Pathophysiology and Outcomes of Endothelium Function in Coronary Microvascular Diseases: A Systematic Review of Randomized Controlled Trials and Multicenter Study
Abstract
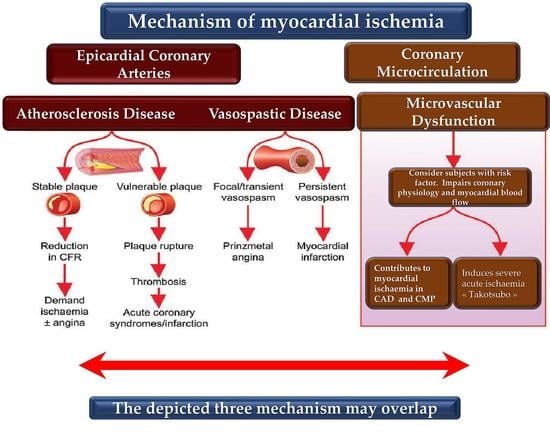
:1. Introduction
2. Methods
Data Collection, Merging, and Endpoint of the Study
3. Results
3.1. Endothelial Modulation of the Vascular Tone Insight on Blood Vessel Size-Dependent
Contribution of the EDRF
3.2. Evidence from Coronary Microvascular Disease Deploying
3.2.1. Insights on Mechanisms, Prevalence, and Clinical Significance of CMD
3.2.2. Lesson from CMD: A Systemic Vascular Disease Not Confined to the Heart
3.3. Impact of Primary Coronary Microcirculatory Dysfunction on Vulnerable Patients
3.3.1. Simultaneous Occurrence of Endothelium-Dependent CMD and Advanced Coronary Atherosclerosis: A Dangerous Mixture
3.3.2. Endothelium-Dependent CMD and Local Low Shear Stress
3.3.3. Vulnerable Microcirculation Opinion
4. A look at the Clinic and Therapy
4.1. Smoking and Vaping as a Risk Factor for Coronary Macrovascular and Microvascular Diseases
4.2. To Exceed or Not in the Treatment with NO?
4.3. An Overview of Clinical Trials Suggesting an Approach Focused on Endothelial Function and Coronary Microvascular Function
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sabe, S.A.; Feng, J.; Sellke, F.W.; Abid, M.R. Mechanisms and clinical implications of endothelium-dependent vasomotor dys-function in coronary microvasculature. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H819–H841. [Google Scholar] [CrossRef] [PubMed]
- Crea, F.; Lanza, G.A.; Camici, P.G. Mechanisms of Coronary Microvascular Dysfunction. In Coronary Microvascular Dysfunction; Springer: Milan, Italy, 2014; pp. 31–47. [Google Scholar] [CrossRef]
- Crea, F.; Lanza, G.A. New Light on a Forgotten Disease: Vasospastic Angina. J. Am. Coll. Cardiol. 2011, 58, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Beijk, M.A.; Vlastra, W.V.; Delewi, R.; van de Hoef, T.P.; Boekholdt, S.M.; Sjauw, K.D.; Piek, J.J. Myocardial infarction with non-obstructive coronary arteries: A focus on vasospastic angina. Neth. Hear. J. 2019, 27, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Shimokawa, H. Coronary Vasomotion Abnormalities; Springer: Singapore, 2021; 155p. [Google Scholar]
- Kikuchi, Y.; Yasuda, S.; Aizawa, K.; Tsuburaya, R.; Ito, Y.; Takeda, M.; Nakayama, M.; Ito, K.; Takahashi, J.; Shimokawa, H. Enhanced Rho-Kinase Activity in Circulating Neutrophils of Patients with Vasospastic Angina: A Possible Biomarker for Diagnosis and Disease Activity Assessment. J. Am. Coll. Cardiol. 2011, 58, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Nihei, T.; Takahashi, J.; Hao, K.; Kikuchi, Y.; Odaka, Y.; Tsuburaya, R.; Nishimiya, K.; Matsumoto, Y.; Ito, K.; Miyata, S.; et al. Prognostic impacts of Rho-kinase activity in circulating leucocytes in patients with vasospastic angina. Eur. Heart J. 2018, 39, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Matsumoto, Y.; Takanami, K.; Ota, H.; Nishimiya, K.; Sugisawa, J.; Tsuchiya, S.; Amamizu, H.; Uzuka, H.; Suda, A.; et al. Coronary Adventitial and Perivascular Adipose Tissue Inflammation in Patients with Vasospastic Angina. J. Am. Coll. Cardiol. 2018, 71, 414–425. [Google Scholar] [CrossRef]
- Mohri, M.; Shimokawa, H.; Hirakawa, Y.; Masumoto, A.; Takeshita, A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J. Am. Coll. Cardiol. 2002, 41, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Masumoto, A.; Mohri, M.; Shimokawa, H.; Urakami, L.; Usui, M.; Takeshita, A. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002, 105, 1545–1547. [Google Scholar] [CrossRef] [Green Version]
- Kataruka, A.; Maynard, C.C.; Kearney, K.E.; Mahmoud, A.; Bell, S.; Doll, J.A.; McCabe, J.M.; Bryson, C.; Gurm, H.S.; Jneid, H.; et al. Temporal Trends in Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting: Insights from the Washington Cardiac Care Outcomes Assessment Program. J. Am. Hear. Assoc. 2020, 9, e015317. [Google Scholar] [CrossRef]
- Alkhouli, M.; Alqahtani, F.; Kalra, A.; Gafoor, S.; Alhajji, M.; Alreshidan, M.; Holmes, D.R.; Lerman, A. Trends in characteristics and outcomes of patients undergoing coronary revascu-larization in the United States, 2003–2016. JAMA Netw. Open 2020, 3, e1921326. [Google Scholar] [CrossRef]
- Shah, S.J.; Lam, C.S.P.; Svedlund, S.; Saraste, A.; Hage, C.; Tan, R.S.; Beussink-Nelson, L.; Ljung Faxén, U.; Fermer, M.L.; Broberg, M.A.; et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur. Heart J. 2018, 39, 3439–3450. [Google Scholar] [CrossRef]
- Svedlund, S.; Saraste, A.; Faxén, U.L.; Benson, L.; Fermer, M.L.; Gan, L.M.; Shah, S.J.; Lam, C.S.P.; Lund, L.H. Association of coronary microvascular dysfunction with heart failure hospitalizations and mortality in heart failure with preserved ejection fraction: A follow-up in the PROMIS-HFpEF study. J. Card. Fail. 2020, 26, 1016–1021. [Google Scholar]
- Ahmad, A.; Corban, M.T.; Toya, T.; Verbrugge, F.H.; Sara, J.D.; Lerman, L.O.; Borlaug, B.A.; Lerman, A. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 23, 765–772. [Google Scholar] [CrossRef]
- Crea, F.; Merz, C.N.B.; Beltrame, J.F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Camici, P.G. On behalf of the Coronary Vasomotion Disorders International Study Group (COVADIS) The parallel tales of microvascular angina and heart failure with preserved ejection fraction: A paradigm shift. Eur. Hear. J. 2016, 38, 473–477. [Google Scholar] [CrossRef]
- Dryer, K.; Gajjar, M.; Narang, N.; Lee, M.; Paul, J.; Shah, A.P.; Nathan, S.; Butler, J.; Davidson, C.J.; Fearon, W.F.; et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am. J. Physiol. Circ. Physiol. 2018, 314, H1033–H1042. [Google Scholar] [CrossRef]
- Suhrs, H.E.; Schroder, J.; Bové, K.B.; Mygind, N.D.; Frestad, D.; Michelsen, M.M.; Lange, T.; Gustafsson, I.; Kastrup, J.; Prescott, E. Inflammation, non-endothelial dependent coronary microvascular function and diastolic function-are they linked ? PLoS ONE 2020, 15, e0236035. [Google Scholar] [CrossRef]
- Yang, J.H.; Obokata, M.; Reddy, Y.N.V.; Redfield, M.M.; Lerman, A.; Borlaug, B.A. Endothelium-dependent and independent coro-nary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2020, 22, 432–441. [Google Scholar] [CrossRef]
- Von Mering, G.O.; Arant, C.B.; Wessel, T.R.; McGorray, S.P.; Merz, C.N.B.; Sharaf, B.L.; Smith, K.M.; Olson, M.B.; Johnson, B.D.; Sopko, G.; et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: Results from the national heart, lung, and blood institute-sponsored. Women’s ischemia syndrome evaluation (WISE). Circulation 2004, 109, 722–725. [Google Scholar] [CrossRef] [Green Version]
- Lerman, A.; Sopko, G. Women and cardiovascular heart disease: Clinical implications from the Women’s Ischemia Syndrome Evaluation (WISE) Study. Are we smarter? J. Am. Coll. Cardiol. 2006, 47 (Suppl. 3), S59–S62. [Google Scholar] [CrossRef] [Green Version]
- Murphy, V.L.; Naya, M.; Taqueti, V.R.; Foster, C.R.; Gaber, M.; Hainer, J.; Dorbala, S.; Blankstein, R.; Rimoldi, O.; Camici, P.G.; et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014, 129, 2518–2527. [Google Scholar] [CrossRef]
