Neurological Benefits, Clinical Challenges, and Neuropathologic Promise of Medical Marijuana: A Systematic Review of Cannabinoid Effects in Multiple Sclerosis and Experimental Models of Demyelination
Abstract
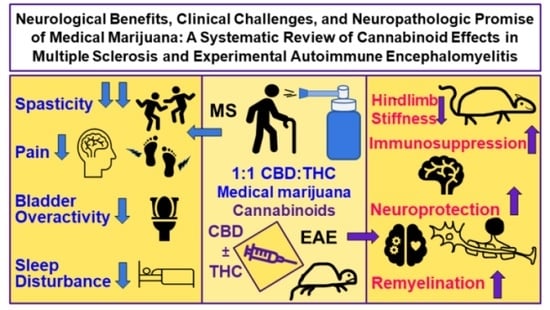
:1. Introduction
1.1. Clinical Subtypes of MS
1.2. Clinicopathologic Correlations in MS
1.3. Prevalence and Risk Factors of MS
1.4. Experimental Animal Models of MS
1.5. Therapeutic Challenges and Biomarker Research in MS
1.6. The Potential of Cannabinoids in MS
1.7. Reviewing Human and Animal Studies
2. Methods
3. Results
4. Discussion
4.1. Certainty of Evidence for Medical Marijuana Effects on MS Symptom Outcomes
4.2. Elucidating the Molecular Mechanisms of Medical Marijuana That May Be Therapeutic in MS
4.3. Mechanisms That Would Inhibit Innate Immune Assaults on CNS White Matter and Cortex
4.4. Mechanisms That Would Downregulate Antigen-Specific Adaptive Immune Responses within the CNS
4.5. Mechanisms That Would Promote Oligodendrocyte Survival
4.6. Mechanisms That Would Provide Neuroprotection and Stimulate Axonal Regeneration
4.7. Mechanisms That Would Support CNS Remyelination
4.8. Challenges and Opportunities of Cannabinoid Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kuhlmann, T.; Ludwin, S.; Prat, A.; Antel, J.; Brück, W.; Lassmann, H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017, 133, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.J.; Evans, R.; Griffiths, L.; Watkins, L.M.; Rees, M.I.; Magliozzi, R.; Allen, I.; McDonnell, G.; Kee, R.; Naughton, M.; et al. Meningeal inflammation and cortical demyelination in acute multiple sclerosis. Ann Neurol. 2018, 84, 829–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raeli, C.; Magliozzi, R.; Roncaroli, F.; Nicholas, R.; Howell, O.W.; Reynolds, R. B cell rich meningeal inflammation associates with increased spinal cord pathology in multiple sclerosis. Brain Pathol. 2020, 30, 779–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.P.; Ben-Zacharia, A.; Harris, C.; Smrtka, J. Multiple sclerosis, relapses, and the mechanism of action of adrenocorticotropic hormone. Front. Neurol. 2013, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and treatment of multiple sclerosis: A review. JAMA 2021, 325, 765. [Google Scholar] [CrossRef] [PubMed]
- Zwibel, H.L. Contribution of impaired mobility and general symptoms to the burden of multiple sclerosis. Adv. Ther. 2009, 26, 1043–1057. [Google Scholar] [CrossRef] [Green Version]
- Cameron, M.H.; Wagner, J.M. Gait abnormalities in multiple sclerosis: Pathogenesis, evaluation, and advances in treatment. Curr. Neurol. Neurosci. Rep. 2011, 11, 507–515. [Google Scholar] [CrossRef]
- Sadeghi Bahmani, D.; Kesselring, J.; Papadimitriou, M.; Bansi, J.; Pühse, U.; Gerber, M.; Shaygannejad, V.; Holsboer-Trachsler, E.; Brand, S. In Patients With Multiple Sclerosis, Both Objective and Subjective Sleep, Depression, Fatigue, and Paresthesia Improved After 3 Weeks of Regular Exercise. Front. Psychiatry 2019, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Nolan, R.C.; Akhand, O.; Rizzo, J.R.; Galetta, S.L.; Balcer, L.J. Evolution of visual outcomes in clinical trials for multiple sclerosis disease-modifying therapies. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2018, 38, 202–209. [Google Scholar] [CrossRef]
- Giedraitiene, N.; Drukteiniene, E.; Kizlaitiene, R.; Cimbalas, A.; Asoklis, R.; Kaubrys, G. Cognitive decline in multiple sclerosis is related to the progression of retinal atrophy and presence of oligoclonal bands: A 5-year follow-up study. Front. Neurol. 2021, 12, 678735. [Google Scholar] [CrossRef]
- Rusz, J.; Benova, B.; Ruzickova, H.; Novotny, M.; Tykalova, T.; Hlavnicka, J.; Uher, T.; Vaneckova, M.; Andelova, M.; Novotna, K.; et al. Characteristics of motor speech phenotypes in multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 19, 62–69. [Google Scholar] [CrossRef]
- De Looze, C.; Moreau, N.; Renié, L.; Kelly, F.; Ghio, A.; Rico, A.; Audoin, B.; Viallet, F.; Pelletier, J.; Petrone, C. Effects of cognitive impairment on prosodic parameters of speech production planning in multiple sclerosis. J. Neuropsychol. 2019, 13, 22–45. [Google Scholar] [CrossRef] [Green Version]
- Pula, J.H.; Newman-Toker, D.E.; Kattah, J.C. Multiple sclerosis as a cause of the acute vestibular syndrome. J. Neurol. 2013, 260, 1649–1654. [Google Scholar] [CrossRef]
- Zecca, C.; Riccitelli, G.C.; Disanto, G.; Singh, A.; Digesu, G.A.; Panicari, L.; Puccini, F.; Mattioli, M.; Tubaro, A.; Gobbi, C. Urinary incontinence in multiple sclerosis: Prevalence, severity and impact on patients’ quality of life. Eur. J. Neurol. 2016, 23, 1228–1234. [Google Scholar] [CrossRef]
- Al Dandan, H.B.; Coote, S.; McClurg, D. Prevalence of lower urinary tract symptoms in people with multiple sclerosis: A systematic review and meta-analysis. Int. J. MS Care 2020, 22, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Preziosi, G.; Gordon-Dixon, A.; Emmanuel, A. Neurogenic bowel dysfunction in patients with multiple sclerosis: Prevalence, impact, and management strategies. Degener. Neurol. Neuromuscul. Dis. 2018, 8, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lew-Starowicz, M.; Gianotten, W.L. Sexual dysfunction in patients with multiple sclerosis. Handb. Clin. Neurol. 2015, 130, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Mirabelli, E.; Elkabes, S. Neuropathic pain in multiple sclerosis and its animal models: Focus on mechanisms, knowledge gaps and future directions. Front. Neurol. 2021, 12, 793745. [Google Scholar] [CrossRef]
- Benedict, R.H.; Zivadinov, R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2011, 7, 332–342. [Google Scholar] [CrossRef]
- Rocca, M.A.; Amato, M.P.; de Stefano, N.; Enzinger, C.; Geurts, J.J.; Penner, I.K.; Rovira, A.; Sumowski, J.F.; Valsasina, P.; Filippi, M. MAGNIMS Study Group Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet. Neurol. 2015, 14, 302–317. [Google Scholar] [CrossRef]
