Rapid Voltammetric Screening Method for the Assessment of Bioflavonoid Content Using the Disposable Bare Pencil Graphite Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Instrumentation
2.3. Procedures
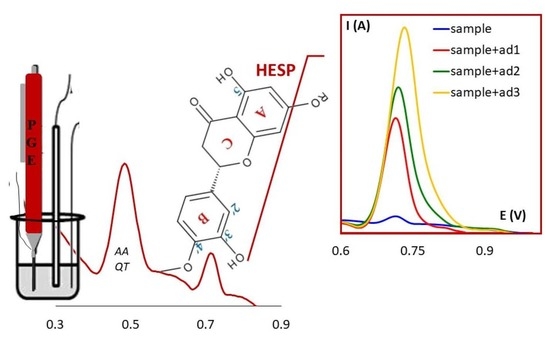
3. Results and Discussion
3.1. Selection of the Optimum Measurement Conditions
3.1.1. The Working Electrode
3.1.2. Solutions and Supporting Electrolyte
3.2. Hesperidin Voltammetric Behavior at the Pencil Graphite Electrode
3.3. Hesperidine Voltammetric Quantification at the Pencil Graphite Electrode
3.3.1. Hesperidin Differential Pulse Voltammetric Determination at PGE
3.3.2. Adsorptive Differential Pulse Voltammetric Determination
3.4. Precision of HESP Voltammetric Response at the Pencil Graphite Electrode
3.5. Interference Studies
3.6. Recovery Studies and Analytical Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P.; Donzelli, A. Hesperidin and SARS-CoV-2: New light on the healthy function of citrus fruits. Antioxidants 2020, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a neuroprotective agent: A review of animal and clinical evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.; Xiang, L.; Ishihara, S.; Matsuura, A.; Sakagami, Y.; Qi, J. Anti-aging effects of hesperidin on Saccharomyces cerevisiae via inhibition of reactive oxygen species and UTH1 gene expression. Biosci. Biotechnol. Biochem. 2012, 76, 640–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiğit, A.; Yardım, Y.; Şentürk, Z. Square-wave adsorptive stripping voltammetric determination of hesperidin using a boron-doped diamond electrode. J. Anal. Chem. 2020, 75, 656–661. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Teichenne, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of hesperidin on cardiovascular disease risk factors: The role of intestinal microbiota on hesperidin bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F. A mechanistic review of the anticancer potential of hesperidin, a natural flavonoid from citrus fruits. Nutr. Res. 2021, 92, 21–31. [Google Scholar] [CrossRef]
- Xiong, H.; Wang, J.; Ran, Q.; Lou, G.; Peng, C.; Gan, Q.; Hu, J.; Sun, J.; Yao, R.; Huang, Q. Hesperidin: A therapeutic agent for obesity. Drug Des. Dev. Ther. 2019, 13, 3855–3866. [Google Scholar] [CrossRef] [Green Version]
- Matos, A.L.; Bruno, D.F.; Ambrósio, A.F.; Santos, P.F. The benefits of flavonoids in diabetic retinopathy. Nutrients 2020, 12, 3169. [Google Scholar] [CrossRef]
- Xie, L.; Gu, Z.; Liu, H.; Jia, B.; Wang, Y.; Cao, M.; Song, R.; Zhang, Z.; Bian, Y. The anti-depressive effects of hesperidin and the relative mechanisms based on the NLRP3 inflammatory signaling pathway. Front. Pharmacol. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Kim, J.; Wie, M.-B.; Ahn, M.; Tanaka, A.; Matsuda, H.; Shin, T. Benefits of hesperidin in central nervous system disorders: A review. Anat. Cell Biol. 2019, 52, 369–377. [Google Scholar] [CrossRef]
- Kotru, S.; Klimuntowski, M.; Ridha, H.; Uddin, Z.; Askhar, A.A.; Singh, G.; Howlader, M.M.R. Electrochemical sensing: A prognostic tool in the fight against COVID-19. Trends Anal. Chem. 2021, 136, 116198. [Google Scholar] [CrossRef]
