1. Introduction
Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. It is characterized by perturbations in immune, humoral, and circulatory function, which result in increased mortality, and thus needs urgent care, compared with ‘straightforward infections’ [
1]. A recent publication based on the Global Burden of Disease, Injuries, and Risk Factors Study estimated that sepsis accounts for almost 20% of all global deaths worldwide [
2]. Clinical management of patients with sepsis relies on early recognition of the syndrome, allowing for the rapid institution of antibiotics, source control, and resuscitation with fluids. Vasoactive drugs also constitute a mainstay of treatment for life-threatening hypotension [
1]. While treating hypotension is important for survival, the administration of vasopressors, particularly in excess, can compound blood flow disruptions to organs in sepsis, and the resultant organ hypoperfusion may offset the survival benefits of normotension.
A challenge in studying circulatory changes in sepsis is that vascular dysfunction may be localized to certain vascular beds, contributing to focal regions of impaired blood flow, whereas other regions remain functionally intact [
3]. Indeed, compared with global hemodynamic variables, microcirculatory alterations are a stronger predictor of survival outcomes in septic patients [
4], underscoring the importance of microvascular regulation and regional control of blood flow in sepsis. Blood vessels rely on neurogenic, metabolic, humoral, and paracrine mediators to control vascular tone and regulate local blood flow. The pathophysiology of vascular dysfunction in sepsis is complex, and the mechanisms dictating blood flow to the respective organs may be such that certain tissues are more vulnerable to blood flow disruptions before others. Although alterations in organ blood flow and, presumably, vascular dysfunction in sepsis, have been reported in the literature [
5,
6], a fundamental knowledge gap related to the inherent differences in susceptibility to vascular dysfunction among vascular beds and the timeline by which this occurs in sepsis still exists.
Here, we characterized the systemic hemodynamics and regional blood flow changes that occur as sepsis progresses to septic shock. We simultaneously measured systemic blood pressure (BP) as well as blood flow to the brain, kidneys, and mesentery prior to and following induction of sepsis. We further evaluated hemodynamic alterations in response to exogenous pharmacological agents to measure in vivo vascular function in real time. We hypothesized that hemodynamics changes in response to vasoactive agents will decline with the progression of sepsis and the drop in drug-induced alterations in regional blood flow will precede the changes in systemic hemodynamics.
2. Materials and Methods
2.1. Animals and Treatments
The experimental protocols described herein conform to the guidelines of the Canadian Council on Animal Care, as well as the NIH Guide for the Care and Use of Laboratory Animals, and were approved by the University of Alberta Animal Care and Use Committee. C57Bl/6 male mice (10–12 weeks old) were purchased from Charles River Laboratories Inc. (Saint-Constant, QC, Canada) and group-housed in the Animal Care Facility at the University of Alberta. Environmentally, a 12:12 h light–dark cycle (06:00–18:00) at an ambient temperature of 23 °C with 40–60% relative humidity was maintained, and mice had ad libitum access to food and water.