- Oreja-Guevara, C.; Ayuso Blanco, T.; Brieva Ruiz, L.; Hernández Pérez, M.Á.; Meca-Lallana, V.; Ramió-Torrentà, L. Cognitive dysfunctions and assessments in multiple sclerosis. Front. Neurol. 2019, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Dlugonski, D.; Pilutti, L.; Sandroff, B.; McAuley, E. Premorbid physical activity predicts disability progression in relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2012, 323, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Walker, I.D.; Gonzalez, E.W. Review of intervention studies on depression in persons with multiple sclerosis. Issues Ment. Health Nurs. 2007, 28, 511–531. [Google Scholar] [CrossRef] [PubMed]
- Bamer, A.M.; Johnson, K.L.; Amtmann, D.; Kraft, G.H. Prevalence of sleep problems in individuals with multiple sclerosis. Mult. Scler. 2008, 14, 1127–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braley, T.J.; Boudreau, E.A. Sleep disorders in multiple sclerosis. Curr. Neurol. Neurosci. Rep. 2016, 16, 50. [Google Scholar] [CrossRef]
- Veauthier, C.; Gaede, G.; Radbruch, H.; Wernecke, K.D.; Paul, F. Poor Sleep in Multiple Sclerosis Correlates with Beck depression inventory values, but not with polysomnographic data. Sleep Disord. 2016, 2016, 8378423. [Google Scholar] [CrossRef] [Green Version]
- Freedman, M.S. Evidence for the efficacy of interferon beta-1b in delaying the onset of clinically definite multiple sclerosis in individuals with clinically isolated syndrome. Ther. Adv. Neurol. Disord. 2014, 7, 279–288. [Google Scholar] [CrossRef] [Green Version]
- National Multiple Sclerosis Society Symptoms & Diagnosis. Treating MS—Medications. 2022. Available online: https://www.nationalmssociety.org (accessed on 18 February 2022).
- Lublin, F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014, 72, 1–5. [Google Scholar] [CrossRef]
- Lublin, F.D.; Coetzee, T.; Cohen, J.A.; Marrie, R.A.; Thompson, A.J.; International Advisory Committee on Clinical Trials in MS. The 2013 clinical course descriptors for multiple sclerosis: A clarification. Neurology 2020, 94, 1088–1092. [Google Scholar] [CrossRef]
- Luca Chisari, C.G.; Zanghì, A.; Patti, F. Early-onset alcohol dependence and multiple sclerosis: Diagnostic challenges. Int. J. Environ. Res. Public Health 2021, 18, 5588. [Google Scholar] [CrossRef]
- Luchetti, S.; Fransen, N.L.; van Eden, C.G.; Ramaglia, V.; Mason, M.; Huitinga, I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: A retrospective autopsy cohort analysis. Acta Neuropathol. 2018, 135, 511–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbatemarco, J.R.; Griffin, A.; Jones, N.G.; Hartman, J.; McKee, K.; Wang, Z.; Nagel, S.J.; Machado, A.G.; Bethoux, F. Long-term outcomes of intrathecal baclofen in ambulatory multiple sclerosis patients: A single-center experience. Mult. Scler. 2021, 27, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zacharia, A.B. Therapeutics for multiple sclerosis symptoms. Mt. Sinai J. Med. 2011, 78, 176–191. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magyari, M. Gender differences in multiple sclerosis epidemiology and treatment response. Dan. Med. J. 2016, 63, B5212. [Google Scholar]
- Popescu, B.F.; Pirko, I.; Lucchinetti, C.F. Pathology of multiple sclerosis: Where do we stand? Continuum 2013, 19, 901–921. [Google Scholar] [CrossRef]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Reviews Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef]
- Cotsapas, C.; Mitrovic, M. Genome-wide association studies of multiple sclerosis. Clin. Transl. Immunol. 2018, 7, e1018. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr Virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Jakimovski, D.; Ramanathan, M.; Weinstock-Guttman, B.; Bergsland, N.; Ramasamay, D.P.; Carl, E.; Dwyer, M.G.; Zivadinov, R. Higher EBV response is associated with more severe gray matter and lesion pathology in relapsing multiple sclerosis patients: A case-controlled magnetization transfer ratio study. Mult. Scler. 2020, 26, 322–332. [Google Scholar] [CrossRef]
- Lanz, T.V.; Brewer, R.C.; Ho, P.P.; Moon, J.-S.; Jude, K.M.; Fernandez, D.; Fernandes, R.A.; Gomez, A.M.; Nadj, G.-S.; Bartley, C.M.; et al. Clonally expanded B Cells in Multiple Sclerosis bind EBV EBNA1 and GlialCAM. Nature 2022. [Google Scholar] [CrossRef] [PubMed]
- Zdimerova, H.; Murer, A.; Engelmann, C.; Raykova, A.; Deng, Y.; Gujer, C.; Rühl, J.; McHugh, D.; Caduff, N.; Naghavian, R.; et al. Attenuated immune control of Epstein–Barr virus in humanized mice is associated with the multiple sclerosis risk factor HLA-DR15. Eur. J. Immunol. 2021, 51, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Nali, L.H.; Olival, G.S.; Montenegro, H.; da Silva, I.T.; Dias-Neto, E.; Naya, H.; Spangenberg, L.; Penalva-de-Oliveira, A.C.; Romano, C.M. Human endogenous retrovirus and multiple sclerosis: A review and transcriptome findings. Mult. Scler. Relat. Disord. 2022, 57, 103383. [Google Scholar] [CrossRef] [PubMed]
- Mameli, G.; Poddighe, L.; Mei, A.; Uleri, E.; Sotgiu, S.; Serra, C.; Manetti, R.; Dolei, A. Expression and activation by Epstein Barr virus of human endogenous retroviruses-W in blood cells and astrocytes: Inference for multiple sclerosis. PLoS ONE 2012, 7, e44991. [Google Scholar] [CrossRef] [PubMed]
- Mameli, G.; Madeddu, G.; Mei, A.; Uleri, E.; Poddighe, L.; Delogu, L.G.; Maida, I.; Babudieri, S.; Serra, C.; Manetti, R.; et al. Activation of MSRV-type endogenous retroviruses during infectious mononucleosis and Epstein-Barr virus latency: The missing link with multiple sclerosis? PLoS ONE 2013, 8, e78474. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Simpson-Yap, S.; Taylor, B.; Blizzard, L.; Lucas, R.; Ponsonby, A.-L.; Broadley, S.; van der Mei, I. Markers of Epstein-Barr virus and human herpesvirus-6 infection and multiple sclerosis clinical progression. Mult. Scler. Relat. Disord. 2022, 59, 103561. [Google Scholar] [CrossRef]
- Kohl, H.M.; Castillo, A.R.; Ochoa-Repáraz, J. The microbiome as a therapeutic target for multiple sclerosis: Can genetically engineered probiotics treat the disease? Diseases 2020, 8, 33. [Google Scholar] [CrossRef]