- Sara, J.D.; Widmer, R.J.; Matsuzawa, Y.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prevalence of Coronary Microvascular Dysfunction Among Patients with Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Hansen, H.S.; Sechtem, U.; Prescott, E.; Ong, P. Sex-Related Differences in Vasomotor Function in Patients With Angina and Unobstructed Coronary Arteries. J. Am. Coll. Cardiol. 2017, 70, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Motiejūnaitė, J.; Akiyama, E.; Cohen-Solal, A.; Maggioni, A.P.; Mueller, C.; Choi, D.J.; Kavoliūnienė, A.; Čelutkienė, J.; Parenica, J.; Lassus, J.; et al. The association of long-term outcome and biological sex in patients with acute heart failure from different geographic regions. Eur. Heart J. 2020, 41, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; Michelsen, M.M.; Mygind, N.D.; E Suhrs, H.; Bove, K.B.; Bechsgaard, D.F.; Aziz, A.; Gustafsson, I.; Kastrup, J.; Prescott, E. Coronary flow velocity reserve predicts adverse prognosis in women with angina and no obstructive coronary artery disease: Results from the iPOWER study. Eur. Hear. J. 2020, 42, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Zhou, W.; Bajaj, N.; Gupta, A.; Sun, Y.-P.; Divakaran, S.; Bibbo, C.; Hainer, J.; Taqueti, V.; Dorbala, S.; Blankstein, R.; et al. Coronary microvascular dysfunction, left ventricular remodeling, and clinical outcomes in aortic stenosis. J. Nucl. Cardiol. 2019, 28, 579–588. [Google Scholar] [CrossRef]
- Kakuta, K.; Dohi, K.; Yamamoto, T.; Fujimoto, N.; Shimoyama, T.; Umegae, S.; Ito, M. Coronary Microvascular Dysfunction Restored After Surgery in Inflammatory Bowel Disease: A Prospective Observational Study. J. Am. Heart Assoc. 2021, 10, e019125. [Google Scholar] [CrossRef]
- Antoniades, C.; Shirodaria, C.; Leeson, P.; Antonopoulos, A.; Warrick, N.; Van-Assche, T.; Cunnington, C.; Tousoulis, D.; Pillai, R.; Ratnatunga, C.; et al. Association of plasma asymmetrical dimethylarginine (ADMA) with elevated vascular superoxide production and endothelial nitric oxide synthase uncoupling: Implications for endothelial function in human atherosclerosis. Eur. Heart J. 2009, 30, 1142–1150. [Google Scholar] [CrossRef] [Green Version]
- Antoniades, C.; Demosthenous, M.; Tousoulis, D.; Antonopoulos, A.; Vlachopoulos, C.; Toutouza, M.; Marinou, K.; Bakogiannis, C.; Mavragani, K.; Lazaros, G.; et al. Role of Asymmetrical Dimethylarginine in Inflammation-Induced Endothelial Dysfunction in Human Atherosclerosis. Hypertension 2011, 58, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Con-tributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef]
- Kakuta, K.; Dohi, K.; Sato, Y.; Yamanaka, T.; Kawamura, M.; Ogura, T.; Nakamori, S.; Fujimoto, N.; Fujii, E.; Yamada, N. Chronic in-flammatory disease is an independent risk factor for coronary flow velocity reserve impairment unrelated to the processes of coronary artery calcium deposition. J. Am. Soc. Echocardiogr. 2016, 29, 173–180. [Google Scholar] [CrossRef]
- Piaserico, S.; Osto, E.; Famoso, G.; Zanetti, I.; Gregori, D.; Poretto, A.; Iliceto, S.; Peserico, A.; Tona, F. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis 2016, 251, 25–30. [Google Scholar] [CrossRef]
- Weber, B.; Perez-Chada, L.M.; Divakaran, S.; Brown, J.M.; Taqueti, V.; Dorbala, S.; Blankstein, R.; Liao, K.; Merola, J.F.; Di Carli, M. Coronary microvascular dysfunction in patients with psoriasis. J. Nucl. Cardiol. 2020, 29, 37–42. [Google Scholar] [CrossRef]
- Vita, T.; Murphy, D.J.; Osborne, M.T.; Bajaj, N.S.; Keraliya, A.; Jacob, S.; Martinez, A.J.D.; Nodoushani, A.; Bravo, P.; Hainer, J.; et al. Association between Nonalcoholic Fatty Liver Disease at CT and Coronary Microvascular Dysfunction at Myocardial Perfusion PET/CT. Radiology 2019, 291, 330–337. [Google Scholar] [CrossRef]
- Gao, B.; Zhu, D.; Xie, J.; Wu, B.; Xu, P.; Liu, J.; Tong, X.; Chen, R.; Zhu, L.; Zhou, L.; et al. A clinical trial for computed tomography myocardial perfusion based non-invasive index of microcirculatory resistance (MPBIMR): Rationale and trial design. Am. J. Transl. Res. 2022, 14, 5552–5562. [Google Scholar]
- Zhai, C.; Fan, H.; Zhu, Y.; Chen, Y.; Shen, L. Coronary functional assessment in non-obstructive coronary artery disease: Present situation and future direction. Front. Cardiovasc. Med. 2022, 9, 934279. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L. Ischemia and no obstructive coronary artery disease (INOCA): Developing evidence-based therapies and research agenda for the next decade. Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef]
- Lee, B.K.; Lim, H.S.; Fearon, W.F.; Yong, A.S.; Yamada, R.; Tanaka, S.; Lee, D.P.; Yeung, A.C.; Tremmel, J. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation 2015, 131, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Ford, T.; Yii, E.; Sidik, N.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; et al. Ischemia and No Obstructive Coronary Artery Disease. Circ. Cardiovasc. Interv. 2019, 12, e008126. [Google Scholar] [CrossRef]
- Suda, A.; Takahashi, J.; Hao, K.; Kikuchi, Y.; Shindo, T.; Ikeda, S.; Sato, K.; Sugisawa, J.; Matsumoto, Y.; Miyata, S.; et al. Coronary Functional Abnormalities in Patients With Angina and Nonobstructive Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 74, 2350–2360. [Google Scholar] [CrossRef]
- Hillis, L.D.; Smith, P.K.; Anderson, J.L.; Bittl, J.A.; Bridges, C.R.; Byrne, J.G.; Cigarroa, J.E.; Disesa, V.J.; Hiratzka, L.F.; Hunter, A.M., Jr.; et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2011, 58, e123–e210. [Google Scholar] [PubMed]
- Hannan, E.L.; Racz, M.J.; Gold, J.; Cozzens, K.; Stamato, N.J.; Powell, T.; Hibberd, M.; Walford, G.; American College of Cardiology; American Heart Association. Adherence of catheterization laboratory cardiologists to American College of Cardiology/American Heart Association guidelines for percutaneous coronary interventions and coronary artery bypass graft surgery: What happens in actual practice? Circulation 2010, 121, 267–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Head, S.J.; Kaul, S.; Mack, M.J.; Serruys, P.W.; Taggart, D.P.; Holmes, J.D.R.; Leon, M.B.; Marco, J.; Bogers, A.J.J.C.; Kappetein, A.P. The rationale for Heart Team decision-making for patients with stable, complex coronary artery disease. Eur. Heart J. 2013, 34, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Peterffy, Á.; Molnár, F.; Sipos, D.; Maros, T.; Kőszegi, Z. Thirty-five-year angiographic follow-up of the first coronary bypass surgery by internal mammary artery in Hungary. Orv Hetil. 2020, 161, 354–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hlatky, M.A.; Boothroyd, D.B.; Bravata, D.M.; Boersma, E.; Booth, J.; Brooks, M.M.; Carrié, D.; Clayton, T.C.; Danchin, N.; Flather, M.; et al. Coronary artery bypass surgery compared with percutaneous coronary in-terventions for multivessel disease: A collaborative analysis of individual patient data from ten randomised trials. Lancet 2009, 373, 1190–1197. [Google Scholar] [CrossRef]