2.2. Systemic and Regional Hemodynamic Assessments
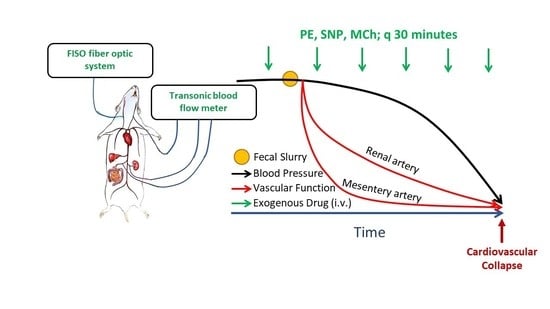
After 1 week of acclimation, mice were anesthetized with inhaled isoflurane (2.5% induction, 1.5% maintenance in pure O2) and kept spontaneously breathing via a nose cone on a heated surgical platform connected to a circulating water bath. A rectal thermometer was used to monitor body temperature. Mice were then instrumented with a fiberoptic pressure sensor (FISO-LS-PT9, FISO Technologies Inc., Quebec, QC, Canada) in the right carotid artery for continuous BP and heart rate (HR) measurements. Micro-renathane catheters (0.25 mm OD; Braintree Scientific, Braintree MA) fused to polyethylene tubing (Intramedic, Becton Dickson, I.D. 0.28 mm × O.D. 0.61 mm) were inserted into the left and right femoral veins for fluid and drug delivery, respectively. In subgroups of mice, regional blood flow measurements were made simultaneously with systemic hemodynamics (blood flow was assessed in one single region in each mouse). For these mice, a laparotomy was performed, and a perivascular flow probe (calibrated 0.5 PSL Nano-Prob, Transonic Systems Inc., Ithaca, NY, USA) was positioned around either the left renal or SMA; the abdomen of these mice was then re-sutured. In a separate group of mice, a perivascular flow probe was placed around the left common carotid artery, and the incision was then sutured closed. After instrumentation, mice were given 30 min to stabilize, after which systemic and regional hemodynamics were recorded continuously using PowerLab and LabChart Pro8 software (ADInstruments, Colorado Springs, CO, USA). Baseline hemodynamic parameters were recorded for 15 min prior to administration of a fecal slurry (FS) solution (see below) and continuously thereafter for up to 4 h. For systemic hemodynamics and blood flow patterns, representative waveform segments within each 60 s interval were analyzed and used as a discrete data point; 30 such discrete consecutive time points were then averaged to reflect each 30-min time interval. To analyze hemodynamic responses to vasoactive agents, the maximum change in BP, HR, or organ blood flow that occurred in the 30 s window following injection was quantified; this time-course captured the maximal changes prior to observing compensatory reflexes (e.g., HR changes due to baroreceptor reflexes). The regional vascular resistance index was calculated as a quotient of ΔBP/Δblood flow in that region. Mice were euthanized by exsanguination via excision of the heart under inhaled isoflurane anesthesia at the end of the experiment.
2.3. Fecal Slurry Preparation and Induction of Polymicrobial Sepsis in Mice
FS was prepared by collecting the cecal contents of untreated euthanized mice. Cecal contents were suspended in a 5% dextrose solution and then filtered through a 100 µm nylon mesh (to remove large particles). Filtered suspensions were adjusted to yield a final concentration of 80 mg/mL. FS samples were aliquoted and frozen in liquid nitrogen and stored at −80 °C; each aliquot only underwent a single freeze–thaw cycle and was then discarded. For this study, all FS aliquots were taken from a single batch of FS. To induce polymicrobial sepsis, FS was injected at a dose of 1.3 mg/g body weight (BW) IP. Control mice received the equivalent volume of saline.
2.4. Hemodynamic Effects of Vasoactive Agents
To assess vascular function in vivo with the progression of sepsis, we administered repeat bolus doses of vasoactive drugs (vasoconstrictors and vasodilators) that cause measurable but transient changes in BP. Changes in hemodynamic responses over time were interpreted as alterations in vascular responsiveness to these agents, indicative of changes in vascular function. Briefly, in a subgroup of instrumented mice (as described above), systemic and regional hemodynamic responses to bolus intravenous injections of a single dose of phenylephrine (PE, 10 µg/kg BW), methylcholine (MCh, 1 µg/kg BW), and sodium nitroprusside (SNP, 5 µg/kg BW) were repeatedly assessed. Vasoactive agents were administered prior to injection of the FS (or vehicle) and every 30 min thereafter for up to 4 h. Doses were selected on the basis of our pilot studies in non-septic mice showing that their injection caused pronounced (~15–25 mmHg) but transient (<30 s) changes in BP with minimal effects on HR (<5%). With minimal effects on HR, BP effects could be attributed primarily to changes in vascular tone. The experimental procedure is shown in
Scheme 1.