- Scolding, N.J.; Jones, J.; Compston, D.A.; Morgan, B.P. Oligodendrocyte susceptibility to injury by T-cell perforin. Immunology 1990, 70, 6–10. [Google Scholar]
- Zeine, R.; Pon, R.; Ladiwala, U.; Antel, J.P.; Filion, L.G.; Freedman, M.S. Mechanism of gamma-delta T cell-induced human oligodendrocyte cytotoxicity: Relevance to multiple sclerosis. J. Neuroimmunol. 1998, 87, 49–61. [Google Scholar] [CrossRef]
- Zeine, R.; Cammer, W.; Barbarese, E.; Liu, C.-C.; Raine, C.S. Structural dynamics of oligodendrocyte lysis by perforin in culture: Relevance to multiple sclerosis. J. Neurosci. Res. 2001, 64, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Stockton, M.E. Premyelinating oligodendrocytes: Mechanisms underlying cell survival and integration. Front. Cell Dev. Biol. 2021, 9, 714169. [Google Scholar] [CrossRef]
- Procaccini, C.; de Rosa, V.; Pucino, V.; Formisano, L.; Matarese, G. animal models of multiple sclerosis. Eur. J. Pharmacol. 2015, 759, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Burrows, D.J.; McGown, A.; Jain, S.A.; de Felice, M.; Ramesh, T.M.; Sharrack, B.; Majid, A. Animal models of multiple sclerosis: From rodents to zebrafish. Mult. Scler. J. 2019, 25, 306–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faber, H.; Kurtoic, D.; Krishnamoorthy, G.; Weber, P.; Pütz, B.; Müller-Myhsok, B.; Weber, F.; Andlauer, T. Gene Expression in Spontaneous Experimental Autoimmune Encephalomyelitis Is Linked to Human Multiple Sclerosis Risk Genes. Front. Immunol. 2020, 11, 2165. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Dieu, R.S.; Wais, V.; Sørensen, M.Z.; Marczynska, J.; Dubik, M.; Kavan, S.; Thomassen, M.; Burton, M.; Kruse, T.; Khorooshi, R.; et al. Central nervous system-endogenous TLR7 and TLR9 induce different immune responses and effects on experimental autoimmune encephalomyelitis. Front. Neurosci. 2021, 15, 685645. [Google Scholar] [CrossRef]
- Khorooshi, R.; Mørch, M.T.; Holm, T.H.; Berg, C.T.; Dieu, R.T.; Dræby, D.; Issazadeh-Navikas, S.; Weiss, S.; Lienenklaus, S.; Owens, T. Induction of endogenous type I interferon within the central nervous system plays a protective role in experimental autoimmune encephalomyelitis. Acta Neuropathol. 2015, 130, 107–118. [Google Scholar] [CrossRef]
- Jensen, M.A.; Arnason, B.G.; Toscas, A.; Noronha, A. Preferential increase of IL-2R+ CD4+ T cells and CD45RB- CD4+ T cells in the central nervous system in experimental allergic encephalomyelitis. J. Neuroimmunol. 1992, 38, 255–261. [Google Scholar] [CrossRef]
- Zeine, R.; Owens, T. Loss rather than downregulation of CD4+ T cells as a mechanism for remission from experimental allergic encephalomyelitis. J. Neuroimmunol. 1993, 44, 193–198. [Google Scholar] [CrossRef]
- Renno, T.; Zeine, R.; Girard, J.M.; Gillani, S.; Dodelet, V.; Owens, T. Selective enrichment of Th1 CD45RBlow CD4+ T cells in autoimmune infiltrates in experimental allergic encephalomyelitis. Int. Immunol. 1994, 6, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.H.; Hardin-Pouzet, H.; Verge, G.; Owens, T. Astrocytes and microglia express inducible nitric oxide synthase in mice with experimental allergic encephalomyelitis. J. Neuroimmunol. 1997, 74, 121–129. [Google Scholar] [CrossRef]
- Miller, S.D.; Karpus, W.J.; Davidson, T.S. Experimental autoimmune encephalomyelitis in the mouse. Curr. Protoc. Immunol. 2010, 88, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kap, Y.S.; Laman, J.D.; ’t Hart, B.A. Experimental autoimmune encephalomyelitis in the common marmoset, a bridge between rodent EAE and multiple sclerosis for immunotherapy development. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2010, 5, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.; Ahn, M.; Matsumoto, Y. Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: Recent insights from macrophages. Anat. Cell Biol. 2012, 45, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirko, I.; Gamez, J.; Johnson, A.J.; Macura, S.I.; Rodriguez, M. Dynamics of MRI lesion development in an animal model of viral-induced acute progressive CNS demyelination. NeuroImage 2004, 21, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.; Tanaka, H.; Hasanovic, F.; Tsunoda, I. Theiler’s virus infection: Pathophysiology of demyelination and neurodegeneration. Pathophysiol. Off. J. Int. Soc. Pathophysiol. 2011, 18, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakimov, V.; Schweiger, F.; Zhan, J.; Behrangi, N.; Horn, A.; Schmitz, C.; Hochstrasser, T.; Kipp, M. Continuous cuprizone intoxication allows active experimental autoimmune encephalomyelitis induction in C57BL/6 mice. Histochem. Cell Biol. 2019, 152, 119–131. [Google Scholar] [CrossRef]
- Oveland, E.; Ahmad, I.; Lereim, R.R.; Kroksveen, A.C.; Barsnes, H.; Guldbrandsen, A.; Myhr, K.-M.; Bø, L.; Berven, F.S.; Wergeland, S. Cuprizone and EAE mouse frontal cortex proteomics revealed proteins altered in multiple sclerosis. Sci. Rep. 2021, 11, 7174. [Google Scholar] [CrossRef]
- Plemel, J.R.; Michaels, N.J.; Weishaupt, N.; Caprariello, A.V.; Keough, M.B.; Rogers, J.A.; Yukseloglu, A.; Lim, J.; Patel, V.V.; Rawji, K.S.; et al. Mechanisms of lysophosphatidylcholine-induced demyelination: A primary lipid disrupting myelinopathy. Glia 2018, 66, 327–347. [Google Scholar] [CrossRef]
- Zarzuelo-Romero, M.J.; Pérez-Ramírez, C.; Cura, Y.; Carrasco-Campos, M.I.; Marangoni-Iglecias, L.M.; Ramírez-Tortosa, M.C.; Jiménez-Morales, A. Influence of genetic polymorphisms on clinical outcomes of glatiramer acetate in multiple sclerosis patients. J. Pers. Med. 2021, 11, 1032. [Google Scholar] [CrossRef] [PubMed]
- Landi, D.; Signori, A.; Cellerino, M.; Fenu, G.; Nicoletti, C.G.; Ponzano, M.; Mancuso, E.; Fronza, M.; Ricchiuto, M.E.; Boffa, G.; et al. What happens after fingolimod discontinuation? A multicentre real-life experience. J. Neurol. 2022, 269, 796–804. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Merkel, B.; Brown, J.W.L.; Ryerson, L.Z.; Kister, I.; Malpas, C.B.; Horakova, D.; Spelman, T.; Izquierdo, G.; Eichau, S.; et al. Timing of high-efficacy therapy for multiple sclerosis: A retrospective observational cohort study. Lancet Neurol. 2020, 19, 307–316. [Google Scholar] [CrossRef]