- Al-Lamee, R.; Thompson, D.; Dehbi, H.M.; Sen, S.; Tang, K.; Davies, J.; Keeble, T.; Mielewczik, M.; Kaprielian, R.; Malik, I.S.; et al. Percutaneous coronary intervention in stable angina (ORBITA): A double-blind, randomised controlled trial. Lancet 2018, 391, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Shimokawa, H. 2014 Williams Harvey Lecture: Importance of coronary vasomotion abnormalities—From bench to bedside. Eur. Hear. J. 2014, 35, 3180–3193. [Google Scholar] [CrossRef] [Green Version]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H.C. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2015, 219, 22–96. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Feletou, M.; Vanhoutte, P.M. EDHF: An update. Clin. Sci. 2009, 117, 139–155. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Chen, X.Y.; Dong, H.; Xu, F. Cyclopiazonic Acid-Induced Ca2+ Store Depletion Initiates Endothelium-Dependent Hyperpolarization-Mediated Vasorelaxation of Mesenteric Arteries in Healthy and Colitis Mice. Front. Physiol. 2021, 12, 639857. [Google Scholar] [CrossRef]
- Delgado, N.T.B.; Rouver, W.D.N.; Freitas-Lima, L.C.; Vieira-Alves, I.; Lemos, V.S.; dos Santos, R.L. Sex Differences in the Vasodilation Mediated by G Protein-Coupled Estrogen Receptor (GPER) in Hypertensive Rats. Front. Physiol. 2021, 12, 659291. [Google Scholar] [CrossRef]
- Campbell, W.B.; Fleming, I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflügers Arch. Eur. J. Physiol. 2010, 459, 881–895. [Google Scholar] [CrossRef] [Green Version]
- Campbell, W.B.; Gebremedhin, D.; Pratt, P.F.; Harder, D.R. Identification of Epoxyeicosatrienoic Acids as Endothelium-Derived Hyperpolarizing Factors. Circ. Res. 1996, 78, 415–423. [Google Scholar] [CrossRef]
- Charles, R.; Eaton, P. Redox Regulation of Soluble Epoxide Hydrolase—Implications for Cardiovascular Health and Disease. Cells 2022, 11, 1932. [Google Scholar] [CrossRef]
- Feugray, G.; Pereira, T.; Iacob, M.; Moreau-Grangé, L.; Prévost, G.; Brunel, V.; Joannidès, R.; Bellien, J.; Duflot, T. Determination of Lipoxygenase, CYP450, and Non-Enzymatic Metabolites of Arachidonic Acid in Essential Hypertension and Type 2 Diabetes. Metabolites 2022, 12, 859. [Google Scholar] [CrossRef]
- Fisslthaler, B.; Popp, R.; Kiss, L.; Potente, M.; Harder, D.R.; Fleming, I.; Busse, R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 1999, 401, 493–497. [Google Scholar] [CrossRef]
- Taylor, H.J.; Chaytor, A.T.; Edwards, D.H.; Griffith, T.M. Gap Junction-Dependent Increases in Smooth Muscle cAMP Underpin the EDHF Phenomenon in Rabbit Arteries. Biochem. Biophys. Res. Commun. 2001, 283, 583–589. [Google Scholar] [CrossRef]
- Griffith, T.M.; Chaytor, A.T.; Taylor, H.J.; Giddings, B.D.; Edwards, D.H. cAMP facilitates EDHF-type relaxations in conduit arteries by enhancing electrotonic conduction via gap junctions. Proc. Natl. Acad. Sci. USA 2002, 99, 6392–6397. [Google Scholar] [CrossRef] [Green Version]
- Griffith, T.M.; Chaytor, A.T.; Edwards, D.H. The obligatory link: Role of gap junctional communication in endotheli-um-dependent smooth muscle hyperpolarization. Pharmacol. Res. 2004, 49, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Endothelial Ion Channels and Cell-Cell Communication in the Microcirculation. Front. Physiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Dora, K.A.; Gardener, M.J.; Garland, C.J.; Weston, A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 1998, 396, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H.A.; Tare, M.; Parkington, H.C. Endothelial potassium channels, endothelium-dependent hyperpolarization and the regulation of vascular tone in health and disease. Clin. Exp. Pharmacol. Physiol. 2004, 31, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shackelford, R.E.; Shen, X.; Dominic, P.; Kevil, C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2022. [Google Scholar] [CrossRef]
- Cheng, Z.; Shen, X.; Jiang, X.; Shan, H.; Cimini, M.; Fang, P.; Ji, Y.; Park, J.Y.; Drosatos, K.; Yang, X.; et al. Hyperhomocysteinemia potentiates diabetes-impaired EDHF-induced vascular relaxation: Role of insufficient hydrogen sulfide. Redox Biol. 2018, 16, 215–225. [Google Scholar] [CrossRef]
- Tang, G.; Yang, G.; Jiang, B.; Ju, Y.; Wu, L.; Wang, R. H2S Is an Endothelium-Derived Hyperpolarizing Factor. Antioxidants Redox Signal. 2013, 19, 1634–1646. [Google Scholar] [CrossRef]
- Mustafa, A.K.; Sikka, G.; Gazi, S.K.; Steppan, J.; Jung, S.M.; Bhunia, A.K.; Barodka, V.M.; Gazi, F.K.; Barrow, R.K.; Wang, R.; et al. Hydrogen sulfide as endothelium derived hyperpolarizing factor sulfhydrates potas-sium channels. Circ. Res. 2011, 109, 1259–1268. [Google Scholar] [CrossRef]
- Dubuis, E.; Gautier, M.; Melin, A.; Rebocho, M.; Girardin, C.; Bonnet, P.; Vandier, C. Chronic carbon monoxide enhanced IbTx-sensitive currents in rat resistance pulmonary artery smooth muscle cells. Am. J. Physiol. Cell. Mol. Physiol. 2002, 283, L120–L129. [Google Scholar] [CrossRef] [Green Version]
- Barbé, C.; Dubuis, E.; Rochetaing, A.; Kreher, P.; Bonnet, P.; Vandier, C. A 4-AP-sensitive current is enhanced by chronic carbon monoxide exposure in coronary artery myocytes. Am. J. Physiol. Circ. Physiol. 2002, 282, H2031–H2038. [Google Scholar] [CrossRef] [Green Version]
- Fujiki, T.; Shimokawa, H.; Morikawa, K.; Kubota, H.; Hatanaka, M.; Talukder, M.H.; Matoba, T.; Takeshita, A.; Sunagawa, K. Endothelium-Derived Hydrogen Peroxide Accounts for the Enhancing Effect of an Angiotensin-Converting Enzyme Inhibitor on Endothelium-Derived Hyperpolarizing Factor–Mediated Responses in Mice. Arter. Thromb. Vasc. Biol. 2005, 25, 766–771. [Google Scholar] [CrossRef]
- Matoba, T.; Shimokawa, H.; Nakashima, M.; Hirakawa, Y.; Mukai, Y.; Hirano, K.; Kanaide, H.; Takeshita, A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Investig. 2000, 106, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Miura, H.; Liu, Y.; Gutterman, D.D. Human Coronary Arteriolar Dilation to Bradykinin Depends on Membrane Hyperpolarization. Circulation 1999, 99, 3132–3138. [Google Scholar] [CrossRef] [Green Version]
- Miura, H.; Gutterman, D.D. Human coronary arteriolar dilation to arachidonic acid depends on cytochrome P-450 monooxy-genase and Ca2+− activated K+ channels. Circ Res. 1998, 83, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Miura, H.; Wachtel, R.E.; Liu, Y.; LoberizaJr, F.R.; Saito, T.; Miura, M.; Gutterman, D.D. Flow-Induced Dilation of Human Coronary Arterioles. Circulation 2001, 103, 1992–1998. [Google Scholar] [CrossRef] [Green Version]
- Oltman, C.L.; Weintraub, N.L.; VanRollins, M.; Dellsperger, K.C. Epoxyeicosatrienoic Acids and Dihydroxyeicosatrienoic Acids Are Potent Vasodilators in the Canine Coronary Microcirculation. Circ. Res. 1998, 83, 932–939. [Google Scholar] [CrossRef] [Green Version]
- Edwards, G.; Thollon, C.; Gardener, M.J.; Félétou, M.; Vilaine, J.-P.; Vanhoutte, P.M.; Weston, A.H. Role of gap junctions and EETs in endothelium-dependent hyperpolarization of porcine coronary artery. J. Cereb. Blood Flow Metab. 2000, 129, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Gauthier, K.M.; Edwards, E.M.; Falck, J.R.; Reddy, D.S.; Campbell, W.B. 14,15-epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension 2005, 45, 666–671. [Google Scholar] [CrossRef] [Green Version]
- Bény, J.-L.; Schaad, O. An evaluation of potassium ions as endothelium-derived hyperpolarizing factor in porcine coronary arteries. J. Cereb. Blood Flow Metab. 2000, 131, 965–973. [Google Scholar] [CrossRef]
