Implication of Sphingolipid Metabolism Gene Dysregulation and Cardiac Sphingosine-1-Phosphate Accumulation in Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Tissue Samples
2.2. Patient Characteristics
2.3. RNA Extraction and Integrity, mRNA-Seq, and ncRNA-Seq Analysis
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Statistical Methods
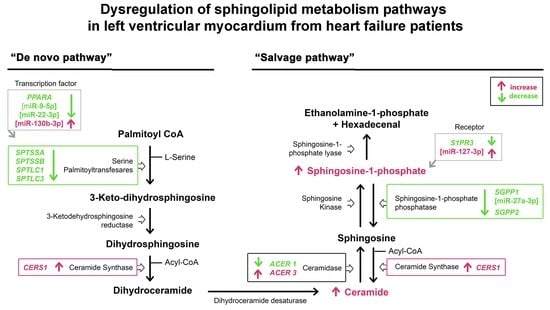
3. Results
3.1. Clinical Characteristics of HF Patients
3.2. mRNA Expression of the Sphingolipid Metabolism Genes
3.3. Expression of the miRNAs Involved in the Regulation of Sphingolipid Metabolism
3.4. Sphingolipid Levels in Heart Tissue
3.5. Relationships between Molecular Heart Tissue Levels and Ventricular Parameters of HF Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Yu, Y.; Sun, S.; Duerksen-Hughes, P.J. Ceramide and other sphingolipids in cellular responses. Cell Biochem. Biophys. 2004, 40, 323–350. [Google Scholar] [CrossRef]
- Sasset, L.; Zhang, Y.; Dunn, T.M.; Di Lorenzo, A. Sphingolipid De Novo Biosynthesis: A Rheostat of Cardiovascular Homeostasis. Trends Endocrinol. Metab. TEM 2016, 27, 807–819. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.X.; Fryer, R.M.; Hsu, A.K.; Zou, A.P.; Gross, G.J.; Campbell, W.B.; Li, P.L. Production and metabolism of ceramide in normal and ischemic-reperfused myocardium of rats. Basic Res. Cardiol. 2001, 96, 267–274. [Google Scholar] [CrossRef]
- Beresewicz, A.; Dobrzyn, A.; Gorski, J. Accumulation of specific ceramides in ischemic/reperfused rat heart; effect of ischemic preconditioning. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2002, 53, 371–382. [Google Scholar]
- Zhou, Y.T.; Grayburn, P.; Karim, A.; Shimabukuro, M.; Higa, M.; Baetens, D.; Orci, L.; Unger, R.H. Lipotoxic heart disease in obese rats: Implications for human obesity. Proc. Natl. Acad. Sci. USA 2000, 97, 1784–1789. [Google Scholar] [CrossRef] [Green Version]
- Park, T.S.; Hu, Y.; Noh, H.L.; Drosatos, K.; Okajima, K.; Buchanan, J.; Tuinei, J.; Homma, S.; Jiang, X.C.; Abel, E.D.; et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res. 2008, 49, 2101–2112. [Google Scholar] [CrossRef] [Green Version]
- Foo, R.S.; Mani, K.; Kitsis, R.N. Death begets failure in the heart. J. Clin. Investig. 2005, 115, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Karliner, J.S.; Honbo, N.; Summers, K.; Gray, M.O.; Goetzl, E.J. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J. Mol. Cell. Cardiol. 2001, 33, 1713–1717. [Google Scholar] [CrossRef]
- Knapp, M.; Baranowski, M.; Czarnowski, D.; Lisowska, A.; Zabielski, P.; Gorski, J.; Musial, W. Plasma sphingosine-1-phosphate concentration is reduced in patients with myocardial infarction. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2009, 15, CR490–CR493. [Google Scholar]
- Baranowski, M.; Gorski, J. Heart sphingolipids in health and disease. Adv. Exp. Med. Biol. 2011, 721, 41–56. [Google Scholar] [CrossRef]
- Tarazon, E.; Gil-Cayuela, C.; Manzanares, M.G.; Roca, M.; Lago, F.; Gonzalez-Juanatey, J.R.; Sanchez-Lacuesta, E.; Martinez-Dolz, L.; Portoles, M.; Rosello-Lleti, E. Circulating Sphingosine-1-Phosphate as A Non-Invasive Biomarker of Heart Transplant Rejection. Sci. Rep. 2019, 9, 13880. [Google Scholar] [CrossRef] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Rickham, P.P. Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br. Med. J. 1964, 2, 177. [Google Scholar] [CrossRef] [Green Version]