- Suleman, S.; Shukla, S.K.; Malhotra, N.; Bukkitgar, S.D.; Shetti, N.P.; Pilloton, R.; Narang, J.; Tan, Y.N.; Aminabhavi, T.M. Point of care detection of COVID-19: Advancement in biosensing and diagnostic methods. Chem. Eng. J. 2021, 414, 128759. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.D.; Yang, X.J.; Chen, B.Z.; Chao, Y.X.; Hu, P.J.; Cai, Y.; Wu, B.; Wei, M.Y. Identification and determination of chemical constituents of Citrus reticulata semen through ultra high performance liquid chromatography combined with Q Exactive Orbitrap tandem mass spectrometry. J. Sep. Sci. 2020, 43, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Baira, E.; Dagla, I.; Siapi, E.; Zoumpoulakis, P.; Tsarbopoulos, A.; Simitzis, P.; Goliomytis, M.; Deligeorgis, S.G.; Skaltsounis, A.-L.; Gikas, E. Development and validation of a UPLC–ESI (-)–MS/MS methodology for the simultaneous quantification of hesperidin, naringin, and their aglycones in chicken tissue samples. J. AOAC Int. 2020, 103, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kral, K.; Sontag, G. Elektrochemische Oxydation von Flavonoiden an einer glasartigen Kohlenstoffelektrode. Mikrochim. Acta 1982, 78, 29–41. [Google Scholar] [CrossRef]
- Temerk, Y.M.; Ibrahim, M.S.; Kotb, M.; Schuhmann, W. Renewable pencil electrodes for highly sensitive anodic stripping voltammetric determination of 3-hydroxyflavone and morin in bulk form and in biological fluids. Electroanalysis 2013, 25, 1381–1387. [Google Scholar] [CrossRef]
- Temerk, Y.M.; Ibrahim, M.S.; Schuhmann, W. Simultaneous anodic adsorptive stripping voltammetric determination of luteolin and 3-hydroxyflavone in biological fluids using renbewable pencil graphite electrode. Electroanalysis 2019, 31, 1095–1103. [Google Scholar] [CrossRef]
- David, I.G.; Oancea, A.G.; Buleandra, M.; Popa, D.E.; Iorgulescu, E.E.; Ciobanu, A.M. Disposable pencil graphite electrode for diosmin voltammetric analysis. Micromachines 2021, 12, 351. [Google Scholar] [CrossRef]
- David, I.G.; Litescu, S.C.; Popa, D.E.; Buleandra, M.; Iordache, L.; Albu, C.; Alecu, A.; Penu, R.L. Voltammetric analysis of naringenin at a disposable pencil graphite electrode—Application to polyphenol content determination in citrus juice. Anal. Methods 2018, 10, 5763–5772. [Google Scholar] [CrossRef]
- Šafranko, S.; Stanković, A.; Asserghine, A.; Jakovljević, M.; Hajra, S.; Nundy, S.; Medvidović-Kosanović, M.; Jokić, S. Electroactivated disposable pencil graphite electrode—New, cost-effective, and sensitive electrochemical detection of bioflavonoid hesperidin. Electroanalysis 2020, 33, 1063–1071. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhang, L.; Chen, G. Carbon nanotube-phenolic resin composite electrode fabricated by far Infrared-assisted crosslinking for enhanced amperometric detection. Electroanalysis 2019, 31, 756–765. [Google Scholar] [CrossRef]
- Sims, M.J.; Li, Q.; Kachoosangi, R.T.; Wildgoose, G.G.; Compton, R.G. Using multiwalled carbon nanotube modified electrodes for the adsorptive striping voltammetric determination of hesperidin. Electrochim. Acta 2009, 54, 5030–5034. [Google Scholar] [CrossRef]
- Zhupanova, A.; Guss, E.; Ziyatdinova, G.; Budnikov, H. Simultaneous voltammetric determination of flavanones using an electrode based on functionalized single-walled carbon nanotubes and polyaluminon. Anal. Lett. 2020, 53, 2170–2189. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Bhakta, A.K.; Mekhalif, Z. Nano-graphene-platelet/Brilliant-green composite coated carbon paste electrode interface for electrocatalytic oxidation of flavanone Hesperidin. Microchem. J. 2021, 160, 105768. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Wang, H.; Lu, W.; Guo, M. Highly sensitive detection of hesperidin using AuNPs/rGO modified glassy electrode. Analyst 2018, 143, 297–303. [Google Scholar] [CrossRef]