- McNeish, A.J.; Wilson, W.S.; Martin, W. Dominant role of an endothelium-derived hyperpolarizing factor (EDHF)-like vasodilator in the ciliary vascular bed of the bovine isolated perfused eye. J. Cereb. Blood Flow Metab. 2001, 134, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Nelli, S.; Wilson, W.S.; Laidlaw, H.; Llano, A.; Middleton, S.; Price, A.G.; Martin, W. Evaluation of potassium ion as the endotheli-um-derived hyperpolarizing factor (EDHF) in the bovine coronary artery. Br. J. Pharmacol. 2003, 139, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Bosnjak, J.J.; Ning, G.; Saito, T.; Miura, M.; Gutterman, D.D. Role for Hydrogen Peroxide in Flow-Induced Dilation of Human Coronary Arterioles. Circ. Res. 2003, 92, e31–e40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Bubolz, A.H.; Mendoza, S.; Zhang, D.X.; Gutterman, D.D. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res. 2011, 108, 566–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matoba, T.; Shimokawa, H.; Morikawa, K.; Kubota, H.; Kunihiro, I.; Urakami-Harasawa, L.; Mukai, Y.; Hirakawa, Y. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary mi-crovessels. Arterioscler Thromb. Vasc Biol. 2003, 23, 1224–1230. [Google Scholar] [CrossRef] [Green Version]
- Yada, T.; Shimokawa, H.; Hiramatsu, O.; Haruna, Y.; Morita, Y.; Kashihara, N.; Shinozaki, Y.; Mori, H.; Goto, M.; Ogasawara, Y.; et al. Cardioprotective role of endogenous hydrogen peroxide during ischemia-reperfusion injury in canine coronary microcir-culation in vivo. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1138–H1146. [Google Scholar] [CrossRef] [Green Version]
- Yada, T.; Shimokawa, H.; Hiramatsu, O.; Shinozaki, Y.; Mori, H.; Goto, M.; Ogasawara, Y.; Kajiya, F. Important Role of Endogenous Hydrogen Peroxide in Pacing-Induced Metabolic Coronary Vasodilation in Dogs In Vivo. J. Am. Coll. Cardiol. 2007, 50, 1272–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yada, T.; Shimokawa, H.; Tachibana, H. Endothelium-dependent hyperpolarization-mediated vasodilatation compensates nitric oxide-mediated endothelial dysfunction during ischemia in diabetes-induced canine coronary collateral microcirculation in vivo. Microcirculation 2018, 25, e12456. [Google Scholar] [CrossRef]
- Mani, S.; Li, H.; Untereiner, A.; Wu, L.; Yang, G.; Austin, R.C.; Dickhout, J.G.; Lhoták, Š.; Meng, Q.H.; Wang, R. Decreased Endogenous Production of Hydrogen Sulfide Accelerates Atherosclerosis. Circulation 2013, 127, 2523–2534. [Google Scholar] [CrossRef] [Green Version]
- Chai, Q.; Lu, T.; Wang, X.-L.; Lee, H.-C. Hydrogen sulfide impairs shear stress-induced vasodilation in mouse coronary arteries. Pflügers Arch. Eur. J. Physiol. 2014, 467, 329–340. [Google Scholar] [CrossRef]
- Naik, J.S.; Osmond, J.M.; Walker, B.R.; Kanagy, N.L. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Am. J. Physiol. Circ. Physiol. 2016, 311, H1437–H1444. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Shimokawa, H.; Yasutake, H.; Fujii, K.; Owada, M.K.; Nakaike, R.; Fukumoto, Y.; Takayanagi, T.; Nagao, T.; Egashira, K.; Fujishima, M.; et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endotheli-um-dependent relaxations in rat mesenteric circulation. J. Cardiovasc. Pharmacol. 1996, 28, 703–711. [Google Scholar] [CrossRef]
- Urakami-Harasawa, L.; Shimokawa, H.; Nakashima, M.; Egashira, K.; Takeshita, A. Importance of endothelium-derived hy-perpolarizing factor in human arteries. J. Clin. Investig. 1997, 100, 2793–2799. [Google Scholar] [CrossRef] [Green Version]
- Godo, S.; Sawada, A.; Saito, H.; Ikeda, S.; Enkhjargal, B.; Suzuki, K.; Tanaka, S.; Shimokawa, H. Disruption of Physiological Balance Between Nitric Oxide and Endothelium-Dependent Hyperpolarization Impairs Cardiovascular Homeostasis in Mice. Arter. Thromb. Vasc. Biol. 2016, 36, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Crea, F.; Lanza, G.A.; Camici, P.G. Physiology of Coronary Microcirculation. In Coronary Microvascular Dysfunction; Springer: Milan, Italy, 2014; pp. 3–30. [Google Scholar]
- Shimokawa, H. Reactive oxygen species in cardiovascular health and disease: Special references to nitric oxide, hydrogen peroxide, and Rho-kinase. J. Clin. Biochem. Nutr. 2020, 66, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, Y.; Stepp, D.W.; Chilian, W.M. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am. J. Physiol. Circ. Physiol. 2000, 279, H459–H465. [Google Scholar] [CrossRef]
- Burgoyne, J.R.; Prysyazhna, O.; Rudyk, O.; Eaton, P. cGMP-dependent activation of protein kinase G precludes disulfide activa-tion: Implications for blood pressure control. Hypertension 2012, 60, 1301–1308. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, J.; Sawada, A.; Nakajima, S.; Noda, K.; Takaki, A.; Shimokawa, H. Mechanisms for enhanced endothelium-derived hy-perpolarizing factor-mediated responses in microvessels in mice. Circ. J. 2012, 76, 1768–1779. [Google Scholar] [CrossRef] [Green Version]
- Cassar, A.; Chareonthaitawee, P.; Rihal, C.S.; Prasad, A.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Lack of correlation between non-invasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ. Cardiovasc. Interv. 2009, 2, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Iribarren, A.; Diniz, M.A.; Merz, C.N.B.; Shufelt, C.; Wei, J. Are we any WISER yet? Progress and contemporary need for smart trials to include women in coronary artery disease trials. Contemp. Clin. Trials 2022, 117, 10662. [Google Scholar] [CrossRef]
- Mileva, N.; Nagumo, S.; Mizukami, T.; Sonck, J.; Berry, C.; Gallinoro, E.; Monizzi, G.; Candreva, A.; Munhoz, D.; Vassilev, D.; et al. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients with Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, 023207. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, H.R.; Merz, C.N.B.; Berry, C.; Samuel, R.; Saw, J.; Smilowitz, N.R.; de Souza, A.C.D.A.; Sykes, R.; Taqueti, V.R.; Wei, J. Coronary Arterial Function and Disease in Women with No Obstructive Coronary Arteries. Circ. Res. 2022, 130, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M.A.; Löffler, A.I.; Ouellette, M.; Smith, L.; Kramer, C.M.; Bourque, J.M. Coronary Microvascular Dysfunction, Microvascular Angina, and Treatment Strategies. JACC Cardiovasc. Imaging 2015, 8, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Ong, P.; Camici, P.G.; Beltrame, J.F.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Merz, C.N.B.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018, 250, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Johnson, N.P. Coronary Physiology Beyond Coronary Flow Reserve in Microvascular Angina. J. Am. Coll. Cardiol. 2018, 72, 2642–2662. [Google Scholar] [CrossRef]
- Rocco, E.; Grimaldi, M.C.; Maino, A.; Cappannoli, L.; Pedicino, D.; Liuzzo, G.; Biasucci, L.M. Advances and Challenges in Biomarkers Use for Coronary Microvascular Dysfunction: From Bench to Clinical Practice. J. Clin. Med. 2022, 11, 2055. [Google Scholar] [CrossRef]
- Odaka, Y.; Takahashi, J.; Tsuburaya, R.; Crea, F.; Shimokawa, H.; Sechtem, U.; Kaski, J.C.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). Plasma concentration of serotonin is a novel biomarker for coronary mi-crovascular dysfunction in patients with suspected angina and unobstructive coronary arteries. Eur. Heart J. 2017, 38, 489–496. [Google Scholar]
- Feuer, D.S.; Handberg, E.M.; Mehrad, B.; Wei, J.; Merz, C.N.B.; Pepine, C.J.; Keeley, E.C. Microvascular Dysfunction as a Systemic Disease: A Review of the Evidence. Am. J. Med. 2022, 135, 1059–1068. [Google Scholar] [CrossRef]