2.5. Arterial Blood Gas Analysis
In a separate cohort, otherwise non-instrumented mice were anesthetized with isoflurane (2.5% induction, 1.5% maintenance in pure O2) and implanted with a catheter (Micro-renathane, 0.25 mm OD (Braintree Scientific, Braintree, MA, USA) fused to polyethylene tubing (Intramedic, Becton Dickson, Becton, NJ, USA, I.D. 0.28 mm × O.D. 0.61 mm)) in the left carotid artery for blood collection. Because blood gas analysis required sample volumes of ~150 µL, serial assessments were not performed due to the potential impact of repeated sampling on hemodynamics and overall survival. Arterial blood was collected under anesthesia in calcium-balanced heparin-coated syringes (Blood Gas Monovette, Sarstedt Inc., Montreal, QC, Canada) at 0.5 h, 1 h, 2 h, and 4 h post-injection of FS or the vehicle, then immediately analyzed by a blood gas analyzer (AB Flex 80, Radiometer Canada, Mississauga, ON, Canada).
2.6. Echocardiography
Cardiac function was assessed by transthoracic echocardiography (Vevo 2100, Visualsonics, Toronto, ON, Canada) using a linear array transducer ranging from 18 to 38 MHz (MS400, Visualsonics, Toronto, ON, Canada). A single operator blinded to the experimental groups performed all echocardiographic measurements. Animals were anesthetized with isoflurane (2.5% induction, 1.5% maintenance in pure O2) and then fixed to a heated platform. The chest was depilated and echocardiography was performed under isoflurane anesthesia in the supine position using a heated ultrasound transmission gel. The heart was imaged via two-dimensional B mode in a parasternal short axis view and once a clear view had been established, an M mode cursor was placed perpendicular to the anterior and posterior wall of the left ventricle and parallel to the septum. Left ventricle internal diameters were obtained during the end-diastole and end-systole. Ejection fraction and stroke volume were then calculated from the ventricular volumes computed from M mode images; heart rates were measured in the same cardiac cycles.
2.7. Intravenous Administration of Lipopolysaccharide (LPS) to Mice
In a separate cohort of mice (instrumented with a carotid pressure sensor for BP measurements and perivascular flow probes to measure carotid or SMA blood flow, as described above), LPS (7 mg/kg) or an equivalent volume of saline was injected intravenously in the left femoral vein. In these mice, continuous systemic and regional hemodynamics, and hemodynamic responses to repeat administration of single doses of SNP were assessed prior to and for 4 h following administration of LPS. The dose of LPS was chosen in line with pilot studies showing a comparable mortality within 4 h to FS injection (at 1.3 mg/g BW).
2.8. Statistical Analysis
Due to the exploratory nature of the work, there were different endpoints assessed throughout the study, including systemic and regional hemodynamics, response to vasoactive drugs as a measure of in vivo vascular function, heart function, and changes in lactate and arterial blood gas parameters. Sample sizes for various endpoints were calculated on the basis of perceived differences and variances from pilot experiments. The confidence level and power were set at 95% (α = 0.05) and 80%, respectively. Data were analyzed by two-way analysis of variance for the effects of FS and time, followed by the Holm–Sidak post hoc test for multiple comparisons using Prism 8.0 (GraphPad Software, Inc., San Diego, CA, USA); p < 0.05 was considered statistically significant.
4. Discussion
The objective of this study was to evaluate the intrinsic differences in susceptibility to dysfunction among vascular beds in sepsis, as well as the timeline at which the vascular dysfunction occurs. Using a murine model of FIP, we showed that the development of vascular dysfunction and regional blood flow perturbation in sepsis is not uniform; instead, certain vascular beds (notably the mesenteric vasculature) exhibit dysregulation before the others, and this underlying dysfunction precedes the BP decline and impaired blood flow to these organs.
The FIP model of polymicrobial sepsis is commonly used and generally well characterized, though the degree of peritonitis induced in the present study was comparatively severe, causing circulatory collapse within 6 h of FS injection. FIP altered cardiovascular function, as observed by increasing HR and diminishing BP over time. The former may constitute an adaptive response to mitigate the fall in cardiac output [
9,
10]. However, since the cardiac changes did not temporally coincide with the fall in BP, it is likely that a progressive loss of systemic vascular resistance was the primary factor associated with circulatory compromise herein [
11].
