Graphene/Epoxy Coating as Multifunctional Material for Aircraft Structures
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials

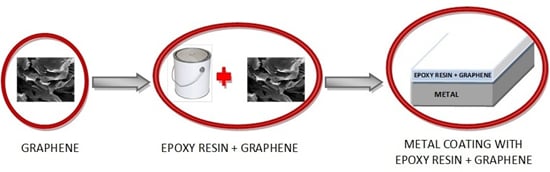
2.2. Graphene/Epoxy Coating Preparation

2.3. Characterizations
2.3.1. Electrochemical Tests
2.3.2. Thermal Analysis
2.3.3. Adhesion Test
2.3.4. Contact Angle Test
3. Results and Discussion
3.1. Electrochemical Test




3.2. Thermal Analysis

3.3. Adhesion Test
3.4. Contact Angle Test
| Sample | Contact Angle (degree) |
|---|---|
| Unfilled water epoxy resin | 60.4 ± 1 |
| Graphene/epoxy resin | 75.3 ± 1 |

4. Conclusions
- The EIS test demonstrate that the addition of graphene nanoflakes significantly changes the barrier properties of epoxy resin.
- The results of capacitance analysis and water contact angle measurements show that graphene improves the barrier properties of neat epoxy resin.
- The DSC and adhesion tests demonstrate no negligible influence of graphene on the resin curing process or on adhesion properties of the coating, respectively.
- Graphene, added to a non-anticorrosive coating system, has an impressive effect on the corrosion performance of material.
Author Contributions
Conflicts of Interest
References
- Du, J.; Cheng, H.M. The fabrication, properties, and uses of graphene/polymer composites. Macromol. Chem. Phys. 2012, 213, 1060–1077. [Google Scholar] [CrossRef]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposite. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Jang, B.Z.; Zhamu, A. Processing of nanographene platelets (NGPs) and NGP nanocomposites: A review. J. Mater. Sci. 2008, 43, 5092–5101. [Google Scholar] [CrossRef]
- Yasmin, A.; Luo, J.J.; Daniel, I.M. Processing of expanded graphite reinforced polymer nanocomposites. Comp. Sci. Technol. 2006, 66, 1182–1189. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, M.; Shen, Z.; Zhao, X.; Zhang, X.; Ma, S. Graphene-reinforced epoxy resin with enhanced atomic oxygen erosion resistance. J. Mater. Sci. 2013, 48, 2416–2423. [Google Scholar] [CrossRef]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical property and structure of covalent functionalised graphene/epoxy nanocomposites. Sci. Rep. 2014, 4, 4375. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, G.; Romeo, V.; Cannavaro, I.; Roncato, D.; Martoraa, B.; Gosso, M. Graphene-polymer composites. Mater. Sci. Eng. 2012, 40, 012018. [Google Scholar]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z.; Silveraiah, V.S.G. Graphene nanoplatelets as novel reinforcement filler in poly(lactid acid)/epoxidized palm oil green nanocomposites: Mechanical properties. Int. J. Mol. Sci. 2012, 13, 10920–10934. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Gupta, R.K. Trends and frontiers in graphene-based polymer nanocomposites. Plast. Eng. 2011, 67, 32–42. [Google Scholar]
- Kim, J.Y.; Kim, T.Y.; Suk, J.W.; Chou, H.; Jang, J.H.; Lee, J.H.; Kholmanov, I.N.; Akinwande, D.; Ruoff, R.S. Enhanced dielectric performance in polymer composite films with carbon nanotube-reduced graphene oxide hybrid filler. Small 2014, 10, 3405–3411. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.; Qiu, H.; Dai, Y.; Zheng, Q.; Li, J.; Yang, J. Self-aligned graphene as anticorrosive barrier in waterborne polyurethane composite coatings. J. Mater. Chem. A 2014, 2, 14139–14145. [Google Scholar] [CrossRef]
- Chang, K.C.; Hsu, M.H.; Lu, H.I.; Lai, M.C.; Liu, P.J.; Hsu, C.H.; Ji, W.F.; Chuang, T.L.; Wei, Y.; Yeh, J.M.; et al. Room-temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitor for cold-rolled steel. Carbon 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Chang, K.C.; Hsu, C.H.; Lu, H.I.; Ji, W.F.; Chang, C.H.; Li, W.Y.; Chuang, T.L.; Yeh, J.M.; Liu, W.R.; Tsai, M.H. Advanced anticorrosive coatings prepared from electroactive polyimide/graphene nanocomposites with synergistic effects of redox catalytic capability and gas barrier properties. Express Polym. Lett. 2014, 8, 243–255. [Google Scholar] [CrossRef]
- Singh, B.P.; Nayak, S.; Nanda, K.K.; Jena, B.K.; Bhattachariee, S.; Besra, L. The production of a corrosion resistant graphene reinforced composite coating on copper by electrophoretic deposition. Carbon 2013, 61, 47–56. [Google Scholar] [CrossRef]
- Bellucci, F.; Nicodemo, L.; Nicolais, L. Transport properties of polymeric materials as determined by electrical capacitance method. Polym. News 1992, 17, 272–277. [Google Scholar]
- Bonnel, K.; le Pen, C.; Pébère, N. E.I.S. characterization of protective coatings on aluminium alloys. Electrochim. Acta 1999, 44, 4259–4267. [Google Scholar] [CrossRef]
- Bonora, P.L.; Defloriana, F.; Fedrizzi, L. Electrochemical impedance spectroscopy as tool for investigating underpaint corrosion. Electrochim. Acta 1996, 41, 1073–1082. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Zhang, J.; Cao, C. Studies of water transport behavior and impedance models of epoxy-coated metals in NaCl solution by EIS. Prog. Org. Coat. 2004, 51, 145–151. [Google Scholar] [CrossRef]
- Juttner, K. Electrochemical impedance spectroscopy (EIS) of corrosion process on inhomogeneous surfaces. Electrochim. Acta 1990, 35, 1501–1508. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monetta, T.; Acquesta, A.; Bellucci, F. Graphene/Epoxy Coating as Multifunctional Material for Aircraft Structures. Aerospace 2015, 2, 423-434. https://doi.org/10.3390/aerospace2030423
Monetta T, Acquesta A, Bellucci F. Graphene/Epoxy Coating as Multifunctional Material for Aircraft Structures. Aerospace. 2015; 2(3):423-434. https://doi.org/10.3390/aerospace2030423
Chicago/Turabian StyleMonetta, Tullio, Annalisa Acquesta, and Francesco Bellucci. 2015. "Graphene/Epoxy Coating as Multifunctional Material for Aircraft Structures" Aerospace 2, no. 3: 423-434. https://doi.org/10.3390/aerospace2030423
APA StyleMonetta, T., Acquesta, A., & Bellucci, F. (2015). Graphene/Epoxy Coating as Multifunctional Material for Aircraft Structures. Aerospace, 2(3), 423-434. https://doi.org/10.3390/aerospace2030423