- Iaffaldano, P.; Lucisano, G.; Caputo, F.; Paolicelli, D.; Patti, F.; Zaffaroni, M.; Brescia Morra, V.; Pozzilli, C.; de Luca, G.; Inglese, M.; et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther. Adv. Neurol. Disord. 2021, 14, 175628642110195. [Google Scholar] [CrossRef] [PubMed]
- Rafiee Zadeh, A.; Askari, M.; Azadani, N.N.; Ataei, A.; Ghadimi, K.; Tavoosi, N.; Falahatian, M. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 1. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 95–104. [Google Scholar] [PubMed]
- Rafiee Zadeh, A.; Ghadimi, K.; Ataei, A.; Askari, M.; Sheikhinia, N.; Tavoosi, N.; Falahatian, M. Mechanism and adverse effects of multiple sclerosis drugs: A review article. Part 2. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 105–114. [Google Scholar]
- Dobson, R.; Ramagopalan, S.; Davis, A.; Giovannoni, G. Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: A meta-analysis of prevalence, prognosis and effect of latitude. J. Neurol. Neurosurg. Psychiatry 2013, 84, 909–914. [Google Scholar] [CrossRef]
- Munger, K.L.; Hongell, K.; Åivo, J.; Soilu-Hänninen, M.; Surcel, H.M.; Ascherio, A. 25-hydroxyvitamin D deficiency and risk of MS among women in the finnish maternity cohort. Neurology 2017, 89, 1578–1583. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the redox status in multiple sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for peripheral biomarkers in neurodegenerative diseases: The Tryptophan-Kynurenine metabolic pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- Biernacki, T.; Sandi, D.; Bencsik, K.; Vécsei, L. Kynurenines in the pathogenesis of multiple sclerosis: Therapeutic perspectives. Cells 2020, 9, 1564. [Google Scholar] [CrossRef] [PubMed]
- Cofield, S.S.; Salter, A.; Tyry, T.; Crowe, C.; Cutter, G.R.; Fox, R.J.; Marrie, R.A. Perspectives on marijuana use and effectiveness: A survey of NARCOMS participants. Neurology. Clin. Pract. 2017, 7, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Jackson, S.J.; Pryce, G. Cannabinoid control of neuroinflammation related to multiple sclerosis. Br. J. Pharmacol. 2007, 152, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011, 163, 1507–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilliard, A.; Stott, C.; Wright, S.; Guy, G.; Pryce, G.; Al-Izki, S.; Bolton, C.; Giovannoni, G. Evaluation of the effects of Sativex (THC BDS: CBD BDS) on inhibition of spasticity in a chronic relapsing experimental allergic autoimmune encephalomyelitis: A model of multiple sclerosis. ISRN Neurol. 2012, 2012, 802649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, J.M.; Kummari, E.; Sherman, J.; Yang, E.J.; Dhital, S.; Gilfeather, C.; Yray, G.; Morgan, T.; Kaplan, B.L.F. CBD suppression of EAE is correlated with early inhibition of splenic IFN-γ + CD8+ T cells and modest inhibition of neuroinflammation. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2021, 16, 346–362. [Google Scholar] [CrossRef]
- Pryce, G.; Ahmed, Z.; Hankey, D.J.; Jackson, S.J.; Croxford, J.L.; Pocock, J.M.; Ledent, C.; Petzold, A.; Thompson, A.J.; Giovannoni, G.; et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain A. J. Neurol. 2003, 126, 2191–2202. [Google Scholar] [CrossRef]
- Wade, D.T.; Robson, P.; House, H.; Makela, P.; Aram, J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin. Rehabil. 2003, 17, 21–29. [Google Scholar] [CrossRef]
- Killestein, J.; Uitdehaag, B.M.; Polman, C.H. Cannabinoids in multiple sclerosis: Do they have a therapeutic role? Drugs 2004, 64, 1–11. [Google Scholar] [CrossRef]
- Smith, P.F. New approaches in the management of spasticity in multiple sclerosis patients: Role of cannabinoids. Ther. Clin. Risk Manag. 2010, 6, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Zajicek, J.P.; Apostu, V.I. Role of cannabinoids in multiple sclerosis. CNS Drugs 2011, 25, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, T.; Honce, J.M. Cannabis and multiple sclerosis-the way forward. Front. Neurol. 2017, 8, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiurchiù, V.; van der Stelt, M.; Centonze, D.; Maccarrone, M. The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: Clues for other neuroinflammatory diseases. Prog. Neurobiol. 2018, 160, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.; Cameron, M. Cannabinoids for treatment of MS symptoms: State of the evidence. Curr. Neurol. Neurosci. Rep. 2018, 18, 50. [Google Scholar] [CrossRef]
- Croxford, J.L.; Miller, S.D. Immunoregulation of a viral model of multiple sclerosis using the synthetic cannabinoid R+WIN55,212. J. Clin. Investig. 2003, 111, 1231–1240. [Google Scholar] [CrossRef] [Green Version]
- Arévalo-Martín, A.; Vela, J.M.; Molina-Holgado, E.; Borrell, J.; Guaza, C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 2511–2516. [Google Scholar] [CrossRef]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [Green Version]
- Grotenhermen, F.; Müller-Vahl, K. The therapeutic potential of cannabis and cannabinoids. Dtsch. Arztebl. Int. 2012, 109, 495–501. [Google Scholar] [CrossRef]
- Rodrigues, R.S.; Lourenço, D.M.; Paulo, S.L.; Mateus, J.M.; Ferreira, M.F.; Mouro, F.M.; Moreira, J.B.; Ribeiro, F.F.; Sebastião, A.M.; Xapelli, S. Cannabinoid Actions on Neural Stem Cells: Implications for Pathophysiology. Molecules 2019, 24, 1350. [Google Scholar] [CrossRef] [Green Version]
- Jones, É.; Vlachou, S. A critical review of the role of the cannabinoid compounds δ9-tetrahydrocannabinol (δ9-THC) and cannabidiol (CBD) and their combination in multiple sclerosis treatment. Molecules 2020, 25, 4930. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. Available online: http://prisma-statement.org/documents/PRISMA_2020_checklist.pdf (accessed on 1 August 2021). [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2 (Updated February 2021); Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 1 August 2021).