- Tığ, G.A.; Bolat, E.Ö.; Zeybek, B.; Pekyardımcı, Ş. Hesperidin-dsDNA interaction based on electrochemically reduced graphene oxide and poly-(2,6-pyridinedicarboxylic acid) modified glassy carbon electrode. Hacettepe J. Biol. Chem. 2016, 44, 487–497. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, R.; Lin, H.; Wei, Q.; Hu, F.; Yang, X. Novel plant flavonoid electrochemical sensor based on in-situ and controllable double-layered membranes modified electrode. PLoS ONE 2020, 15, e0237583. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z. Application of electrochemical sensors based on carbon nanomaterials for detection of flavonoids. Nanomaterials 2020, 10, 2020. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Yakupova, E.; Davletshin, R. Voltammetric determination of hesperidin on the electrode modified with SnO2 nanoparticles and surfactants. Electroanalysis 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Sun, B.; Hou, X.; Li, D.; Gou, Y.; Hu, F.; Li, W.; Shi, X. Electrochemical sensing and high selective detection of hesperidin with molecularly imprinted polymer based on ultrafine activated carbon. J. Electrochem. Soc. 2019, 166, B1644. [Google Scholar] [CrossRef]
- Sun, D.; Wang, F.; Wu, K.; Chen, J.; Zhou, Y. Electrochemical determination of hesperidin using mesoporous SiO2 modified electrode. Microchim. Acta 2009, 167, 35–39. [Google Scholar] [CrossRef]
- David, I.G.; Buleandra, M.; Popa, D.E.; Bîzgan, A.-M.C.; Moldovan, Z.; Badea, I.-A.; Iorgulescu, E.E.; Tekiner, T.A.; Basaga, H. Voltammetric determination of polyphenolic content as rosmarinic acid equivalent in tea samples using pencil graphite electrodes. J. Food Sci. Technol. 2016, 53, 2589–2596. [Google Scholar] [CrossRef] [Green Version]
- Obendorf, D.; Reichart, E. Determination of hesperidin by cathodic stripping voltammetry in orange juice and Helopyrin, a phytopharmaceutical preparation. Electroanalysis 1995, 7, 1075–1081. [Google Scholar] [CrossRef]
- Yardım, Y.; Keskin, E.; Sentürk, Z. Voltammetric determination of mixtures of caffeine and chlorogenic acid in beverage samples using a boron-doped diamond electrode. Talanta 2013, 116, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, Ö.; Dilgin, D.G.; Ertek, B.; Dil, Y. Differential pulse voltammetric determination of eugenol at a pencil graphite electrode. Mater. Sci. Eng. C 2016, 160, 156–162. [Google Scholar] [CrossRef]
- Alves, G.F.; Lisboa, T.P.; de Faria, L.V.; de Farias, D.M.; Costa Matos, M.A.; Matos, R.C. Disposable Pencil Graphite Electrode for Ciprofloxacin Determination in Pharmaceutical Formulations by Square Wave Voltammetry. Electroanalysis 2021, 33, 543–549. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Milicevic, A.; Novak Jovanovic, I. The relationship between the first oxidation potential and changes in electronic structures upon the electrochemical oxidation of flavonoids: Approach to O-glycosyl, galloyl and methoxy substituents. J. Mol. Liq. 2021, 335, 116223. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.; Enache, T.A.; Gil, E.D.S.; Oliveira-Brett, A.M. Natural phenolic antioxidants electrochemistry: Towards a new food science methodology. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1680–1726. [Google Scholar] [CrossRef]