The gradual decline in BP was accompanied by impaired regional blood flow patterns. While we observed differences among regions, these were less pronounced than expected, given that cerebral and renal blood flow are autoregulated, whereas blood flow to the intestine is less so [
12,
13,
14]. Indeed, the linear decline in blood flow in the mesenteric circulation mirrored the gradual decline in BP, consistent with a progressive decline in perfusion pressure. In contrast, carotid and renal blood flow in FIP mice exhibited a biphasic pattern, characterized by an initial plateau phase (reflecting autoregulation), followed by a progressive decline thereafter (wherein the fall in perfusion pressure exceeded the autoregulatory range) [
15]. It should be noted that while the majority of studies have reported reduced renal blood flow in experimental models of sepsis, others have reported unchanged or even increased blood flow to the kidneys [
16]. This apparent discrepancy may be reconciled by the fact that cardiac output is a primary determinant of blood flow patterns to the kidneys [
16]. Reduced cardiac output, as seen in the present study, is often accompanied by decreased renal blood flow, while preserved or high cardiac output predicted unchanged or increased renal blood flow in experimental models of sepsis [
16]. As differences in wet and dry tissue weights were comparable between the two groups (data not shown), increased vascular permeability and tissue edema are less likely to be attributed to the increased vascular resistance and decreased BP, consistent with other models of sepsis [
17].
The impaired organ blood flow was associated with a number of systemic metabolic changes, including an increase in plasma lactate levels with concurrent metabolic acidosis [
8], which is associated with increased mortality in both emergency departments and hospitalized patients [
18]. Though elevated lactate levels could also reflect respiratory compromise, the increased blood oxygen content suggested that the increased lactate levels were primarily due to the reduced oxygen uptake and/or release rather than oxygen transport [
7]. While the observed increase in oxygen content in FIP mice was due to hemoconcentration, the reduced partial pressure of O
2 and the increased lactate levels, in conjunction with impaired organ blood flow in FIP mice, indicate the development of ischemic hypoxia in septic mice.
The gradual decline in regional blood flow occurring after 1.5 h was greater in magnitude than the drop in perfusion pressure (as reflected by the increased regional vascular resistance) without pronounced tissue edema, suggesting a progressive decline in the vascular control mechanisms that maintain tonic vasodilation. To gain additional insights into how the vascular mechanisms regulating organ blood flow were affected in sepsis, we assessed hemodynamic responses to several vasoactive agents—an approach that integrated all regulatory systems at play within a particular vascular bed, including those regulating the vascular tone of resistance arterioles. Thus, the blood flow through that vascular bed is influenced by all the homeostatic mechanisms at play in all downstream vessels. To the best of our knowledge, the present study was the first to interrogate the mechanisms of vascular dysfunction by using in vivo methods to serially assess vascular function over time by administering vasoactive agents. The doses of vasoactive agents used caused minimal changes in HR; therefore, the BP effects could be attributed predominantly to changes in vascular resistance. In non-septic control mice, PE caused a transient rise in BP, reflecting increased systemic vasoconstriction, whereas SNP and MCh resulted in hypotensive responses, reflecting a systemic vasodilatory effect. In FIP mice, BP responses to tested vasoactive agents began to diminish 2–2.5 h post-injection of FS, suggesting a time-dependent loss of vascular reactivity (i.e., vasoplegia), but loss of responsiveness was similar with each pharmacological agent. In contrast, the analysis of regional blood flow patterns revealed marked differences. In control mice, none of the agents had marked effects on carotid or renal blood flow, except for PE, reflecting intact autoregulatory systems. Interestingly, direct pharmacological agonism with PE, SNP, and MCh has been shown to affect blood flow in these beds [
19,
20,
21,