- Schünemann, H.J.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H.; Cochrane GRADEing Methods Group. Completing ‘summary of findings’ tables and grading the certainty of the evidence. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd: Chichester, UK, 2019; pp. 375–402. [Google Scholar] [CrossRef]
- D’hooghe, M.; Willekens, B.; Delvaux, V.; D’haeseleer, M.; Guillaume, D.; Laureys, G.; Nagels, G.; Vanderdonckt, P.; van Pesch, V.; Popescu, V. Sativex® (nabiximols) cannabinoid oromucosal spray in patients with resistant multiple sclerosis spasticity: The Belgian experience. BMC Neurol. 2021, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Sorosina, M.; Clarelli, F.; Ferrè, L.; Osiceanu, A.M.; Unal, N.T.; Mascia, E.; Martinelli, V.; Comi, G.; Benigni, F.; Esposito, F.; et al. Clinical response to nabiximols correlates with the downregulation of immune pathways in multiple sclerosis. Eur. J. Neurol. 2018, 25, 934–970. [Google Scholar] [CrossRef]
- Flachenecker, P.; Henze, T.; Zettl, U.K. Nabiximols (THC/CBD oromucosal spray, Sativex®) in clinical practice—Results of a multicenter, non-interventional study (MOVE 2) in patients with multiple sclerosis spasticity. Eur. Neurol. 2014, 71, 271–279. [Google Scholar] [CrossRef]
- Flachenecker, P.; Henze, T.; Zettl, U. Long-term effectiveness and safety of nabiximols (tetrahydrocannabinol/cannabidiol oromucosal spray) in clinical practice. Eur. Neurol. 2014, 72, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Feneberg, W.; Meier, M.; Pöllmann, W. Clinical experience with THC:CBD oromucosal spray in patients with multiple sclerosis-related spasticity. Int. J. Neurosci. 2014, 124, 652–656. [Google Scholar] [CrossRef]
- Paolicelli, D.; Direnzo, V.; Manni, A.; D’Onghia, M.; Tortorella, C.; Zoccolella, S.; Di Lecce, V.; Iaffaldano, A.; Trojano, M. Long-term data of efficacy, safety, and tolerability in a real-life setting of THC/CBD oromucosal spray-treated multiple sclerosis patients. J. Clin. Pharmacol. 2016, 56, 845–851. [Google Scholar] [CrossRef]
- Novotna, A.; Mares, J.; Ratcliffe, S.; Novakova, I.; Vachova, M.; Zapletalova, O.; Gasperini, C.; Pozzilli, C.; Cefaro, L.; Comi, G. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (sativex ®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis: Sativex for refractory spasticity in MS. Eur. J. Neurol. 2011, 18, 1122–1131. [Google Scholar] [CrossRef]
- Collin, C.; Ehler, E.; Waberzinek, G.; Alsindi, Z.; Davies, P.; Powell, K.; Notcutt, W.; O’Leary, C.; Ratcliffe, S.; Nováková, I.; et al. A double-blind, randomized, placebo-controlled, parallel-group study of sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol. Res. 2010, 32, 451–459. [Google Scholar] [CrossRef]
- Collin, C.; Davies, P.; Mutiboko, I.K.; Ratcliffe, S. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur. J. Neurol. 2007, 14, 290–296. [Google Scholar] [CrossRef]
- Turri, M.; Teatini, F.; Donato, F.; Zanette, G.; Tugnoli, V.; Deotto, L.; Bonetti, B.; Squintani, G. Pain modulation after oromucosal cannabinoid spray (SATIVEX®) in patients with multiple sclerosis: A study with quantitative sensory testing and laser-evoked potentials. Medicines 2018, 5, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, M.; Naro, A.; Leo, A.; Sessa, E.; D’Aleo, G.; Bramanti, P.; Calabrò, R.S. Evaluating Sativex® in neuropathic pain management: A clinical and neurophysiological assessment in multiple sclerosis. Pain Med. 2016, 17, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langford, R.M.; Mares, J.; Novotna, A.; Vachova, M.; Novakova, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2013, 260, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, G.; Aponte, R.; Bruzzese, D.; Guarcello, G.; Manzo, V.; Napolitano, M.; Moreggia, O.; Chiariello, F.; Florio, C. THC/CBD oromucosal spray in patients with multiple sclerosis overactive bladder: A pilot prospective study. Neurol. Sci. 2018, 39, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Kavia, R.B.; de Ridder, D.; Constantinescu, C.S.; Stott, C.G.; Fowler, C.J. Randomized controlled trial of Sativex to treat detrusor overactivity in multiple sclerosis. Mult. Scler. 2010, 16, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Braley, T.J.; Whibley, D.; Alschuler, K.N.; Ehde, D.M.; Chervin, R.D.; Clauw, D.J.; Williams, D.; Kratz, A.L. Cannabinoid use among Americans with MS: Current trends and gaps in knowledge. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front. Immunol. 2018, 9, 1782. [Google Scholar] [CrossRef]
- González-García, C.; Torres, I.M.; García-Hernández, R.; Campos-Ruíz, L.; Esparragoza, L.R.; Coronado, M.J.; Grande, A.G.; García-Merino, A.; Sánchez López, A.J. Mechanisms of action of cannabidiol in adoptively transferred experimental autoimmune encephalomyelitis. Exp. Neurol. 2017, 298, 57–67. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Gao, F.; Kaushansky, N.; Coppola, G.; Vogel, Z. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J. Neuroinflamm. 2016, 13, 136. [Google Scholar] [CrossRef] [Green Version]
- Kong, W.; Li, H.; Tuma, R.F.; Ganea, D. Selective CB2 receptor activation ameliorates EAE by reducing th17 differentiation and immune cell accumulation in the CNS. Cell. Immunol. 2014, 287, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Martin, B.R.; Adler, M.W.; Razdan, R.J.; Kong, W.; Ganea, D.; Tuma, R.F. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2009, 4, 249–259. [Google Scholar] [CrossRef]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Shriver, L.P.; Ledent, C.; Cheng, X.; Carrier, E.J.; Mann, M.K.; Giovannoni, G.; et al. Direct suppression of cns autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Aguado, T.; Resel, E.; Chara, J.C.; Matute, C.; Mato, S.; Guzman, M.; Palazuelos, J. Δ9-tetrahydrocannabinol promotes functional remyelination in the mouse brain. Br. J. Pharmacol. 2021, 178, 4176–4192. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Roig, J.; Agbemenyah, H.Y.; Celarain, N.; Quintana, E.; Ramió-Torrentà, L.; Havemann-Reinecke, U. Dose-dependent effect of cannabinoid WIN-55,212-2 on myelin repair following a demyelinating insult. Sci. Rep. 2020, 10, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mecha, M.; Feliú, A.; Machín, I.; Cordero, C.; Mestre, L.; de Castro, F.; Clemente, D.; Guaza, C. 2-AG limits Theiler’s virus induced acute neuroinflammation by modulating microglia and promoting MDSCS. Glia 2018, 66, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Arevalo-Martin, A.; Molina-Holgado, E.; Guaza, C. A CB1/CB2 receptor agonist, WIN 55,212-2, exerts its therapeutic effect in a viral autoimmune model of multiple sclerosis by restoring self-tolerance to myelin. Neuropharmacology 2012, 63, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Mestre, L.; Docagne, F.; Correa, F.; Loría, F.; Hernangómez, M.; Borrell, J.; Guaza, C. A cannabinoid agonist interferes with the progression of a chronic model of multiple sclerosis by downregulating adhesion molecules. Mol. Cell. Neurosci. 2009, 40, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Pertwee, R.G. Cannabinoids and multiple sclerosis. Pharmacol. Ther. 2002, 95, 165–174. [Google Scholar] [CrossRef]