- Morrow, A.J.; Ford, T.J.; Mangion, K.; Kotecha, T.; Rakhit, R.; Galasko, G.; Hoole, S.; Davenport, A.; Kharbanda, R.; Ferreira, V.M.; et al. Rationale and design of the Medical Research Council’s Precision Medicine with Zibotentan in Microvascular Angina (PRIZE) trial. Am. Heart J. 2020, 229, 70–80. [Google Scholar] [CrossRef]
- Abraham, G.R.; Morrow, A.J.; Oliveira, J.; Weir-McCall, J.R.; Davenport, E.E.; Berry, C.; Davenport, A.P.; Hoole, S.P. Mechanistic study of the effect of Endothelin SNPs in microvascular angina—Protocol of the PRIZE Endothelin Sub-Study. IJC Heart Vasc. 2022, 39, 100980. [Google Scholar] [CrossRef]
- Ford, T.J.; Corcoran, D.; Padmanabhan, S.; Aman, A.; Rocchiccioli, P.; Good, R.; McEntegart, M.; Maguire, J.J.; Watkins, S.; Eteiba, H.; et al. Genetic dysregulation of endothelin-1 is impli-cated in coronary microvascular dysfunction. Eur. Heart J. 2020, 41, 3239–3252. [Google Scholar] [CrossRef]
- Jukema, R.A.; de Winter, R.W.; van Diemen, P.A.; Driessen, R.S.; Danser, A.J.; Garrelds, I.M.; Raijmakers, P.G.; van de Ven, P.M.; Knaapen, P.; Danad, I.; et al. The relation of RAAS activity and endothelin-1 levels to coronary atherosclerotic burden and microvascular dysfunction in chest pain patients. Atherosclerosis 2022, 347, 47–54. [Google Scholar] [CrossRef]
- Naya, M.; Aikawa, T.; Manabe, O.; Obara, M.; Koyanagawa, K.; Katoh, C.; Tamaki, N. Elevated serum endothelin-1 is an inde-pendent predictor of coronary microvascular dysfunction in nonobstructive territories in patients with coronary artery disease. Heart Vessels 2021, 36, 917–923. [Google Scholar] [CrossRef]
- Gibbs, T.; Tapoulal, N.; Shanmuganathan, M.; Burrage, M.K.; Borlotti, A.; Banning, A.P.; Choudhury, R.P.; Neubauer, S.; Kharbanda, R.K.; Ferreira, V.M.; et al. OxAMI (Oxford Acute Myocardial Infarction) Study Neuropeptide-Y Levels in ST-Segment-Elevation Myocardial Infarction: Relationship with Coronary Microvascular Function, Heart Failure, and Mortality. J. Am. Heart Assoc. 2022, 11, e024850. [Google Scholar] [CrossRef]
- Rosano, G.M.; Tousoulis, D.; McFadden, E.; Clarke, J.; Davies, G.J.; Kaski, J.C. Effects of neuropeptide Y on coronary artery vaso-motion in patients with microvascular angina. Int. J. Cardiol. 2017, 238, 123–127. [Google Scholar] [CrossRef]
- Fopiano, K.A.; Jalnapurkar, S.; Davila, A.C.; Arora, V.; Bagi, Z. Coronary Microvascular Dysfunction and Heart Failure with Pre-served Ejection Fraction—Implications for Chronic Inflammatory Mechanisms. Curr. Cardiol. Rev. 2022, 18, e310821195986. [Google Scholar] [CrossRef]
- Godo, S.; Takahashi, J.; Yasuda, S.; Shimokawa, H. The role of inflammation in coronary epicardial and microvascular dysfunc-tion. Eur. Cardiol. 2021, 16, e13. [Google Scholar] [CrossRef]
- Yan, B.; Wang, H.; Tan, Y.; Fu, W. microRNAs in Cardiovascular Disease: Small Molecules but Big Roles. Curr. Top. Med. Chem. 2019, 19, 1918–1947. [Google Scholar] [CrossRef]
- Van Rooij, E.; Olson, E.N. MicroRNA therapeutics for cardiovascular disease: Opportunities and obstacles. Nat. Rev. Drug Discov. 2012, 11, 860–872. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, N.; Chen, W.; Wang, Z. Leveraging Extracellular Non-coding RNAs to Diagnose and Treat Heart Diseases. J. Cardiovasc. Transl. Res. 2022, 15, 456–468. [Google Scholar] [CrossRef]
- Li, D.; Yang, P.; Xiong, Q.; Song, X.; Yang, X.; Liu, L.; Yuan, W.; Rui, Y.-C. MicroRNA-125a/b-5p inhibits endothelin-1 expression in vascular endothelial cells. J. Hypertens. 2010, 28, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Jaguszewski, M.; Osipova, J.; Ghadri, J.R.; Napp, L.C.; Widera, C.; Franke, J.; Fijalkowski, M.; Nowak, R.; Fijalkowska, M.; Volkmann, I.; et al. A signature of circulating microRNAs dif-ferentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur. Heart J. 2014, 35, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Al-Badri, A.; Kim, J.H.; Liu, C.; Mehta, P.K.; Quyyumi, A.A. Peripheral Microvascular Function Reflects Coronary Vascular Function. Arter. Thromb. Vasc. Biol. 2019, 39, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Nishimiya, K.; Suda, A.; Fukui, K.; Hao, K.; Takahashi, J.; Matsumoto, Y.; Mitsuishi, K.; Watanabe, T.; Ohyama, K.; Sugisawa, J.; et al. Prognostic Links Between OCT-Delineated Coronary Morphologies and Coronary Functional Abnormalities in Patients with INOCA. JACC Cardiovasc. Interv. 2021, 14, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Pepine, C.J.; Anderson, R.D.; Sharaf, B.L.; Reis, S.E.; Smith, K.M.; Handberg, E.M.; Johnson, B.D.; Sopko, G.; Bairey Merz, C.N. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood. Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010, 55, 2825–2832. [Google Scholar] [CrossRef] [Green Version]
- AlBadri, A.; Merz, C.N.B.; Johnson, B.D.; Wei, J.; Mehta, P.K.; Cook-Wiens, G.; Reis, S.E.; Kelsey, S.F.; Bittner, V.; Sopko, G.; et al. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J. Am. Coll. Cardiol. 2019, 73, 684–693. [Google Scholar] [CrossRef]
- Ford, T.J.; Stanley, B.; Good, R.; Rocchiccioli, P.; McEntegart, M.; Watkins, S.; Eteiba, H.; Shaukat, A.; Lindsay, M.; Robertson, K.; et al. Stratified medical therapy using invasive coronary function testing in angina: The CorMicA trial. J. Am. Coll. Cardiol. 2018, 72, 2841–2855. [Google Scholar] [CrossRef]
- Ong, P.; Safdar, B.; Seitz, A.; Hubert, A.; Beltrame, J.F.; Prescott, E. Diagnosis of coronary microvascular dysfunction in the clinic. Cardiovasc. Res. 2020, 116, 841–855. [Google Scholar] [CrossRef]
- Kumar, S.; Mehta, P.K.; Eshtehardi, P.; Hung, O.Y.; Koh, J.S.; Kumar, A.; Al-Badri, A.; Rabah, R.; D’Souza, M.; Gupta, S.; et al. Functional coronary angiography in symptomatic patients with no obstructive coronary artery disease. Catheter. Cardiovasc. Interv. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Nowroozpoor, A.; Gutterman, D.; Safdar, B. Is microvascular dysfunction a systemic disorder with common biomarkers found in the heart, brain, and kidneys?—A scoping review. Microvasc. Res. 2020, 134, 104123. [Google Scholar] [CrossRef]
- Ohura-Kajitani, S.; Shiroto, T.; Godo, S.; Ikumi, Y.; Ito, A.; Tanaka, S.; Sato, K.; Sugisawa, J.; Tsuchiya, S.; Suda, A.; et al. Marked impairment of endothelium-dependent digital vasodilatations in patients with microvascular angina: Evidence for systemic small artery disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1400–1412. [Google Scholar] [CrossRef]
- Ford, T.; Rocchiccioli, P.; Good, R.; McEntegart, M.; Eteiba, H.; Watkins, S.; Shaukat, A.; Lindsay, M.; Robertson, K.; Hood, S.; et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur. Hear. J. 2018, 39, 4086–4097. [Google Scholar] [CrossRef]
- Godo, S.; Takahashi, J.; Yasuda, S.; Shimokawa, H. Endothelium in Coronary Macrovascular and Microvascular Diseases. J. Cardiovasc. Pharmacol. 2021, 78 (Suppl. 6), S19–S29. [Google Scholar] [CrossRef]
- Behroozian, A.; Beckman, J.A. Microvascular Disease Increases Amputation in Patients With Peripheral Artery Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 534–540. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study Group (COVADIS). International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef] [Green Version]