22], suggesting that the direct vascular effects of these agonists interfere with autoregulatory mechanisms in these vascular beds. That no such changes were observed in non-septic control mice may be related to the low doses of agents used in the present study. In contrast, the superimposable blood flow profiles between the control and FIP mice with all administered agents suggests that carotid blood flow is less prone to perturbations when vascular control systems go awry in sepsis. Though reduced responsiveness to all agents was observed in the renal artery, these differences did not manifest as quickly as in the SMA, again possibly due to compensation by autoregulatory mechanisms. In the mesenteric bed, reduced and increased blood flow with PE and SNP, respectively, can be attributed to direct actions on vascular resistance, rather than their effects on perfusion pressure. Although the effects of PE and SNP waned over time even in non-septic mice, likely reflecting the well described phenomena of tachyphylaxis with these agents [
23,
24], the time-course in septic animals was far more rapid. In septic mice, SMA blood flow changes in response to PE and SNP were disturbed within 0.5 h post-injection of FS, suggesting that sepsis causes a marked acceleration in vascular unresponsiveness to these drugs. The rapid onset of vasoplegia in the SMA could not be attributed to the proximity of the intraperitoneal FS injection, as a similar time-course of dysfunction was observed in mice systemically injected with LPS. Interestingly, the observed SMA blood flow dysregulation in response to vasoactive drugs was concurrent with the immediate rise in plasma lactate levels. This shows that metabolic and vascular derangements occur early on in sepsis before changes in systemic hemodynamics are apparent. Therefore, regional hemodynamic assessment is critical in determining the status of vascular integrity for the timely institution of potential treatments before permanent vasculature damage and organ dysfunction occur. These novel findings are important for devising intervention strategies to specifically promote blood flow in dysfunctional regions while preventing life-threatening hypotension.
SNP stimulates soluble guanyl cyclase (sGC) in smooth muscle cells, catalyzing the dephosphorylation of guanosine triphosphate to cyclic guanosine monophosphate, which induces smooth muscle relaxation through several mechanisms, including sequestering calcium and activation of cell membrane potassium channels [
25,
26]. In conditions of oxidative stress, oxidation of the ferrous heme in sGC generates heme-free sGC [
27], which is susceptible to ubiquitination and subsequent breakdown [
28]. Although heme-free sGC has preserved basal activity, it cannot be activated by nitric oxide (NO) [
29]. The reversal of blood flow in the mesentery within 0.5 h of FIP suggests that a disturbed sGC–cyclic guanosine monophosphate signaling pathway is evident in this vascular bed as a result of FIP in mice earlier than in other vascular beds.
PE, the α1 selective adrenergic receptor agonist, causes vasoconstriction and increases systemic vascular resistance and arterial blood pressure. Vascular hyporesponsiveness to vasopressors has been characterized in septic models [
30]. Dysfunction, desensitization and reduction of α receptors; inactivation of catecholamines by oxidation, and adrenal insufficiency are among the suggested mechanisms for vascular hyporesponsiveness to vasopressors [
31,
32,
33]. A reduction in the number of hepatic α1 adrenergic receptors has been reported in a rat model of sepsis [
34]. The hypothalamic–pituitary–adrenal axis, the main neuroendocrine structure regulating the adaptive response to different stressors, is responsible for catecholamine secretion, vasopressin release, and cytokine activation in sepsis. Hypothalamic–pituitary–adrenal axis dysfunction results in reduced production of the corticotropin-releasing hormone, adrenocorticotropic hormone, and cortisol, or causes relative hormonal deficiency by affecting their receptors [
35,
36]. The immediate effect of FIP on reversing SMA blood flow response to PE may suggest that mesenteric α1 receptors are affected earlier than these receptors in other vascular beds following FIP in mice.
Interestingly, MCh caused a pronounced lowering of SMA blood flow, suggesting that its effect of reducing perfusion pressure predominates over its direct vasodilatory effects in the mesenteric circulation [
37]. By extension, this suggests that the BP-lowering effect of MCh may be mediated by vasodilation of blood vessels supplying the skeletal muscle, which muscarinic receptors are known to populate [
38], rather than directly affecting the mesenteric circulation. With the progression of sepsis, changes in the responsiveness to MCh were relatively slower compared with those to SNP, despite the reliance on NO–sGC signaling [
37]. This discrepancy may be reconciled by the fact that MCh can elicit vasodilation via multiple mechanisms (e.g., NO, EDH, etc.); in pathological conditions, alternative mechanisms may compensate for the loss of vascular regulatory systems.