- Hu, J.; Li, Q.; Tan, X. Study on the adsorptive behavior of hesperidin and its adsorptive stripping voltammetry. Anal. Lett. 1996, 29, 1779–1789. [Google Scholar] [CrossRef]
- Temerk, Y.M.; Ibrahim, M.S.; Kotb, M. Square-wave cathodic adsorptive stripping voltammetric determination of 3-hydroxyflavone, morin and hesperidin in bulk form and biological fluids in absence and presence of Cu(II). J. Braz. Chem. Soc. 2011, 11, 2056–2064. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xu, X.; Bin, Q.; Ling, J.; Chen, G. A new method for fabrication of an integrated indium tin oxide electrode on electrophoresis microchips with amperometric detection and its application for determination of synephrine and hesperidin in pericarpium citri reticulatae. Electrophoresis 2006, 27, 4174–4181. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 5th ed.; Pearson Prentice Hall: Edinburgh, UK, 2005. [Google Scholar]
- AOAC International. Appendix F: Guidelines for Standard Method Performance Requirements; AOAC International: Rockville, MD, USA, 2012; pp. 1–17. [Google Scholar]
- Blasco, A.J.; Rogerio, M.; González, M.; Escarpa, A. “Electrochemical Index” as a screening method to determine “total polyphenolics” in foods: A proposal. Anal. Chim. Acta 2005, 539, 237–244. [Google Scholar] [CrossRef]
- Phong, N.H.; Toan, T.T.T.; Tinh, M.X.; Tuyen, T.N.; Mau, T.X.; Khieu, D.Q. Simultaneous voltammetric determination of ascorbic acid, paracetamol, and caffeine using electrochemically reduced graphene-oxide-modified electrode. J. Nanomater. 2018, 2018, 5348016. [Google Scholar] [CrossRef]
- Oliviera Brett, A.M.; Ghica, M.-E. Electrochemical oxidation of quercetin. Electroanalysis 2003, 15, 1745–1750. [Google Scholar] [CrossRef] [Green Version]









| Working Electrode | Epa2 (V) | S (A × L/mol × cm2) |
|---|---|---|
| PGE/2B | 0.722 | 1.501 |
| PGE/B | 0.715 | 1.255 |
| PGE/HB | 0.708 | 1.918 |
| PGE/H | 0.715 | 2.563 |
| PGE/2H | 0.715 | 2.201 |
| GCE | 0.742 | 0.411 |
| Pt | no characteristic peak |
| Peak | Ip = f(v) | Ip = f(v1/2) | log Ip = f(log v) |
|---|---|---|---|
| First potential scan | |||
| a2 (Epa2 ~0.800 V) | Non-linear | Ipa2 = 2.00 × 10−5v1/2 –5.00 × 10−7; R2 = 0.9893 | logIpa2 = 0.6229log v–4.6846; R2 = 0.9660 |
| a3 (Epa3 ~1.230 V) | Ipa3 = 1.00 × 10−5v + 7.00 × 10−7; R2 = 0.9895 | Ipa3 = 1.00 × 10−5v1/2–2.00 × 10−6; R2 = 0.9611 | logIpa3 = 1.0999log v–4.7387; R2 = 0.9841 |
| c1 (Epc1 ~0.480 V) | Ipc1 = −2.00 × 10−5v–6.00 × 10−7; R2 = 0.9675 | Ipc1 = −1.00 × 10−5v1/2 + 2.00 × 10−6; R2 = 0.9893 | logIpc1 = 0.8294log–4.8235; R2 = 0.9927 |
| Second potential scan | |||
| a1 (Epa1 ~0.530 V) | Ipa1 = 2.00 × 10−5v + 4.00 × 10−7; R2 = 0.9904 | Ipa1 = 2.00 × 10−5v1/2–2.00 × 10−6; R2 = 0.9913 | logIpa1 = 0.9721log v–4.7088; R2 = 0.9647 |
| a2 (Epa2 ~0.800 V) | Non-linear | Ipa2 = 2.00 × 10−5v1/2–8.00 × 10−7; R2 = 0.9931 | logIpa2 = 0.6055log v–4.7675; R2 = 0.9860 |
| a3 (Epa3 ~1.230 V) | Ipa3 = 2.00 × 10−5v + 4.00 × 10−7; R2 = 0.9889 | Ipa3 = 2.00 × 10−5v1/2–2.00 × 10−6; R2 = 0.9824 | logIpa3 = 0.8533log v–4.4697; R2 = 0.9833 |
| c1 (Epc1 ~0.480 V) | Ipc1 = −2.00 × 10−5v–5.00 × 10−7; R2 = 0.9898 | Ipc1 = −2.00 × 10−5v1/2 + 2.00 × 10−6; R2 = 0.9935 | logIpc1 = 0.9024log v–4.6985; R2 = 0.9934 |
| Technique | Electrode | Linear Range (mol/L) | Limit of Detection (mol/L) | Sample | Ref. |
|---|---|---|---|---|---|