- Godo, S.; Corban, M.T.; Toya, T.; Gulati, R.; Lerman, L.O.; Lerman, A. Association of coronary microvascular endothelial dysfunction with vulnerable plaque characteristics in early coronary atherosclerosis. EuroIntervention 2020, 16, 387–394. [Google Scholar] [CrossRef]
- Al Suwaidi, J.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R., Jr.; Lerman, A. Long-Term Follow-Up of Patients With Mild Coronary Artery Disease and Endothelial Dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef] [Green Version]
- Siasos, G.; Sara, J.D.; Zaromytidou, M.; Park, K.H.; Coskun, A.U.; Lerman, L.O.; Oikonomou, E.; Maynard, C.C.; Fotiadis, D.; Stefanou, K.; et al. Local Low Shear Stress and Endothelial Dysfunction in Patients with Nonobstructive Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2018, 71, 2092–2102. [Google Scholar] [CrossRef] [PubMed]
- Al Badri, A.; Eshtehardi, P.; Hung, O.Y.; Bouchi, Y.; Khawaja, S.; Mercado, K.; Corban, M.T.; Mehta, P.K.; Shaw, L.J.; Samady, H. Coronary microvascular dsfunction is associated with significant plaque burden and diffuse epicardial atherosclerotic disease. J. Am. Coll. Cardiol. Interv. 2019, 12, 1519–1520. [Google Scholar] [CrossRef]
- Usui, E.; Yonetsu, T.; Kanaji, Y.; Hoshino, M.; Yamaguchi, M.; Hada, M.; Fukuda, T.; Sumino, Y.; Ohya, H.; Hamaya, R.; et al. Optical Coherence Tomography–Defined Plaque Vulnerability in Relation to Functional Stenosis Severity and Microvascular Dysfunction. JACC Cardiovasc. Interv. 2018, 11, 2058–2068. [Google Scholar] [CrossRef]
- Abe, J.-I.; Berk, B.C. Novel Mechanisms of Endothelial Mechanotransduction. Arter. Thromb. Vasc. Biol. 2014, 34, 2378–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Li, Y.-S.; Chien, S. Shear Stress–Initiated Signaling and Its Regulation of Endothelial Function. Arter. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Thondapu, V.; Shishikura, D.; Dijkstra, J.; Zhu, S.J.; Revalor, E.; Serruys, P.W.; van Gaal, W.J.; Poon, E.K.W.; Ooi, A.; Barlis, P. Non-Newtonian Endothelial Shear Stress Simulation: Does It Matter ? Front. Cardiovasc. Med. 2022, 9, 835270. [Google Scholar] [CrossRef]
- Corban, M.T.; Eshtehardi, P.; Suo, J.; McDaniel, M.C.; Timmins, L.H.; Rassoul-Arzrumly, E.; Maynard, C.; Mekonnen, G.; King, S., 3rd; Quyyumi, A.A.; et al. Combination of plaque burden, wall shear stress, and plaque phenotype has incre-mental value for prediction of coronary atherosclerotic plaque progression and vulnerability. Atherosclerosis 2014, 232, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of Endothelial Shear Stress in the Natural History of Coronary Atherosclerosis and Vascular Remodeling: Molecular, Cellular, and Vascular Behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef] [Green Version]
- Siasos, G.; Tsigkou, V.; Zaromytidou, M.; Sara, J.D.; Varshney, A.; Coskun, A.U.; Lerman, A.; Stone, P.H. Role of local coronary blood flow patterns and shear stress on the development of microvascular and epicardial endothelial dysfunction and coronary plaque. Curr. Opin. Cardiol. 2018, 33, 638–644. [Google Scholar] [CrossRef]
- Lerman, A.; Holmes, D.R.; Herrmann, J.; Gersh, B.J. Microcirculatory dysfunction in ST-elevation myocardial infarction: Cause, consequence, or both ? Eur. Heart J. 2007, 28, 788–797. [Google Scholar] [CrossRef] [Green Version]
- Paciaroni, M.; Bogousslavsky, J. Connecting Cardiovascular Disease and Dementia: Further Evidence. J. Am. Heart Assoc. 2013, 2, e000656. [Google Scholar] [CrossRef] [Green Version]
- Fuster, V. The vulnerable patient: Providing a lens into the interconnected diseases of the heart and brain. J. Am. Coll. Cardiol. 2015, 66, 1077–1078. [Google Scholar] [CrossRef] [Green Version]
- Xiang, D.; Kleber, F.X. Smoking and hyperlipidemia are important risk factors for coronary artery spasm. Chin. Med. J. 2003, 116, 510–513. [Google Scholar]
- Sugiishi, M.; Takatsu, F. Cigarette smoking is a major risk factor for coronary spasm. Circulation 1993, 87, 76–79. [Google Scholar] [CrossRef] [Green Version]
- Takagi, Y.; Takahashi, J.; Yasuda, S.; Miyata, S.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; et al. Prognostic stratification of patients with vasospastic angina: A comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J. Am. Coll. Cardiol. 2013, 62, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, J.; Nihei, T.; Takagi, Y.; Miyata, S.; Odaka, Y.; Tsunoda, R.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: Multicentre registry study of the Japanese coronary spasm association. Eur. Hear. J. 2014, 36, 228–237. [Google Scholar] [CrossRef]
- Murohara, T.; Kugiyama, K.; Ohgushi, M.; Sugiyama, S.; Yasue, H. Cigarette smoke extract contracts isolated porcine coronary arteries by super-oxide anionmediated degradation of EDRF. Am. J. Physiol. 1994, 266, H874–H880. [Google Scholar]
- Morrow, J.D.; Frei, B.; Longmire, A.W.; Gaziano, J.M.; Lynch, S.M.; Shyr, Y.; Strauss, W.E.; Oates, J.A.; Roberts, L.J., 2nd. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995, 332, 1198–1203. [Google Scholar] [CrossRef]
- Fetterman, J.L.; Weisbrod, R.M.; Feng, B.; Bastin, R.; Tuttle, S.T.; Holbrook, M.; Baker, G.; Robertson, R.M.; Conklin, D.J.; Bhatnagar, A.; et al. Flavorings in Tobacco Products Induce Endothelial Cell Dysfunction. Arter. Thromb. Vasc. Biol. 2018, 38, 1607–1615. [Google Scholar] [CrossRef]
- Lee, W.H.; Ong, S.-G.; Zhou, Y.; Tian, L.; Bae, H.R.; Baker, N.; Whitlatch, A.; Mohammadi, L.; Guo, H.; Nadeau, K.C.; et al. Modeling Cardiovascular Risks of E-Cigarettes With Human-Induced Pluripotent Stem Cell–Derived Endothelial Cells. J. Am. Coll. Cardiol. 2019, 73, 2722–2737. [Google Scholar] [CrossRef] [Green Version]
- Woelkart, G.; Kollau, A.; Stessel, H.; Russwurm, M.; Koesling, D.; Schrammel, A.; Schmidt, K.; Mayer, B. Effects of flavoring compounds used in electronic cigarette refill liquids on endothelial and vascular function. PLoS ONE 2019, 14, e0222152. [Google Scholar] [CrossRef] [Green Version]
- Kerr, D.M.I.; Brooksbank, K.J.; Taylor, R.G.; Pinel, K.; Rios, F.J.; Touyz, R.M.; Delles, C. Acute effects of electronic and tobacco cigarettes on vascular and respiratory function in healthy volunteers: A cross-over study. J. Hypertens. 2019, 37, 154–166. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, M.; Nunes, J.P.; Carbone, S. Cardiovascular effects of heat-notburn and electronic-vaping-cigarettes in smokers. Minerva Cardioangiol. 2020, 68, 545–547. [Google Scholar] [CrossRef]
- Biondi Zoccai, G.; Carnevale, R.; Vitali, M.; Tritapepe, L.; Martinelli, O.; Macrina, F.; Bullen, C.; Peruzzi, M.; Cavarretta, E.; Marullo, A.G.; et al. A randomized trial comparing the acute coronary, systemic, and environmental effects of electronic vaping cigarettes versus heat-not-burn cigarettes in smokers of combustible cigarettes undergoing in-vasive coronary assessment: Rationale and design of the SUR-VAPES 3 trial. Minerva Cardioangiol. 2020, 68, 548–555. [Google Scholar] [PubMed]
- Murrell, W. Nitro-glycerine as a remedy for angina pectoris. Lancet 1879, 113, 80–81. [Google Scholar] [CrossRef] [Green Version]
- Golino, M.; Spera, F.R.; Manfredonia, L.; De Vita, A.; Di Franco, A.; Lamendola, P.; Villano, A.; Melita, V.; Mencarelli, E.; Lanza, G.A.; et al. Microvascular ischemia in patients with successful percutaneous coronary inter-vention: Effects of ranolazine and isosorbide-5-mononitrate. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6545–6550. [Google Scholar] [PubMed]
