apical surface of polarized kidney cells. J. Biol. Chem. 2005, 280, 39353–39362. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhong, L.; Deng, J.; Peng, J.; Dan, H.; Zeng, X.; Li, T.; Chen, Q. High expression of ace2 receptor of 2019-ncov on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020, 12, 8. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef]
- Thuy, B.T.P.; My, T.T.A.; Hai, N.T.T.; Hieu, L.T.; Hoa, T.T.; Thi Phuong Loan, H.; Triet, N.T.; Anh, T.T.V.; Quy, P.T.; Tat, P.V.; et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega 2020, 5, 8312–8320. [Google Scholar] [CrossRef]
- Da Silva, J.K.R.; Figueiredo, P.L.B.; Byler, K.G.; Setzer, W.N. Essential oils as antiviral agents, potential of essential oils to treat sars-cov-2 infection: An in-silico investigation. Int. J. Mol. Sci. 2020, 21, 3426. [Google Scholar] [CrossRef]
- Bernardi, S.; Zennaro, C.; Palmisano, S.; Velkoska, E.; Sabato, N.; Toffoli, B.; Giacomel, G.; Buri, L.; Zanconati, F.; Bellini, G.; et al. Characterization and significance of ace2 and mas receptor in human colon adenocarcinoma. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 202–209. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the essential oils and compounds are available from the authors. |




| No | Essential Oil | Botanical Name | IC50 (μg/mL) | Selected Dose (μg/mL) | ACE2 Activity (% of Control) |
|---|---|---|---|---|---|
| 1 | Bergamot oil | Citrus bergamia | 48.58 | 25 | 66.54 *** |
| 2 | Black pepper oil | Piper nigrum | <25 | - | - |
| 3 | Chamomile German blue oil | Matricaria chamomilla | <25 | - | - |
| 4 | Chili oil | Capsicum annum | >200 | - | - |
| 5 | Citronella oil | Cymbopogon winterianus | <25 | - | - |
| 6 | Clary sage oil | Salvia sclarea | 39.22 | - | - |
| 7 | Cypress oil | Cupressus sempervirens | >200 | 50 | 65.12 *** |
| 8 | Elemi oil | Canarium valgare | <25 | - | - |
| 9 | Eucalyptus oil | Eucalyptus globulus | >200 | 50 | 70.50 *** |
| 10 | Fennel oil | Foeniculum vulgare | 97.22 | 25 | 87.3 * |
| 11 | Frankincense oil | Boswellia sp. | 155.91 | 25 | 91.48 |
| 12 | Geranium oil | Pelargonium graveolens | >200 | 50 | 10.63 **** |
| 13 | Ginger oil | Zingiber officinale | 83.03 | 25 | 79.52 ** |
| 14 | Juniper berry oil | Juniperus communis | >200 | 50 | 60.85 *** |
| 15 | Kunzea oil | Kunzea ambigua | <25 | - | - |
| 16 | Lemon oil | Citrus limon | 57.93 | 25 | 24.79 **** |
| 17 | Lavender oil | Lavandula officinalis | 55.65 | - | - |
| 18 | Lime oil | Citrus aurantifolia | 50.98 | - | - |
| 19 | May chang oil | Litsea cubeba | <25 | - | - |
| 20 | Marjoram oil | Origanum majorana | >200 | 50 | 90.11 |
| 21 | Myrtle oil | Myrtus communis | 144.22 | 25 | 93.56 |
| 22 | Neroli oil | Citrus aurantium | 119.52 | 25 | 52.88 *** |
| 23 | Palmarosa oil | Cymbopogon martinii | <25 | - | - |
| 24 | Patchouly oil | Pogostemon cablin | 22.19 | - | - |
| 25 | Peppermint oil | Mentha piperita | 40.96 | - | - |
| 26 | Petitgrain oil | Citrus aurantium | 151.63 | 25 | 80.23 ** |
| 27 | Ravinstra oil | Cinnamomum camphora | 45.58 | - | - |
| 28 | Rosemary oil | Rosmarinus officinalis | 32.44 | - | - |
| 29 | Tangerine oil | Citrus reticulata | 109.31 | 25 | 59.20 *** |
| 30 | Tea tree oil | Mellaleuca alternifolia | >200 | 50 | 71.55 *** |
| No | Compound | RT (min) | Contents (%) | KI | Identification * |
