Fluorination vs. Chlorination: Effect on the Sensor Response of Tetrasubstituted Zinc Phthalocyanine Films to Ammonia
Abstract
:1. Introduction
2. Materials and Methods
Theoretical Calculations
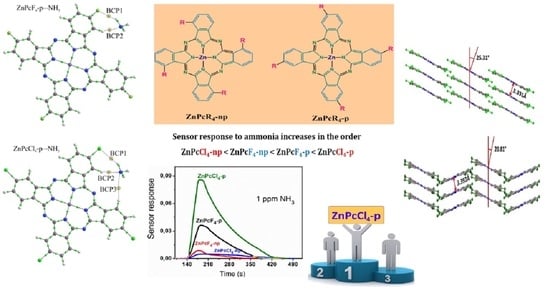
3. Results and Discussion
3.1. Crystal Structure of ZnPcCl4-p and ZnPcCl4-np
3.2. Structure and Morphology of Thin Films
3.3. Sensor Response of ZnPcHal4 Films to Ammonia
3.4. Theoretical Study of the Nature of Bonding between Ammonia and ZnPcHal4 Molecules
3.5. Sensor Characteristics of ZnPcCl4-p and ZnPcF4-p Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Timmer, B.; Olthuis, W.; Van Den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzorati, D.; Mainardi, L.; Sedda, G.; Gasparri, R.; Spaggiari, L.; Cerveri, P. A Review of Exhaled Breath Key Role in Lung Cancer Diagnosis. J. Breath Res. 2017, 13, 034001. [Google Scholar] [CrossRef]
- Yoon, J.W.; Lee, J.H. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2013, 24, 012004. [Google Scholar] [CrossRef] [Green Version]
- Claps, R.; Englich, F.V.; Leleux, D.P.; Richter, D.; Tittel, F.K.; Curl, R.F. Ammonia detection by use of near-infrared diode-laser-based overtone spectroscopy. Appl. Opt. 2001, 40, 4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.J.; Sun, K.; Tao, L.; Khan, M.A.; Zondlo, M.A. Open-path, quantum cascade-laser-based sensor for high-resolution atmospheric ammonia measurements. Atmos. Meas. Tech. 2014, 7, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Sekhar, P.K.; Kysar, J.S. An Electrochemical Ammonia Sensor on Paper Substrate. J. Electrochem. Soc. 2017, 164, B113–B117. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.Y.; Huang, C.P.; Huang, W.T. Gas-detecting properties of surface acoustic wave ammonia sensors. Sens. Actuators B Chem. 2004, 101, 1–7. [Google Scholar] [CrossRef]
- Chen, X.; Li, D.M.; Liang, S.F.; Zhan, S.; Liu, M. Gas sensing properties of surface acoustic wave NH3 gas sensor based on Pt doped polypyrrole sensitive film. Sens. Actuators B Chem. 2013, 177, 364–369. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, X.; Jiang, Y.; Yang, X.; Li, L.; Yu, J. Poly(vinyl alcohol) as a gas accumulation layer for an organic field-effect transistor ammonia sensor. Sens. Actuators B Chem. 2017, 243, 1248–1254. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, X.; Shi, W.; Yang, X.; Li, L.; Yu, J. Poly(3-hexylthiophene)/polystyrene (P3HT/PS) blends based organic field-effect transistor ammonia gas sensor. Sens. Actuators B Chem. 2016, 225, 10–15. [Google Scholar] [CrossRef]
- Besar, K.; Yang, S.; Guo, X.; Huang, W.; Rule, A.M.; Breysse, P.N.; Kymissis, I.J.; Katz, H.E. Printable ammonia sensor based on organic field effect transistor. Org. Electron. 2014, 15, 3221–3230. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Zhu, Y.Q. Room temperature ammonia sensing properties of W18O49 nanowires. Sens. Actuators B Chem. 2009, 137, 27–31. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Aarya, S.; Kumar, Y.; Chahota, R.K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 30, 269–290. [Google Scholar] [CrossRef]
- Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Microwave-based sensors with phthalocyanine films for acetone, ethanol and methanol detection. Sens. Actuators B Chem. 2016, 237, 876–886. [Google Scholar] [CrossRef]
- Chani, M.T.S.; Karimov, K.S.; Ahmad Khalid, F.; Raza, K.; Umer Farooq, M.; Zafar, Q. Humidity sensors based on aluminum phthalocyanine chloride thin films. Phys. E Low-Dimens. Syst. Nanostruct. 2012, 45, 77–81. [Google Scholar] [CrossRef]
- Zhihua, L.; Xucheng, Z.; Jiyong, S.; Xiaobo, Z.; Xiaowei, H.; Tahir, H.E.; Holmes, M. Fast response ammonia sensor based on porous thin film of polyaniline/sulfonated nickel phthalocyanine composites. Sens. Actuators B Chem. 2016, 226, 553–562. [Google Scholar] [CrossRef]
- Parkhomenko, R.G.; Sukhikh, A.S.; Klyamer, D.D.; Krasnov, P.O.; Gromilov, S.; Kadem, B.; Hassan, A.K.; Basova, T.V. Thin Films of Unsubstituted and Fluorinated Palladium Phthalocyanines: Structure and Sensor Response toward Ammonia and Hydrogen. J. Phys. Chem. C 2017, 121, 1200–1209. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V. Thin films of tetrafluorosubstituted cobalt phthalocyanine: Structure and sensor properties. Appl. Surf. Sci. 2016, 372, 79–86. [Google Scholar] [CrossRef]
- Klyamer, D.; Sukhikh, A.; Gromilov, S.; Krasnov, P.; Basova, T. Fluorinated metal phthalocyanines: Interplay between fluorination degree, films orientation, and ammonia sensing properties. Sensors 2018, 18, 2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, T.; Weiler, U.; Kelting, C.; Schlettwein, D.; Makarov, S.; Wöhrle, D.; Abdallah, O.; Kunst, M.; Jaegermann, W. Silicon–organic pigment material hybrids for photovoltaic application. Sol. Energy Mater. Sol. Cells 2007, 91, 1873–1886. [Google Scholar] [CrossRef]
- Oksengendler, I.G.; Kondratenko, N.V.; Luk’yanets, E.A.; Yagupol’skii, L.M. Fluoro-and perfluoro-tert-butyl-substituted phthalocyanines. Zh. Org. Khim 1977, 13, 2234. [Google Scholar]
- Tang, M.L.; Bao, Z. Halogenated Materials as Organic Semiconductors. Chem. Mater. 2010, 23, 446–455. [Google Scholar] [CrossRef]
- Pakhomov, L.G.; Pakhomov, G.L. NO2 interaction with thin film of phthalocyanine derivatives {1}. Synth. Met. 1995, 71, 2299–2300. [Google Scholar] [CrossRef]
- Irie, S.; Hoshino, A.; Kuwamoto, K.; Isoda, S.; Miles, M.J.; Kobayashi, T. Point-on-line coincidence in epitaxial growth of CuPcCl 16 on graphite. Appl. Surf. Sci. 1997, 113–114, 310–315. [Google Scholar] [CrossRef]
- Mittelberger, A.; Kramberger, C.; Meyer, J.C. Insights into radiation damage from atomic resolution scanning transmission electron microscopy imaging of mono-layer CuPcCl16 films on graphene. Sci. Rep. 2018, 8, 4813. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Biskupek, J.; Kurata, H.; Kaiser, U. Critical conditions for atomic resolution imaging of molecular crystals by aberration-corrected HRTEM. Ultramicroscopy 2015, 159, 73–80. [Google Scholar] [CrossRef]
- Bobaru, S.C.; Salomon, E.; Layet, J.-M.; Angot, T. Structural Properties of Iron Phtalocyanines on Ag(111): From the Submonolayer to Monolayer Range. J. Phys. Chem. C 2011, 115, 5875–5879. [Google Scholar] [CrossRef]