Although we have identified that NO signaling and sympathetic stimulation-dependent pathways are altered early regionally in sepsis, detailed studies are required to understand the complex mechanisms governing the susceptibility of certain vascular beds to dysfunction over others. Blood flow responses to administered agents, as performed herein, combine the integrated effects of direct vascular effects, changes in perfusion pressure (caused by the vasoactive effects of agents in other vascular beds), and autoregulatory responses. Due to the systemic nature of sepsis and the breadth of metabolic, circulatory, and immune perturbations characterizing its progression, it is likely that multiple mechanisms are involved. For example, in addition to the disturbed sGC–cyclic guanosine monophosphate signaling pathway [
39], receptor downregulation (due to internalization and decreased expression) is another mechanism underling the development of vasoplegia in sepsis [
32,
33]. Additionally, changes in metabolic function have been reported to directly affect vascular function. Lactic acidosis initiates intracellular signaling cascades in both endothelial and vascular smooth muscle cells, resulting in alterations in the calcium transient and the number of adrenoreceptors on the cell surface [
30,
40], the opening of ATP-sensitive potassium channels [
41], and an increase in the expression of inducible nitric oxide synthase in the endothelium and vascular smooth muscle cells [
42,
43]. In addition to the well-studied effects of metabolic stresses on endothelial cells (and vascular function), emerging data suggest that the vascular endothelium may play a pivotal role in metabolic homeostasis regulation and that endothelial dysfunction directly contributes to the development of metabolic disorders [
44]. The importance of uninterrupted energy production in sepsis has been reported recently [
45]. Thus, the combined effects that result in perturbed vascular signaling and dysregulated energy homeostasis are likely to be multifaceted in sepsis and require further interrogation.
Regardless of the mechanism, vascular dysfunction and vasoplegia are localized to certain regions. We have shown that inherent differences exist between different vascular beds and, surprisingly, the dysfunction in certain beds (mesenteric) progresses more quickly compared with other vascular beds. Moreover, our data suggest that the development of regional vascular dysfunction could be underway without evidence of BP dysregulation or a change in total organ blood flow. Selective targeting of damaged vascular beds to improve regional blood flow [
46] is a potential novel treatment which can eventually prevent or at least delay the onset of multi-organ damage and death in sepsis.
Notable limitations of our study include the lack of an antibiotic treatment in this relatively severe model of sepsis. Antibiotics were withheld because the time period studied herein reflects what could be considered the acute (or hyper-acute) phase of sepsis, in which patients would not typically be treated; in this way, the unabated progression of sepsis to septic shock could be studied. Nevertheless, since antibiotics constitute a mainstay treatment of sepsis, their impact on sepsis progression (and the hemodynamic consequences thereof) remains an important topic. Another limitation was the use of saline as a vehicle, rather than a 5% dextrose solution (in which the FS was prepared). Although our pilot studies revealed that dextrose administration had a minor influence on blood glucose levels in non-septic rats, the use of saline in control mice nonetheless confounds the interpretation of altered glucose handling in FS-treated mice, and follow-up studies are needed to address this point. A final limitation of the study is the use of anesthetic throughout. Although isoflurane is an agent of choice due to its modest cardiovascular, respiratory, and immunomodulatory effects in otherwise healthy individuals [
47,
48], in high-risk patients with pre-existing immune dysfunction or multiple organ failure, the choice/influence of anesthetics on the inflammatory response may have clinical implications [
49]. Therefore, the results presented herein must be interpreted with caution. Notwithstanding, it is noteworthy that ICU patients are often exposed to anesthetic agents for prolonged periods; therefore, the complex side effects of anesthetics may be clinically important in this population.