|---|---|---|---|---|---|
| 1 | α-Pinene | 10.27 | 1.2 | 934 | MS, KI, ST |
| 2 | α-Myrcene | 13.6 | 0.4 | 1004 | MS, KI, ST |
| 3 | Limonene | 14.7 | 0.5 | 1029 | MS, KI, ST |
| 4 | Linalool | 18.5 | 0.87 | 1098 | MS, KI, ST |
| 5 | Phenylethyl alcohol | 18.62 | 0.8 | 1109 | MS, KI, ST |
| 6 | Isopulegol | 20.4 | 1.6 | 1148 | MS, KI, ST |
| 7 | Menthone | 20.74 | 0.7 | 1155 | MS, KI, ST |
| 8 | Citronellal | 20.87 | 0.4 | 1158 | MS, KI, ST |
| 9 | iso-Menthone | 21.16 | 1.1 | 1164 | MS, KI, ST |
| 10 | α-Terpineol | 22.61 | 3.6 | 1192 | MS, KI, ST |
| 11 | Nerol | 24.03 | 3.3 | 1124 | MS, KI, ST |
| 12 | Citronellol | 24.16 | 27.1 | 1153 | MS, KI, ST |
| 13 | Geraniol | 25.23 | 21.4 | 1251 | MS, KI, ST |
| 14 | Neral | 25.98 | 0.2 | 1267 | MS, KI, ST |
| 15 | Citronellyl formate | 26.28 | 7.7 | 1273 | MS, KI, ST |
| 16 | Neryl acetate | 30.91 | 10.5 | 1377 | MS, KI, ST |
| 17 | Aristoene | 33.48 | 0.8 | 1439 | MS, KI, ST |
| 18 | Germacrene D | 35.96 | 0.3 | 1375 | MS, KI |
| 19 | δ-Cadinen | 35.61 | 0.2 | 1514 | MS, KI |
| 20 | Guaiol | 39.65 | 2.7 | 1592 | MS, KI, ST |
| 21 | Eudesmol | 41.8 | 0.6 | 1650 | MS, KI, ST |
| 22 | α-Bisabolol | 42.2 | 4.3 | 1661 | MS, KI |
| No | Compound | RT (min) | Contents (%) | KI | Identification * |
|---|---|---|---|---|---|
| 1 | α-Pinene | 10.32 | 1.4% | 935 | MS, KI, ST |
| 2 | Sabinene | 12.11 | 1.2% | 974 | MS, KI, ST |
| 3 | β-Pinene | 12.29 | 8.6% | 978 | MS, KI, ST |
| 4 | β-Myrcene | 12.94 | 0.6% | 991 | MS, KI, ST |
| 5 | p-Cymene | 14.52 | 3.0% | 1025 | MS, KI, ST |
| 6 | Limonene | 14.75 | 73.0% | 1030 | MS, KI, ST |
| 7 | γ-Terpinene | 16.15 | 9.2% | 1060 | MS, KI, ST |
| 8 | Neral | 24.66 | 0.5% | 1238 | MS, KI, ST |
| 9 | Geranial | 26.03 | 0.7% | 1268 | MS, KI, ST |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senthil Kumar, K.J.; Gokila Vani, M.; Wang, C.-S.; Chen, C.-C.; Chen, Y.-C.; Lu, L.-P.; Huang, C.-H.; Lai, C.-S.; Wang, S.-Y. Geranium and Lemon Essential Oils and Their Active Compounds Downregulate Angiotensin-Converting Enzyme 2 (ACE2), a SARS-CoV-2 Spike Receptor-Binding Domain, in Epithelial Cells. Plants 2020, 9, 770. https://doi.org/10.3390/plants9060770
Senthil Kumar KJ, Gokila Vani M, Wang C-S, Chen C-C, Chen Y-C, Lu L-P, Huang C-H, Lai C-S, Wang S-Y. Geranium and Lemon Essential Oils and Their Active Compounds Downregulate Angiotensin-Converting Enzyme 2 (ACE2), a SARS-CoV-2 Spike Receptor-Binding Domain, in Epithelial Cells. Plants. 2020; 9(6):770. https://doi.org/10.3390/plants9060770
Chicago/Turabian StyleSenthil Kumar, K. J., M. Gokila Vani, Chung-Shuan Wang, Chia-Chi Chen, Yu-Chien Chen, Li-Ping Lu, Ching-Hsiang Huang, Chien-Sing Lai, and Sheng-Yang Wang. 2020. "Geranium and Lemon Essential Oils and Their Active Compounds Downregulate Angiotensin-Converting Enzyme 2 (ACE2), a SARS-CoV-2 Spike Receptor-Binding Domain, in Epithelial Cells" Plants 9, no. 6: 770. https://doi.org/10.3390/plants9060770
APA StyleSenthil Kumar, K. J., Gokila Vani, M., Wang, C. -S., Chen, C. -C., Chen, Y. -C., Lu, L. -P., Huang, C. -H., Lai, C. -S., & Wang, S. -Y. (2020). Geranium and Lemon Essential Oils and Their Active Compounds Downregulate Angiotensin-Converting Enzyme 2 (ACE2), a SARS-CoV-2 Spike Receptor-Binding Domain, in Epithelial Cells. Plants, 9(6), 770. https://doi.org/10.3390/plants9060770