- Fryer, J.R. Electron Crystallography of Phthalocyanines. J. Porphyr. Phthalocyanines 1999, 3, 672–678. [Google Scholar] [CrossRef]
- Sukhikh, A.; Bonegardt, D.; Klyamer, D.; Krasnov, P.; Basova, T. Chlorosubstituted copper phthalocyanines: Spectral study and structure of thin films. Molecules 2020, 25, 1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaraj, T.; Rajalingam, R. Theoretical Studies of the Zeolite-Y Encapsulated Chlorine-Substituted Copper(II)phthalocyanine Complex on the Formation Glycidol from Allyl Alcohol. ACS Omega 2018, 3, 9613–9619. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.R.; Sahu, S.; Sharma, S. Charge transport and prototypical optical absorptions in functionalized zinc phthalocyanine compounds: A density functional study. J. Phys. Org. Chem. 2018, 31, e3785. [Google Scholar] [CrossRef]
- Ling, M.-M.; Bao, Z.; Erk, P. Air-stable n-channel copper hexachlorophthalocyanine for field-effect transistors. Appl. Phys. Lett. 2006, 89, 163516. [Google Scholar] [CrossRef]
- Achar, B.N.; Jayasree, P.K. “Molecular Metals” Based on Copper(II) 2,9,16,23-tetrahalo Substituted Phthalocyanine Derivatives. Synth. React. Inorg. Met. Chem. 2000, 30, 719–733. [Google Scholar] [CrossRef]
- Antunes, E.M.; Nyokong, T. Synthesis and Photophysical Properties of Tetra- and Octasubstituted Phosphorous Oxide Triazatetrabenzcorrole Photosensitizers. Met. Based. Drugs 2008, 2008, 498916. [Google Scholar] [CrossRef] [Green Version]
- Bruker AXS Inc. Bruker Advanced X-ray Solutions; Bruker: Madison, WI, USA, 2004. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Bushmarinov, I.S.; Lyssenko, K.A.; Antipin, M.Y. Atomic energy in the “Atoms in Molecules” theory and its use for solving chemical problems. Russ. Chem. Rev. 2009, 78, 283–302. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. B 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Baerends, E.J.; Ellis, D.E.; Ros, P. Self-consistent molecular Hartree-Fock-Slater calculations I. The computational procedure. Chem. Phys. 1973, 2, 41–51. [Google Scholar] [CrossRef]
- Dunlap, B.I.; Connolly, J.W.D.; Sabin, J.R. On some approximations in applications of Xα theory. J. Chem. Phys. 1979, 71, 3396–3402. [Google Scholar] [CrossRef]
- Van Alsenoy, C. Ab initio calculations on large molecules: The multiplicative integral approximation. J. Comput. Chem. 1988, 9, 620–626. [Google Scholar] [CrossRef]
- Kendall, R.A.; Früchtl, H.A. The impact of the resolution of the identity approximate integral method on modern ab initio algorithm development. Theor. Chem. Acc. 1997, 97, 158–163. [Google Scholar] [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R. Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119–124. [Google Scholar] [CrossRef]
- Eichkorn, K.; Treutler, O.; Öhm, H.; Häser, M.; Ahlrichs, R. Auxiliary basis sets to approximate Coulomb potentials (Chem. Phys. Letters 240 (1995) 283) (PII:0009-2614(95)00621-4). Chem. Phys. Lett. 1995, 242, 652–660. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Riplinger, C.; Sandhoefer, B.; Hansen, A.; Neese, F. Natural triple excitations in local coupled cluster calculations with pair natural orbitals. J. Chem. Phys. 2013, 139, 134101. [Google Scholar] [CrossRef]