- Svendsen, K.B.; Jensen, T.S.; Bach, F.W. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo-controlled crossover trial. BMJ 2004, 329, 253. [Google Scholar] [CrossRef] [Green Version]
- Wissel, J.; Haydn, T.; Müller, J.; Brenneis, C.; Berger, T.; Poewe, W.; Schelosky, L.D. Low dose treatment with the synthetic cannabinoid Nabilone significantly reduces spasticity-related pain: A double-blind placebo-controlled cross-over trial. J. Neurol. 2006, 253, 1337–1341. [Google Scholar] [CrossRef]
- Rog, D.J. Cannabis-based medicines in multiple sclerosis—A review of clinical studies. Immunobiology 2010, 215, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Zajicek, J.; Ball, S.; Wright, D.; Vickery, J.; Nunn, A.; Miller, D.; Cano, M.G.; McManus, D.; Mallik, S.; Hobart, J.; et al. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): A randomised, placebo-controlled trial. Lancet Neurol. 2013, 12, 857–865. [Google Scholar] [CrossRef] [Green Version]
- Freeman, R.M.; Adekanmi, O.; Waterfield, M.R.; Waterfield, A.E.; Wright, D.; Zajicek, J. The effect of cannabis on urge incontinence in patients with multiple sclerosis: A multicentre, randomised placebo-controlled trial (CAMS-LUTS). Int. Urogynecol. J. Pelvic Floor Dysfunct. 2006, 17, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Koppel, B.S.; Brust, J.C.; Fife, T.; Bronstein, J.; Youssof, S.; Gronseth, G.; Gloss, D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Zettl, U.K.; Rommer, P.; Hipp, P.; Patejdl, R. Evidence for the efficacy and effectiveness of THC-CBD oromucosal spray in symptom management of patients with spasticity due to multiple sclerosis. Ther. Adv. Neurol. Disord. 2016, 9, 9–30. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.; Germanos, R.; Weier, M.; Pollard, J.; Degenhardt, L.; Hall, W.; Buckley, N.; Farrell, M. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: A systematic review of reviews. Curr. Neurol. Neurosci. Rep. 2018, 18, 1–12. [Google Scholar] [CrossRef]
- Akgün, K.; Essner, U.; Seydel, C.; Ziemssen, T. Daily practice managing resistant multiple sclerosis spasticity with delta-9-Tetrahydrocannabinol: Cannabidiol oromucosal spray: A systematic review of observational studies. J. Cent. Nerv. Syst. Dis. 2019, 11, 1179573519831997. [Google Scholar] [CrossRef]
- Filippi, M.; Brück, W.; Chard, D.; Fazekas, F.; Geurts, J.J.G.; Enzinger, C.; Hametner, S.; Kuhlmann, T.; Preziosa, P.; Rovira, À.; et al. Association between pathological and MRI findings in multiple sclerosis. Lancet Neurol. 2019, 18, 198–210. [Google Scholar] [CrossRef]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef]
- Holdcroft, A.; Maze, M.; Doré, C.; Tebbs, S.; Thompson, S. A multicenter dose-escalation study of the analgesic and adverse effects of an oral cannabis extract (Cannador) for postoperative pain management. Anesthesiology 2006, 104, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, E.; Randhawa, G.; Longoria, V.; Zeine, R. Medical marijuana effects in movement disorders, focus on huntington disease; a literature review. J. Pharm. Pharm. Sci. 2020, 23, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hess, E.J.; Moody, K.A.; Geffrey, A.L.; Pollack, S.F.; Skirvin, L.A.; Bruno, P.L.; Paolini, J.L.; Thiele, E.A. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 2016, 57, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- United States Food & Drug Administration. FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2018. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms (accessed on 1 January 2021).
- Senn Cannazza, G.; Biagini, G. Receptors and channels possibly mediating the effects of phytocannabinoids on seizures and epilepsy. Pharmaceuticals 2020, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.M.; DasGupta, R.; Dalton, C.; Wiseman, O.J.; Berkley, K.J.; Fowler, C.J. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult. Scler. 2004, 10, 425–433. [Google Scholar] [CrossRef]
- Shannon, S.; Lewis, N.; Lee, H.; Hughes, S. Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm. J. 2019, 23, 18–041. [Google Scholar] [CrossRef] [Green Version]
- Messina, S.; Solaro, C.; Righini, I.; Bergamaschi, R.; Bonavita, S.; Bossio, R.B.; Morra, V.B.; Costantino, G.; Cavalla, P.; Centonze, D.; et al. Sativex in resistant multiple sclerosis spasticity: Discontinuation study in a large population of Italian patients (SA.FE. study). PLoS ONE 2017, 12, e0180651. [Google Scholar] [CrossRef] [Green Version]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef] [Green Version]
- Di Marzo, V.; Stella, N.; Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Reviews. Neurosci. 2015, 16, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Gopher, A.; Almog, S.; Martin, B.R.; Compton, D.R. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamashita, A.; Waku, K. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mecha, M.; Carrilo-Salinas, F.J.; Feliu, A.; Mestre, L.; Guaza, C. Perspectives on cannabis-based therapy of multiple sclerosis: A mini review. Front. Cell. Neurosci. 2020, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and cannabinoid receptors: The story so far. Iscience 2020, 23, 101301. [Google Scholar] [CrossRef]
- Miranzadeh Mahabadi, H.; Bhatti, H.; Laprairie, R.B.; Taghibiglou, C. Cannabinoid receptors distribution in mouse cortical plasma membrane compartments. Mol. Brain 2021, 14, 89. [Google Scholar] [CrossRef]