- Tarazon, E.; Perez-Carrillo, L.; Gimenez-Escamilla, I.; Ramos-Castellanos, P.; Martinez-Dolz, L.; Portoles, M.; Rosello-Lleti, E. Relationships of Telomere Homeostasis with Oxidative Stress and Cardiac Dysfunction in Human Ischaemic Hearts. Antioxidants 2021, 10, 1750. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Kassiotis, C.; Razeghi, P.; Taegtmeyer, H. Return to the fetal gene program protects the stressed heart: A strong hypothesis. Heart Fail. Rev. 2007, 12, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Son, N.H.; Park, T.S.; Yamashita, H.; Yokoyama, M.; Huggins, L.A.; Okajima, K.; Homma, S.; Szabolcs, M.J.; Huang, L.S.; Goldberg, I.J. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J. Clin. Investig. 2007, 117, 2791–2801. [Google Scholar] [CrossRef] [Green Version]
- Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Ceramide in apoptosis: An overview and current perspectives. Biochim. Biophys. Acta 2002, 1585, 114–125. [Google Scholar] [CrossRef]
- Jozefczuk, E.; Guzik, T.J.; Siedlinski, M. Significance of sphingosine-1-phosphate in cardiovascular physiology and pathology. Pharmacol. Res. 2020, 156, 104793. [Google Scholar] [CrossRef]
- Sattler, K.J.; Elbasan, S.; Keul, P.; Elter-Schulz, M.; Bode, C.; Graler, M.H.; Brocker-Preuss, M.; Budde, T.; Erbel, R.; Heusch, G.; et al. Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res. Cardiol. 2010, 105, 821–832. [Google Scholar] [CrossRef]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef]
- Knapp, M.; Zendzian-Piotrowska, M.; Kurek, K.; Blachnio-Zabielska, A. Myocardial infarction changes sphingolipid metabolism in the uninfarcted ventricular wall of the rat. Lipids 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Guillen, N.; Navarro, M.A.; Surra, J.C.; Arnal, C.; Fernandez-Juan, M.; Cebrian-Perez, J.A.; Osada, J. Cloning, characterization, expression and comparative analysis of pig Golgi membrane sphingomyelin synthase 1. Gene 2007, 388, 117–124. [Google Scholar] [CrossRef]
- Albi, E.; Cataldi, S.; Bartoccini, E.; Magni, M.V.; Marini, F.; Mazzoni, F.; Rainaldi, G.; Evangelista, M.; Garcia-Gil, M. Nuclear sphingomyelin pathway in serum deprivation-induced apoptosis of embryonic hippocampal cells. J. Cell. Physiol. 2006, 206, 189–195. [Google Scholar] [CrossRef]
- Tu, R.; Yang, W.; Hu, Z. Inhibition of sphingomyelin synthase 1 affects ceramide accumulation and hydrogen peroxide-induced apoptosis in Neuro-2a cells. Neuroreport 2016, 27, 967–973. [Google Scholar] [CrossRef]
- Separovic, D.; Semaan, L.; Tarca, A.L.; Awad Maitah, M.Y.; Hanada, K.; Bielawski, J.; Villani, M.; Luberto, C. Suppression of sphingomyelin synthase 1 by small interference RNA is associated with enhanced ceramide production and apoptosis after photodamage. Exp. Cell Res. 2008, 314, 1860–1868. [Google Scholar] [CrossRef] [Green Version]
- Separovic, D.; Hanada, K.; Maitah, M.Y.; Nagy, B.; Hang, I.; Tainsky, M.A.; Kraniak, J.M.; Bielawski, J. Sphingomyelin synthase 1 suppresses ceramide production and apoptosis post-photodamage. Biochem. Biophys. Res. Commun. 2007, 358, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Hailemariam, T.K.; Zhou, H.; Li, Y.; Duckworth, D.C.; Peake, D.A.; Zhang, Y.; Kuo, M.S.; Cao, G.; Jiang, X.C. Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization. Biochim. Biophys. Acta 2007, 1771, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- Tafesse, F.G.; Huitema, K.; Hermansson, M.; van der Poel, S.; van den Dikkenberg, J.; Uphoff, A.; Somerharju, P.; Holthuis, J.C. Both sphingomyelin synthases SMS1 and SMS2 are required for sphingomyelin homeostasis and growth in human HeLa cells. J. Biol. Chem. 2007, 282, 17537–17547. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef] [Green Version]