- Kojima, S.; Matsui, K.; Sakamoto, T.; Ishihara, M.; Kimura, K.; Miyazaki, S.; Yamagishi, M.; Tei, C.; Hiraoka, H.; Sonoda, M.; et al. Long-Term Nitrate Therapy After Acute Myocardial Infarction Does not Improve or Aggravate Prognosis. Circ. J. 2007, 71, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Park, T.K.; Cho, S.W.; Oh, M.S.; Lee, D.H.; Seong, C.S.; Gwag, H.B.; Lim, A.Y.; Yang, J.H.; Song, Y.B.; et al. Impact of different nitrate therapies on long-term clinical outcomes of patients with vasospastic angina: A propensity score-matched analysis. Int. J. Cardiol. 2018, 252, 1–5. [Google Scholar] [CrossRef]
- Redfield, M.M.; Anstrom, K.J.; Levine, J.A.; Koepp, G.A.; Borlaug, B.A.; Chen, H.H.; LeWinter, M.M.; Joseph, S.M.; Shah, S.J.; Semigran, M.J.; et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2015, 373, 2314–2324. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Anstrom, K.J.; Lewis, G.D.; Shah, S.J.; Levine, J.A.; Koepp, G.A.; Givertz, M.M.; Felker, G.M.; LeWinter, M.M.; Mann, D.L.; et al. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: The INDIE-HFpEF randomized clinical trial. JAMA 2018, 320, 1764–1773. [Google Scholar] [CrossRef] [Green Version]
- Schiattarella, G.G.; Altamirano, F.; Tong, D.; French, K.M.; Villalobos, E.; Kim, S.Y.; Luo, X.; Jiang, N.; May, H.I.; Wang, Z.V.; et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019, 568, 351–356. [Google Scholar] [CrossRef]
- Saito, H.; Godo, S.; Sato, S.; Ito, A.; Ikumi, Y.; Tanaka, S.; Ida, T.; Fujii, S.; Akaike, T.; Shimokawa, H. Important Role of Endothelial Caveolin-1 in the Protective Role of Endothelium-dependent Hyperpolarization Against Nitric Oxide–Mediated Nitrative Stress in Microcirculation in Mice. J. Cardiovasc. Pharmacol. 2018, 71, 113–126. [Google Scholar] [CrossRef]
- Oemrawsingh, R.M.; Cheng, J.M.; García-García, H.M.; Kardys, I.; van Schaik, R.H.; Regar, E.; van Geuns, R.-J.; Serruys, P.W.; Boersma, E.; Akkerhuis, K.M. High-sensitivity Troponin T in relation to coronary plaque characteristics in patients with stable coronary artery disease; results of the ATHEROREMO-IVUS study. Atherosclerosis 2016, 247, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Taqueti, V.R. Treating coronary microvascular dysfunction as the ‟culprit” lesion in patients with refractory angina: Lessons from CorMicA at 1 year. J. Am. Coll. Cardiol. Interv. 2020, 13, 46–48. [Google Scholar] [CrossRef]
- Sidik, N.P.; McDermott, M.; McEntegart, M.B.; Berry, C. Chest pain without obstructive coronary artery disease: A case series. Eur. Hear. J. Case Rep. 2020, 4, ytaa060. [Google Scholar] [CrossRef] [Green Version]
- Godo, S.; Shimokawa, H. Endothelial functions. Arterioscler Thromb Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [Green Version]
- Pancaldi, E.; Tedino, C.; Riccardi, M.; Alghisi, F.; Cimino, G.; Pascariello, G.; Calvi, E.; Sciatti, E.; Vizzardi, E.; Metra, M. Endothelial function evaluation in idiopathic vs. ischemic dilated cardiomyopathy. Am. J. Cardiovasc. Dis. 2022, 12, 136–142. [Google Scholar]
- Yamamoto, M.; Hara, H.; Moroi, M.; Ito, S.; Nakamura, M.; Sugi, K. Impaired Digital Reactive Hyperemia and the Risk of Restenosis after Primary Coronary Intervention in Patients with Acute Coronary Syndrome. J. Atheroscler. Thromb. 2014, 21, 957–965. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Sugiyama, S.; Sugamura, K.; Nozaki, T.; Ohba, K.; Konishi, M.; Matsubara, J.; Sumida, H.; Kaikita, K.; Kojima, S.; et al. Digital assessment of endothelial function and ischemic heart disease in women. J. Am. Coll. Cardiol. 2010, 55, 1688–1696. [Google Scholar] [CrossRef] [Green Version]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R., Jr.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef] [Green Version]
- Kitta, Y.; Obata, J.-E.; Nakamura, T.; Hirano, M.; Kodama, Y.; Fujioka, D.; Saito, Y.; Kawabata, K.-I.; Sano, K.; Kobayashi, T.; et al. Persistent Impairment of Endothelial Vasomotor Function Has a Negative Impact on Outcome in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2009, 53, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Matsuzawa, Y.; Kwon, T.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2015, 4, e002270. [Google Scholar] [CrossRef] [Green Version]
- Nardone, M.; Miner, S.; McCarthy, M.; Edgell, H. Standard exercise stress testing attenuates peripheral microvascular function in patients with suspected coronary microvascular dysfunction. BMC Sports Sci. Med. Rehabil. 2021, 13, 18. [Google Scholar] [CrossRef]
- Nardone, M.; Miner, S.; McCarthy, M.; Ardern, C.I.; Edgell, H. Noninvasive Microvascular Indices Reveal Peripheral Vascular Abnormalities in Patients With Suspected Coronary Microvascular Dysfunction. Can. J. Cardiol. 2019, 36, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, R.; Fox, K. Insight into the Mode of Action of ACE Inhibition in Coronary Artery Disease. Drugs 2009, 69, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.A.H.; Fujiki, T.; Morikawa, K.; Motoishi, M.; Matsuo, Y.; Hatanaka, M.; Tsutsui, M.; Takeshita, A.; Shimokawa, H. Endothelial Nitric Oxide Synthase-Independent Effects of an ACE Inhibitor on Coronary Flow Response to Bradykinin in Aged Mice. J. Cardiovasc. Pharmacol. 2004, 44, 557–563. [Google Scholar] [CrossRef]
- Bairey Merz, C.N.; Pepine, C.J.; Shimokawa, H.; Berry, C. Treatment of coronary microvascular dysfunction. Cardiovasc. Res. 2020, 116, 856–870. [Google Scholar] [CrossRef]
- Liu, H.; Xie, G.; Huang, W.; Liu, J.; Zhao, N.; Corban, M.T.; Lerman, A.; Wu, Y.; Wang, H. Rationale and design of a multicenter, randomized, patients-blinded two-stage clinical trial on effects of endothelial function test in patients with non-obstructive coronary artery disease (ENDOFIND). Int. J. Cardiol. 2020, 325, 16–22. [Google Scholar] [CrossRef]
- D’Amario, D.; Restivo, A.; Leone, A.M.; Vergallo, R.; Migliaro, S.; Canonico, F.; Galli, M.; Trani, C.; Burzotta, F.; Aurigemma, C.; et al. Ticagrelor and preconditioning in patients with stable coronary artery disease (TAPER-S): A randomized pilot clinical trial. Trials 2020, 21, 192. [Google Scholar] [CrossRef]




| Author/Year | Study Period | Total Number | Cohort (N) | Aims | Type | Findings | |||
|---|---|---|---|---|---|---|---|---|---|
| Mohri et al. (2003) JACC [9] | 1999–2000 | 18 | Saline | Fasudil 13 | Role of Rho-kinase | RCT | Fasudil ameliorates myocardial ischaemia in patients with coronary microvascular spasm by mean of the inhibition of Rho-kinase | ||
| Masumoto et al. (2002) Circulation [10] | 1999–2000 | 20 | Saline 5 | Fasudil 15 | Role of Rho-kinase | RCT | Fasudil was effective in preventing ACh-induced coronary artery spasm and resultant myocardial ischaemia in patients with vasospastic angina. | ||
| Kataruka et al. (2020) JAHA [11] | 2005–2017 | 215 066 | PCI 178 474 | CABG 36 592 | Whether 30 days death ratio increased in PCI vs. CABG | Multicentre COAP database | Clinical acuity increased for patients treated with PCI rather than CABG with increased use of PCI instead of CABG. PCI 30 days death ratio increased (0.98 vs. 1.19, p < 0.0001). CABG decreased (1.21 vs. 0.74, p < 0.0001) | ||