- Molina-Holgado, F.; Molina-Holgado, E.; Guaza, C.; Rothwell, N.J. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide-induced nitric oxide release in astrocyte cultures. J. Neurosci. Res. 2002, 67, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Cabral, G.A.; Marciano-Cabral, F. Cannabinoid receptors in microglia of the central nervous system: Immune functional relevance. J. Leukoc. Biol. 2005, 78, 1192–1197. [Google Scholar] [CrossRef]
- Herkenham, M.; Lynn, A.B.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J. Neurosci. Off. J. Soc. Neurosci. 1991, 11, 563–583. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araque, A.; Castillo, P.E.; Manzoni, O.J.; Tonini, R. Synaptic functions of endocannabinoid signaling in health and disease. Neuropharmacology 2017, 124, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [Green Version]
- Glass, M.; Dragunow, M.; Faull, R.L. Cannabinoid receptors in the human brain: A detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 1997, 77, 299–318. [Google Scholar] [CrossRef]
- Ehrhart, J.; Obregon, D.; Mori, T.; Hou, H.; Sun, N.; Bai, Y.; Klein, T.; Fernandez, F.; Tan, J.; Shytle, R.D. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J. Neuroinflamm. 2005, 2, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maresz, K.; Carrier, E.J.; Ponomarev, E.D.; Hillard, C.J.; Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 2005, 95, 437–445. [Google Scholar] [CrossRef]
- Miller, A.M.; Stella, N. CB2 receptor-mediated migration of immune cells: It can go either way. Br. J. Pharmacol. 2008, 153, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Cabral, G.A.; Ferreira, G.A.; Jamerson, M.J. Endocannabinoids and the immune system in health and disease. Handb. Exp. Pharmacol. 2015, 231, 185–211. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Barroso-Chinea, P.; Rico, A.J.; Conte-Perales, L.; Callén, L.; Roda, E.; Gómez-Bautista, V.; López, I.P.; Lluis, C.; Labandeira-García, J.L.; et al. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J. Psychopharmacol. 2011, 25, 97–104. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Stempel, A.V.; Stumpf, A.; Zhang, H.Y.; Özdoğan, T.; Pannasch, U.; Theis, A.K.; Otte, D.M.; Wojtalla, A.; Rácz, I.; Ponomarenko, A.; et al. Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron 2016, 90, 795–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittel, B.N. Direct suppression of autoreactive lymphocytes in the central nervous system via the CB2 receptor. Br. J. Pharmacol. 2008, 153, 271–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagano, C.; Navarra, G.; Coppola, L.; Bifulco, M.; Laezza, C. Molecular mechanism of cannabinoids in cancer progression. Int. J. Mol. Sci. 2021, 22, 3680. [Google Scholar] [CrossRef]
- Moriconi, A.; Cerbara, I.; Maccarrone, M.; Topai, A. GPR55: Current knowledge and future perspectives of a purported “Type-3” cannabinoid receptor. Curr. Med. Chem. 2010, 17, 1411–1429. [Google Scholar] [CrossRef] [PubMed]
- Marichal-Cancino, B.A.; Fajardo-Valdez, A.; Ruiz-Contreras, A.E.; Mendez-Díaz, M.; Prospero-García, O. Advances in the physiology of GPR55 in the central nervous system. Curr. Neuropharmacol. 2017, 15, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Chiurchiù, V.; Lanuti, M.; De, B.M.; Battistini, L.; Maccarrone, M. The differential characterization of GPR55 receptor in human peripheral blood reveals a distinctive expression in monocytes and NK cells and a proinflammatory role in these innate cells. Int. Immunol. 2015, 27, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Pistis, M.; Melis, M. From surface to nuclear receptors: The endocannabinoid family extends its assets. Curr. Med. Chem. 2010, 17, 1450–1467. [Google Scholar] [CrossRef]
- Xia, R.; Samad, T.A.; Btesh, J.; Jiang, L.H.; Kays, I.; Stjernborg, L.; Dekker, N. TRPV1 signaling: Mechanistic understanding and therapeutic potential. Curr. Top. Med. Chem. 2011, 11, 2180–2191. [Google Scholar] [CrossRef]
- Eljaschewitsch, E.; Witting, A.; Mawrin, C.; Lee, T.; Schmidt, P.M.; Wolf, S.; Hoertnagl, H.; Raine, C.S.; Schneider-Stock, R.; Nitsch, R.; et al. The endocannabinoid anandamide protects neurons during CNS inflammation by induction of MKP-1 in microglial cells. Neuron 2006, 49, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Centonze, D.; Bari, M.; Rossi, S.; Prosperetti, C.; Furlan, R.; Fezza, F.; de Chiara, V.; Battistini, L.; Bernardi, G.; Bernardini, S.; et al. The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain A. J. Neurol. 2007, 130, 2543–2553. [Google Scholar] [CrossRef] [Green Version]
- Di Filippo, M.; Pini, L.A.; Pelliccioli, G.P.; Calabresi, P.; Sarchielli, P. Abnormalities in the cerebrospinal fluid levels of endocannabinoids in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Romero, J.P.; Tolón, R.M.; Clemente, D.; Docagne, F.; Hillard, C.J.; Guaza, C.; Romero, J. Cannabinoid CB1 and CB2 receptors and fatty acid amide hydrolase are specific markers of plaque cell subtypes in human multiple sclerosis. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2396–2402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynoso-Moreno, I.; Tietz, S.; Vallini, E.; Engelhardt, B.; Gertsch, J.; Chicca, A. Selective endocannabinoid reuptake inhibitor WOBE437 reduces disease progression in a mouse model of multiple sclerosis. ACS Pharmacol. Transl. Sci. 2021, 4, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, Á.; Bernal-Chico, A.; Colomer, T.; Rodríguez-Antigüedad, A.; Matute, C.; Mato, S. Gene expression analysis of astrocyte and microglia endocannabinoid signaling during autoimmune demyelination. Biomolecules 2020, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Fransson, J.; Gómez-Conde, A.I.; Romero-Imbroda, J.; Fernández, O.; Leyva, L.; Rodríguez de Fonseca, F.; Chun, J.; Louapre, C.; Van-Evercooren, A.B.; Zujovic, V.; et al. Activation of macrophages by lysophosphatidic acid through the lysophosphatidic acid receptor 1 as a novel mechanism in multiple sclerosis pathogenesis. Mol. Neurobiol. 2021, 58, 470–482. [Google Scholar] [CrossRef] [PubMed]
- González de San Román, E.; Manuel, I.; Ledent, C.; Chun, J.; Rodríguez de Fonseca, F.; Estivill-Torrús, G.; Santín, L.J.; Rodríguez Puertas, R. CB1 and LPA1 receptors relationship in the mouse central nervous system. Front. Mol. Neurosci. 2019, 12. [Google Scholar] [CrossRef]