- Karbowska, J.; Kochan, Z.; Smolenski, R.T. Peroxisome proliferator-activated receptor alpha is downregulated in the failing human heart. Cell. Mol. Biol. Lett. 2003, 8, 49–53. [Google Scholar]
- Ji, R.; Akashi, H.; Drosatos, K.; Liao, X.; Jiang, H.; Kennel, P.J.; Brunjes, D.L.; Castillero, E.; Zhang, X.; Deng, L.Y.; et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2017, 2, e96203. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| mRNA-Seq (n = 26) | ncRNA-Seq (n = 42) | ELISA (n = 36) | |
|---|---|---|---|
| Age (years) | 53 ± 9 | 52 ± 10 | 52 ± 10 |
| Gender male (%) | 96 | 93 | 87 |
| NYHA class | III-IV | III-IV | III-IV |
| BMI (kg/m2) | 27 ± 5 | 26 ± 4 | 27 ± 6 |
| Hemoglobin (mg/dL) | 14 ± 3 | 13 ± 2 | 14 ± 2 |
| Hematocrit (%) | 40 ± 7 | 40 ± 6 | 41 ± 5 |
| Total cholesterol (mg/dL) | 155 ± 39 | 159 ± 45 | 164 ± 50 |
| Prior hypertension (%) | 25 | 31 | 21 |
| Prior smoking (%) | 71 | 71 | 75 |
| Diabetes mellitus (%) | 29 | 30 | 35 |
| LVEF (%) | 21 ± 8 | 21 ± 8 | 22 ± 9 |
| LVESD (mm) | 66 ± 12 | 61 ± 12 | 63 ± 12 |
| LVEDD (mm) | 74 ± 11 | 69 ± 12 | 71 ± 11 |
| Left ventricular mass (g) | 362 ± 142 | 316 ± 120 | 341 ± 109 |
| Left ventricle mass index (g/m2) | 194 ± 76 | 166 ± 60 | 180 ± 65 |
| Duration of disease (months) # | 59 ± 56 | 44 ± 38 | 57 ± 52 |
| LV Mass | LVESD | LVEDD | |
|---|---|---|---|
| CERS1 | r = 0.797 p < 0.0001 | r = 0.561 p = 0.012 | r = 0.601 p = 0.007 |
| S1P | r = −0.550 p = 0.052 | r = −0.552 p = 0.041 | r = −0.541 p = 0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Carrillo, L.; Giménez-Escamilla, I.; Martínez-Dolz, L.; Sánchez-Lázaro, I.J.; Portolés, M.; Roselló-Lletí, E.; Tarazón, E. Implication of Sphingolipid Metabolism Gene Dysregulation and Cardiac Sphingosine-1-Phosphate Accumulation in Heart Failure. Biomedicines 2022, 10, 135. https://doi.org/10.3390/biomedicines10010135
Pérez-Carrillo L, Giménez-Escamilla I, Martínez-Dolz L, Sánchez-Lázaro IJ, Portolés M, Roselló-Lletí E, Tarazón E. Implication of Sphingolipid Metabolism Gene Dysregulation and Cardiac Sphingosine-1-Phosphate Accumulation in Heart Failure. Biomedicines. 2022; 10(1):135. https://doi.org/10.3390/biomedicines10010135
Chicago/Turabian StylePérez-Carrillo, Lorena, Isaac Giménez-Escamilla, Luis Martínez-Dolz, Ignacio José Sánchez-Lázaro, Manuel Portolés, Esther Roselló-Lletí, and Estefanía Tarazón. 2022. "Implication of Sphingolipid Metabolism Gene Dysregulation and Cardiac Sphingosine-1-Phosphate Accumulation in Heart Failure" Biomedicines 10, no. 1: 135. https://doi.org/10.3390/biomedicines10010135
APA StylePérez-Carrillo, L., Giménez-Escamilla, I., Martínez-Dolz, L., Sánchez-Lázaro, I. J., Portolés, M., Roselló-Lletí, E., & Tarazón, E. (2022). Implication of Sphingolipid Metabolism Gene Dysregulation and Cardiac Sphingosine-1-Phosphate Accumulation in Heart Failure. Biomedicines, 10(1), 135. https://doi.org/10.3390/biomedicines10010135