- Riplinger, C.; Neese, F. An efficient and near linear scaling pair natural orbital based local coupled cluster method. J. Chem. Phys. 2013, 138, 034106. [Google Scholar] [CrossRef]
- Pinski, P.; Riplinger, C.; Valeev, E.F.; Neese, F. Sparse maps—A systematic infrastructure for reduced-scaling electronic structure methods. I. An efficient and simple linear scaling local MP2 method that uses an intermediate basis of pair natural orbitals. J. Chem. Phys. 2015, 143, 034108. [Google Scholar] [CrossRef] [Green Version]
- Sandler, I.; Chen, J.; Taylor, M.; Sharma, S.; Ho, J. Accuracy of DLPNO-CCSD(T): Effect of Basis Set and System Size. J. Phys. Chem. A 2021, 125, 1553–1563. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atom in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Klyamer, D.D.; Sukhikh, A.S.; Gromilov, S.A.; Kruchinin, V.N.; Spesivtsev, E.V.; Hassan, A.K.; Basova, T.V. Influence of fluorosubstitution on the structure of zinc phthalocyanine thin films. Macroheterocycles 2018, 11, 304–311. [Google Scholar] [CrossRef]
- Hoshino, A.; Takenaka, Y.; Miyaji, H. Redetermination of the crystal structure of α-copper phthalocyanine grown on KCl. Acta Crystallogr. Sect. B Struct. Sci. 2003, 59, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ballirano, P.; Caminiti, R.; Ercolani, C.; Maras, A.; Orrù, M.A. X-ray powder diffraction structure reinvestigation of the α and β forms of cobalt phthalocyanine and kinetics of the α → β phase transition. J. Am. Chem. Soc. 1998, 120, 12798–12807. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, P.; Ye, J.; Li, Y.; Li, H.; Zhang, X.; Li, R.; Dong, H.; Hu, W.; Kloc, C. Molecular Crystal Engineering: Tuning Organic Semiconductor from p-type to n-type by Adjusting Their Substitutional Symmetry. Adv. Mater. 2017, 29, 1605053. [Google Scholar] [CrossRef]
- Scheidt, W.R.; Dow, W. Molecular Stereochemistry of Phthalocyanatozinc(II). J. Am. Chem. Soc. 1977, 99, 1101–1104. [Google Scholar] [CrossRef]
- Barsan, N.; Simion, C.; Heine, T.; Pokhrel, S.; Weimar, U. Modeling of sensing and transduction for p-type semiconducting metal oxide based gas sensors. J. Electroceram. 2010, 25, 11–19. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Rana, M.K.; Sinha, M.; Panda, S. Gas sensing behavior of metal-phthalocyanines: Effects of electronic structure on sensitivity. Chem. Phys. 2018, 513, 23–34. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, B.; Zhang, H.; Qin, J. Theoretical insight into two-dimensional M-Pc monolayer as an excellent material for formaldehyde and phosgene sensing. Appl. Surf. Sci. 2021, 543, 148805. [Google Scholar] [CrossRef]
- Kuprikova, N.M.; Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.O.; Mrsic, I.; Basova, T.V. Fluorosubstituted lead phthalocyanines: Crystal structure, spectral and sensing properties. Dyes Pigments 2020, 173, 107939. [Google Scholar] [CrossRef]