| Alkhouli et al. (2020) JAMA [12] | 2003–2016 | 12 062 081 | PCI 8 687 338 | CABG 3 374 743 | Whether death increased in PCI vs. CABG | Multicentre observational | Risk-adjusted mortality decreased significantly after CABG but not after PCI. mortality increased in PCI (22.8% to 53.1%) decreased in CABG (5.6% to 3.4%) | ||
| Shah et al. (2018) EHJ [13] | 2015–2018 | 263 202 HFpEF | CMD absent 51 | CMD present 151 | Whether CMD is higher in HFpEF. | Prospective Multicentre PROMIS-HFpEF | High prevalence of CMD (151) in HFpEF [75% (95% confidence interval 69–81%)] in the absence of unrevascularized macrovascular CAD. Smoking p = 0.0006 and atrial fibrillation p = 0.004 in CMD | ||
| Hage et al. (2020) J Card Fail [14] | 2015–2018 | 263 | CMD absent 51 | CMD present 151 | To determine association of CMD with hospitalization and mortality in HFpEF. | Prospective Multicentre PROMIS-HFpEF | CMD was independently associated with primarily CV- and HF-specific events. | ||
| Ahmad et al. (2021) Eur J Heart Fail [15]. | 2010–2019 | 51 | HFpEF 22 | No/HFpEF 29 | Whether exist difference in CFR between HFpEF and No/HFpEF | Prospective Multicentre | Coronary microvascular function is inversely associated with filling pressures. HFpEF was associated to lower CFR (2.5 ± 0.6 vs. 3.2 ± 0.7; p = 0.0003) | ||
| Dryer et al. (2018) Am J Physiol Heart Circ Physiol [17] | 2015–2017 | 44 | HFpEF 30 | No/HFpEF 14 | To evaluate the incidence of coronary microvascular dysfunction in HFpEF | Prospective Multicentre USA | HFpEF had more abnormalities of coronary flow and resistance than to No/HFpEF | ||
| Suhrs et al. (2020) PLoS One [18] | 2012–2017 | 431 | 336 women CAD 180 Db 156 nDb | 95 controls | To evaluate if subclinical inflammation is associated with non-endothelial dependent CMD and diastolic dysfunction. | Prospective iPOWER study | Inflammatory biomarkers increased to both CMD and E/e’ diastolic dysfunction. | ||
| Yang et al. (2020) Eur J Heart Fail [19] | 1993–2015 | 162 | HFpEF 115 | HFnpEF 47 | To evaluate the role of ED and EI mechanism In HFpEF | Observational Multicentre | HFpEF was associated to CMD due to ED and EI mechanisms. Worse diastolic dysfunction is higher in HFpEF and EI-CMD. | ||
| Von Mering et al. (2004) Circulation [20] | 1996–2000 | † 163 | No CAD 74 | Minimal CAD 49 | Severe CAD 40 | Whether Ach influence vasomotion response in woman | RCT WISE | In woman impaired coronary vasomotor response to Ach. Ach independently linked to adverse cardiovascular outcomes (less time free from cardiovascular events (p = 0.004) | |
| Murthy et al. (2014) Circulation [21] | 2006–2010 | 1218 | Men 405 | Women 813 | Whether there is a relative extent to which CMD affects both genders | Retrospective | CMD is not a uniquely female disorder and identifies men and women at increased clinical risk. CFR was a powerful incremental predictor of MACE (hazard ratio 0.80 [95% CI 0.75–086] per 10% increase in CFR; p < 0.0001) | ||
| Sara et al. (2015) JACC Cardio Int [23] | 1993–2012 | 1439 | CBFAch+, CFRAdn+ 520 | CBFAch-, CFRAdn+ 478 | CBFAch+, CFRAdn- 173 | CBFAch−, CFRAdn- 268 | Assessment of the prevalence of CM abnormalities in patients presenting with chest pain and CAD | Prospective | Patients with chest pain and nonobstructive CAD have high prevalence of CM abnormalities |
| Aziz et al. (2017) JACC [24] | 2007–2014 | 1379 | Male 573 | Women 806 | Determine sex differences of vasomotor dysfunction in a European population | Prospective | Female patients have a higher prevalence of vasomotor dysfunction (especially CMD) compared with male patients. Odds ratio 4.2 (95% confidence interval: 3.1 to 5.5; p < 0.001) and 2.3 (95% CI: 1.7 to 3.1; p < 0.001) | ||
| Motiejūnaitė et al. (2020) EHJ [25] | 1995–2008 | * 22 523 γ26 376 | Male 12 589 | Women 9933 | Evaluate the association of sex and 1-year all-cause mortality in patients with AHF in various regions of the world. | Comparative Study | Globally women with AHF have a lower 1-year mortality and less evidenced-based treatment than men. (HR 0.86 (0.79–0.94), p < 0.001 after adjustment) | ||
| Schroder et al. (2021) EHJ [26] | 2003–2008 | All women 1681 | CFVR < 2.25 723 | CFVR ≥ 2.25 958 | Whether assessment of CMD predicts adverse outcome in women with angina and no obstructive CAD. | Prospective iPOWER | CFVR by echocardiography is predictive of adverse outcome in women with angina and no obstructive CAD (HR 1.07; 95% CI 1.03–1.11) | ||
| Kakuta et al. (2021) JAHA [29] | 2015–2018 | 67 | IBD 37 | Control 30 | To investigate the presence and severity CMD in IBD | Retrospective | IBD is associated with CMD, which improved after surgical resection of diseased intestines. | ||
| Lee et al. (2015) Circulation [40] | 2007–2012 | 139 | Men 32 | Woman 107 | To investigate angina in symptomatic patients with nonobstructive CAD by using Ach, IMR, CFR, FFR and IVUS | Prospective | High rate of patients with angina in the absence of obstructive CAD have occult coronary abnormalities | ||
| Ford et al. (2019) Circ Cardiovasc Interv [41] | 2016–2017 | 391 | INOCA 195 | CAD 206 | To determine microvascular and vasospastic angina INOCA | RCT | Higher rate (3/4) of INOCA reveal coronary vasomotion disorders including microvascular and vasospastic angina | ||
| Suda et al. (2019) JACC [42] | 2014–2017 | 187 | Men 113 | Woman 74 | To evaluate the coronary functional abnormalities in both epicardial and microvascular coronary arteries in patients with angina and INOCA. | RCT | Patients with angina and INOCA have both epicardial coronary spasm and increased microvascular resistance. Worse prognosis and Rho-kinase activation may be involved. | ||
| Al-Lamee et al. (2018) Lancet [48] | 2014–2017 | 200 Medically treated angina | PCI 105 | Placebo 95 | To evaluate efficacy of PCI on stable angina | RCT ORBITA | PCI did not increase exercise time (PCI minus placebo 16·6 s, 95% CI -8·9 to 42·0, p = 0·200). | ||
| Maron et al. (2020) NEJM [49] | 5179 | PCI plus OMT | OMT alone | Whether PCI plus OMT is effective compared OMT alone. | RCT ISCHEMIA | PCI is not effective in patients with stable coronary disease and moderate or severe ischaemia. At 5 years, (death, MI, Re-hosp) was 16.4% and 18.2%, respectively (difference, −1.8 percentage points; 95% CI, −4.7 to 1.0) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avtaar Singh, S.S.; Nappi, F. Pathophysiology and Outcomes of Endothelium Function in Coronary Microvascular Diseases: A Systematic Review of Randomized Controlled Trials and Multicenter Study. Biomedicines 2022, 10, 3010. https://doi.org/10.3390/biomedicines10123010
Avtaar Singh SS, Nappi F. Pathophysiology and Outcomes of Endothelium Function in Coronary Microvascular Diseases: A Systematic Review of Randomized Controlled Trials and Multicenter Study. Biomedicines. 2022; 10(12):3010. https://doi.org/10.3390/biomedicines10123010
Chicago/Turabian StyleAvtaar Singh, Sanjeet Singh, and Francesco Nappi. 2022. "Pathophysiology and Outcomes of Endothelium Function in Coronary Microvascular Diseases: A Systematic Review of Randomized Controlled Trials and Multicenter Study" Biomedicines 10, no. 12: 3010. https://doi.org/10.3390/biomedicines10123010
APA StyleAvtaar Singh, S. S., & Nappi, F. (2022). Pathophysiology and Outcomes of Endothelium Function in Coronary Microvascular Diseases: A Systematic Review of Randomized Controlled Trials and Multicenter Study. Biomedicines, 10(12), 3010. https://doi.org/10.3390/biomedicines10123010