| DPV DPAdSV | PGE | 1.00 × 10−7–1.20 × 10−5 5.00 × 10−8–1.00 × 10−6 | 8.58 × 10−8 1.90 × 10−8 | Dietary supplements | This work |
| CSV | HMDE | 1.64 × 10−7–4.10 × 10−5 | 1.37 × 10−7 | Orange juice, phytopharmaceuticals | [34] |
| LSAdSV | HMDE | 5.00 × 10−7–8.00 × 10−6 | 3.00 × 10−7 | [41] | |
| SWCAdSV | HMDE HMDE/Cu2+ | 1.90 × 10−8–6.54 × 10−7 7.40 × 10−7–2.85 × 10−6 9.09 × 10−7–2.85 × 10−6 9.00 × 10−9–1.84 × 10−7 | 7.54 × 10−9 5.76 × 10−8 7.58 × 10−8 4.89 × 10−9 | Bulk Urine Serum Bulk | [42] |
| SWAdSV | BDDE | 4.09 × 10−6–1.15 × 10−4 | 1.20 × 10−6 | Dietary supplements | [5] |
| AD | ITO-EMC | 3.00–45.00 μg/mL | 0.57 μg/mL | Pericarpium Citri reticulatae pericarpium | [43] |
| AD | CNT-PR | 1.00 × 10−6–1.00 × 10−3 | 2.30 × 10−7 | Pericarpium Citri reticulatae | [22] |
| AD | AuNPs/rGO/GCE | 5.00 × 10−8–8.00 × 10−6 | 8.20 × 10−9 | Pericarpium Citri reticulatae, Chinese medicines | [26] |
| DPAdSV | SnO2-CPB/GCE | 1.00 × 10−7–7.50 × 10−5 | 7.70 × 10−8 | Orange juice | [30] |
| DPAdSV | SiO2-CPE | 5.00 × 10−7–2.50 × 10−5 | 2.50 × 10−7 | Chinese medicines | [32] |
| SWAdSV | MWCNT-BPPGE | 2.00 × 10−8–3.00 × 10−5 | 7.30 × 10−9 | Orange juice | [23] |
| DPV | nGp-Bg/MCPE | 1.00 × 10−7–1.00 × 10−4 | 5.00 × 10−8 | Lemon juice, orange rind, peppermint extract | [25] |
| DPV | Polyaluminon/f- SWCNT/GCE | 1.00 × 10−7–2.50 × 10−5 | 2.90 × 10−8 | Orange and grapefruit juice | [24] |
| DPV | PAP-MIP/AuNPs/uaC/GCE | 8.00 × 10−8–3.00 × 10−5 | 4.50 × 10−8 | Chinese medicines | [31] |
| DPV | ERGO/P(PDCA)/dsDNA/GCE | 8.20 × 10−7–8.20 × 10−5 | 2.40 × 10−7 | Serum | [27] |
| DPV | ePGE | 5.00 × 10−7–1.00 × 10−5 | 2.00 × 10−7 | Pharmaceuticals | [21] |
| Technique | DPV (Oxidation) | DPAdSV (Reduction) | ||||
|---|---|---|---|---|---|---|
| Concentration (mol/L) | 1.00 × 10−7 | 1.00 × 10−6 | 1.00 × 10−5 | 5.00 × 10−8 | 3.00 × 10−7 | 1.00 × 10−6 |
| RSD% intra-day | 8.17 | 5.99 | 2.50 | 8.94 | 5.70 | 3.13 |
| RSD% inter-day | 8.55 | 6.41 | 3.27 | 9.38 | 6.01 | 3.36 |
| Claimed bioflavonoids content (mg) | 10 |
| Found by DPV ± SD (mg HESP) | 10.36 ± 0.46 |
| RSD, % | 4.63 |
| Average %R ± SD | 103.58 ± 4.63 |
| Relative error (er%) | 3.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, I.G.; Numan, N.; Buleandră, M.; Popa, D.-E.; Lițescu, S.C.; Riga, S.; Ciobanu, A.M. Rapid Voltammetric Screening Method for the Assessment of Bioflavonoid Content Using the Disposable Bare Pencil Graphite Electrode. Chemosensors 2021, 9, 323. https://doi.org/10.3390/chemosensors9110323
David IG, Numan N, Buleandră M, Popa D-E, Lițescu SC, Riga S, Ciobanu AM. Rapid Voltammetric Screening Method for the Assessment of Bioflavonoid Content Using the Disposable Bare Pencil Graphite Electrode. Chemosensors. 2021; 9(11):323. https://doi.org/10.3390/chemosensors9110323
Chicago/Turabian StyleDavid, Iulia Gabriela, Nimet Numan, Mihaela Buleandră, Dana-Elena Popa, Simona Carmen Lițescu, Sorin Riga, and Adela Magdalena Ciobanu. 2021. "Rapid Voltammetric Screening Method for the Assessment of Bioflavonoid Content Using the Disposable Bare Pencil Graphite Electrode" Chemosensors 9, no. 11: 323. https://doi.org/10.3390/chemosensors9110323
APA StyleDavid, I. G., Numan, N., Buleandră, M., Popa, D. -E., Lițescu, S. C., Riga, S., & Ciobanu, A. M. (2021). Rapid Voltammetric Screening Method for the Assessment of Bioflavonoid Content Using the Disposable Bare Pencil Graphite Electrode. Chemosensors, 9(11), 323. https://doi.org/10.3390/chemosensors9110323