- Chia, L.S.; Du, Y.H.; Palale, S.; Lee, P.S. Interaction of Copper Phthalocyanine with Nitrogen Dioxide and Ammonia Investigation Using X-ray Absorption Spectroscopy and Chemiresistive Gas Measurements. ACS Omega 2019, 4, 10388–10395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koch, U.; Popelier, P.L.A. Characterization of C-H-O hydrogen bonds on the basis of the charge density. J. Phys. Chem. 1995, 99, 9747–9754. [Google Scholar] [CrossRef]
- Kumar, P.S.V.; Raghavendra, V.; Subramanian, V. Bader’s Theory of Atoms in Molecules (AIM) and its Applications to Chemical Bonding. J. Chem. Sci. 2016, 128, 1527–1536. [Google Scholar] [CrossRef]
- Kaya, E.N.; Şenocak, A.; Klyamer, D.D.; Demirbaş, E.; Basova, T.V.; Durmuş, M. Ammonia sensing performance of thin films of cobalt(II) phthalocyanine bearing fluorinated substituents. J. Mater. Sci. Mater. Electron. 2019, 8, 7543–7551. [Google Scholar] [CrossRef]
- Şenoğlu, S.; Özer, M.; Dumludağ, F.; Acar, N.; Salih, B.; Bekaroğlu, Ö. Synthesis, characterization, DFT study, conductivity and effects of humidity on CO2 sensing properties of the novel tetrakis-[2-(dibenzylamino)ethoxyl] substituted metallophthalocyanines. Sens. Actuators B Chem. 2020, 310, 127860. [Google Scholar] [CrossRef]
- Liu, L.; Fei, T.; Guan, X.; Lin, X.; Zhao, H.; Zhang, T. Room temperature ammonia gas sensor based on ionic conductive biomass hydrogels. Sens. Actuators B Chem. 2020, 320, 128318. [Google Scholar] [CrossRef]
- Can Ömür, B. Humidity effect on adsorption kinetics of ammonia onto electrospun SnO2 nanofibers. Mater. Res. Express 2019, 6, 045043. [Google Scholar] [CrossRef]
- Sizun, T.; Bouvet, M.; Suisse, J.M. Humidity effect on ammonia sensing properties of substituted and unsubstituted cobalt phthalocyanines. Talanta 2012, 97, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Banimuslem, H.A.; Kadem, B.Y. Fluorinated chromium phthalocyanine thin films: Characterization and ammonia vapor detection. Chemosensors 2018, 6, 63. [Google Scholar] [CrossRef] [Green Version]
- Basova, T.V.; Mikhaleva, N.S.; Hassan, A.K.; Kiselev, V.G. Thin films of fluorinated 3d-metal phthalocyanines as chemical sensors of ammonia: An optical spectroscopy study. Sens. Actuators B Chem. 2016, 227, 634–642. [Google Scholar] [CrossRef]













| Compound | ZnPcF4-np | ZnPcCl4-p | ZnPcCl4-np |
|---|---|---|---|
| Empirical formula | C32H12F4N8Zn | C32H12Cl4N8Zn | C32H12Cl4N8Zn |
| Formula weight | 649.87 | 715.67 | 715.67 |
| Temperature/K | 150.0 | 150.0 | 150.0 |
| Crystal system | monoclinic | tetragonal | monoclinic |
| Space group | P21/n | I41/a | P21/n |
| a/Å | 14.8240(17) | 38.3949(13) | 14.4126(15) |
| b/Å | 4.8033(7) | 38.3949(13) | 4.8075(5) |
| c/Å | 17.866(2) | 3.6284(2) | 20.331(2) |
| α/° | 90 | 90 | 90 |
| β/° | 108.039(7) | 90 | 110.544(3) |
| γ/° | 90 | 90 | 90 |
| Volume/Å3 | 1209.6(3) | 5348.9(5) | 1319.1(2) |
| Z | 2 | 8 | 2 |
| ρcalcg/cm3 | 1.784 | 1.777 | 1.792 |
| F(000) | 652 | 2864 | 716 |
| Independent reflections | 2293 [Rint = 0.0818, Rsigma = 0.0751] | 4075 [Rint = 0.0575, Rsigma = 0.0371] | 1895 [Rint = 0.0684, Rsigma = 0.0519] |
| Data/restraints/parameters | 2293/0/226 | 4075/0/226 | 1895/310/400 |
| Goodness-of-fit on F2 | 1.014 | 1.120 | 1.042 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0479, wR2 = 0.0800 | R1 = 0.0460, wR2 = 0.0871 | R1 = 0.0581, wR2 = 0.1547 |
| Final R indexes [all data] | R1 = 0.0833, wR2 = 0.0910 | R1 = 0.0553, wR2 = 0.0900 | R1 = 0.1015, wR2 = 0.1806 |
| CCDC deposition № | 2077055 | 2077056 | 2077057 |
| Sensing Layer | Calculated LOD, ppm | Response Time, s | Recovery Time, s |
|---|---|---|---|
| ZnPcF4-np | 0.08 | 35 | 155 |
| ZnPcF4-p | 0.01 | 45 | 210 |
| ZnPcCl4-np | 0.1 | 45 | 280 |
| ZnPcCl4-p | 0.01 | 45 | 260 |
| Structure | BCP | ρ(r), e/Å3 | ∇2ρ(r), e/Å5 | |λ1|/λ3 | he(r), a.u. | Eb, kcal/mol |
|---|---|---|---|---|---|---|
| ZnPcCl4-np···NH3 | 1 | 0.045 | 0.674 | 0.137 | 1.21·10−3 | −0.9 (−0.1 *) |
| 2 | 0.045 | 0.672 | 0.138 | 1.18·10−3 | ||
| 3 | 0.069 | 0.628 | 0.180 | 3.50·10−4 | ||
| ZnPcCl4-p···NH3 | 1 | 0.059 | 0.579 | 0.199 | 2.16·10−4 | −3.5 (−3.7 *) |
| 2 | 0.113 | 0.972 | 0.238 | −6.60·10−5 | ||
| 3 | 0.006 | 0.075 | 0.140 | 2.74·10−4 | ||
| ZnPcF4-np···NH3 | 1 | 0.077 | 1.003 | 0.194 | 2.38·10−4 | −1.5 (−1.0 *) |
| 2 | 0.075 | 0.665 | 0.187 | 2.34·10−4 | ||
| ZnPcF4-p···NH3 | 1 | 0.065 | 0.845 | 0.196 | 3.99·10−4 | −3.2 (−3.1 *) |
| 2 | 0.110 | 0.967 | 0.235 | −4.00⋅10−6 |
| Sensing Layer | Method | Concentration Range, ppm | Minimal Investigated Concentration, ppm | Response/ Recovery Time, s | Temp. Range, °C | Refs. |
|---|---|---|---|---|---|---|
| CuPc | chemiresistive | 0.5–2 | 0.5 | 60/120 (0.5 ppm) | RT | [77] |
| CoPc | chemiresistive | 20–100 | 20 | ~60/240 (23 ppm) | RT | [84] |
| CoPcR8, R is 5-(trifluorome-thyl)-2-mer-captopyridine | chemiresistive | 0.3–50 | 0.3 | 20/40 (5 ppm) | RT | [80] |
| PdPc | chemiresistive | 10–50 | 10 | 25/50 (10 ppm) | RT | [21] |
| FCrPc | chemiresistive | 40–100 | 40 | 10/13 (40 ppm) | 100–400 | [85] |
| ZnPcF16 | SPR * | 100–200 | 100 | 10/30 (100 ppm) | RT | [86] |
| ZnPcF4-p | chemiresistive | 0.1–50 | 0.1 | 45/210 | RT | this work |
| ZnPcCl4-p | chemiresistive | 0.1–50 | 0.1 | 45/260 (1 ppm) | RT | this work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonegardt, D.; Klyamer, D.; Sukhikh, A.; Krasnov, P.; Popovetskiy, P.; Basova, T. Fluorination vs. Chlorination: Effect on the Sensor Response of Tetrasubstituted Zinc Phthalocyanine Films to Ammonia. Chemosensors 2021, 9, 137. https://doi.org/10.3390/chemosensors9060137
Bonegardt D, Klyamer D, Sukhikh A, Krasnov P, Popovetskiy P, Basova T. Fluorination vs. Chlorination: Effect on the Sensor Response of Tetrasubstituted Zinc Phthalocyanine Films to Ammonia. Chemosensors. 2021; 9(6):137. https://doi.org/10.3390/chemosensors9060137
Chicago/Turabian StyleBonegardt, Dmitry, Darya Klyamer, Aleksandr Sukhikh, Pavel Krasnov, Pavel Popovetskiy, and Tamara Basova. 2021. "Fluorination vs. Chlorination: Effect on the Sensor Response of Tetrasubstituted Zinc Phthalocyanine Films to Ammonia" Chemosensors 9, no. 6: 137. https://doi.org/10.3390/chemosensors9060137
APA StyleBonegardt, D., Klyamer, D., Sukhikh, A., Krasnov, P., Popovetskiy, P., & Basova, T. (2021). Fluorination vs. Chlorination: Effect on the Sensor Response of Tetrasubstituted Zinc Phthalocyanine Films to Ammonia. Chemosensors, 9(6), 137. https://doi.org/10.3390/chemosensors9060137