- Dopkins, N.; Miranda, K.; Wilson, K.; Holloman, B.L.; Nagarkatti, P.; Nagarkatti, M. Effects of orally administered cannabidiol on neuroinflammation and intestinal inflammation in the attenuation of experimental autoimmune encephalomyelitis. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2021, 1–18. [Google Scholar] [CrossRef]
- Ni, X.; Geller, E.B.; Eppihimer, M.J.; Eisenstein, T.K.; Adler, M.W.; Tuma, R.F. WIN 55,212-2, a cannabinoid receptor agonist, attenuates leukocyte/endothelial interactions in an experimental autoimmune encephalomyelitis model. Mult. Scler. 2004, 10, 158–164. [Google Scholar] [CrossRef]
- Hegde, V.L.; Nagarkatti, M.; Nagarkatti, P.S. Cannabinoid receptor activation leads to massive mobilization of myeloid-derived suppressor cells with potent immunosuppressive properties. Eur. J. Immunol. 2010, 40, 3358–3371. [Google Scholar] [CrossRef]
- Eisenstein, T.K.; Meissler, J.J. Effects of cannabinoids on T-cell function and resistance to infection. J. Neuroimmune Pharmacol. Off. J. Soc. Neuroimmune Pharmacol. 2015, 10, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Quirant-Sánchez, B.; Mansilla, M.J.; Navarro-Barriuso, J.; Presas-Rodríguez, S.; Teniente-Serra, A.; Fondelli, F.; Ramo-Tello, C.; Martínez-Cáceres, E. Combined therapy of vitamin D3-tolerogenic dendritic cells and interferon-β in a preclinical model of multiple sclerosis. Biomedicines 2021, 9, 1758. [Google Scholar] [CrossRef] [PubMed]
- Angelina, A.; Pérez-Diego, M.; López-Abente, J.; Rückert, B.; Nombela, I.; Akdis, M.; Martín-Fontecha, M.; Akdis, C.; Palomares, O. Cannabinoids induce functional Tregs by promoting tolerogenic DCs via autophagy and metabolic reprograming. Mucosal Immunol. 2022, 15, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.; Seabra, G.; Crippa, J.A.; Hallak, J.E.; Zuardi, A.W.; Martins-De-Souza, D. F198. Effects of cannabinoids on a human oligodendrocyte culture: Implications for schizophrenia. Schizophr. Bull. 2018, 44 (Suppl. 1), 298. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Luo, Q.; Shang, B.; Yang, X.; Zhang, Y.; Pan, Q.; Wu, N.; Tang, W.; Du, D.; Sun, X.; et al. Selective activation of cannabinoid receptor-2 reduces white matter injury via PERK signaling in a rat model of traumatic brain injury. Exp. Neurol. 2022, 347, 113899. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Yu, F.; Wen, J.; Vana, A.; Zhang, Y. Therapeutic potential of a novel cannabinoid agent CB52 in the mouse model of experimental autoimmune encephalomyelitis. Neuroscience 2013, 254, 427–442. [Google Scholar] [CrossRef] [Green Version]
- Olianas, M.C.; Dedoni, S.; Onali, P. Cannabinoid CB1 and CB2 receptors differentially regulate TNF-α-induced apoptosis and LPA1-mediated pro-survival signaling in HT22 hippocampal cells. Life Sci. 2021, 276, 119407. [Google Scholar] [CrossRef]
- Rossi, S.; Furlan, R.; Chiara, V.D.; Muzio, L.; Musella, A.; Motta, C.; Studer, V.; Cavasinni, F.; Bernardi, G.; Martino, G.; et al. Cannabinoid CB1 receptors regulate neuronal TNF-α effects in experimental autoimmune encephalomyelitis. Brain Behav. Immun. 2011, 25, 1242–1248. [Google Scholar] [CrossRef]
- Musella, A.; Sepman, H.; Mandolesi, G.; Gentile, A.; Fresegna, D.; Haji, N.; Conrad, A.; Lutz, B.; Maccarrone, M.; Centonze, D. Pre- and postsynaptic type-1 cannabinoid receptors control the alterations of glutamate transmission in experimental autoimmune encephalomyelitis. Neuropharmacology 2014, 79, 567–572. [Google Scholar] [CrossRef]
- Kuzmina, U.S.; Zainullina, L.F.; Vakhitov, V.A.; Bakhtiyarova, K.Z.; Vakhitova, Y.V. The role of glutamate in the pathogenesis of multiple sclerosis. Neurosci. Behav. Physiol. 2020, 50, 669–675. [Google Scholar] [CrossRef]
- Starost, L.; Lindner, M.; Herold, M.; Xu, Y.K.T.; Drexler, H.C.A.; Heß, K.; Ehrlich, M.; Ottoboni, L.; Ruffini, F.; Stehling, M.; et al. Extrinsic immune cell-derived, but not intrinsic oligodendroglial factors contribute to oligodendroglial differentiation block in multiple sclerosis. Acta Neuropathol. 2020, 140, 715–736. [Google Scholar] [CrossRef]
- Heß, K.; Starost, L.; Kieran, N.W.; Thomas, C.; Vincenten, M.C.J.; Antel, J.; Martino, G.; Huitinga, I.; Healy, L.; Kuhlmann, T. Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol. 2020, 140, 359–375. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Eixarch, H.; Martínez-Sáez, E.; Calvo-Barreiro, L.; Calucho, M.; Castro, Z.; Ortega-Aznar, A.; Ramón, Y.; Cajal, S.; Montalban, X.; et al. Expression of bone morphogenetic proteins in multiple sclerosis lesions. Am. J. Pathol. 2019, 189, 665–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, A.F.; Davies, C.L.; Holloway, R.K.; Ireland, G.; Borger, E.; Soong, D.; Richardson, J.C.; Williams, A.; Pollard, J.W.; Miron, V.E. Central nervous system regeneration is driven by microglia necroptosis and repopulation. Nat. Neurosci. 2019, 22, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- De Faria, O.; Dhaunchak, A.S.; Kamen, Y.; Roth, A.D.; Kuhlmann, T.; Colman, D.R.; Kennedy, T.E. TMEM10 promotes oligodendrocyte differentiation and is expressed by oligodendrocytes in human remyelinating multiple sclerosis plaques. Sci. Rep. 2019, 9, 3606. [Google Scholar] [CrossRef]
- Huerga-Gomez, A.; Aguado, T.; Sanchez-de la Torre, A.; Bernal-Chico, A.; Matute, C.; Mato, S.; Guzmán, M.; Galve-Roperh, I.; Palazuelos, J. Delta(9)-tetrahydrocannabinol promotes oligodendrocyte development and CNS myelination in vivo. Glia 2020, 69, 532–545. [Google Scholar] [CrossRef]
- Piper, B.J.; DeKeuster, R.M.; Beals, B.M.; Cobb, C.M.; Burchman, C.A.; Perkinson, L.; Lynn, S.T.; Nichols, S.D.; Abess, A.T. Substitution of medical cannabis for pharmaceutical agents for pain, anxiety, and sleep. J. Psychopharmacol. 2017, 31, 569–575. [Google Scholar] [CrossRef]
- Ergisi, M.; Erridge, S.; Harris, M.; Kawka, M.; Nimalan, D.; Salazar, O.; Loupasaki, K.; Ali, R.; Holvey, C.; Coomber, R.; et al. An updated analysis of clinical outcome measures across patients from the UK Medical Cannabis Registry. Cannabis Cannabinoid Res. 2022. [Google Scholar] [CrossRef]

| Study | Year | Findings | Formulations |
|---|---|---|---|
| D’hooghe et al. Retrospective Cohort Study [105] | 2021 | A total of 276 patients with MS for at least 6 months and spasticity for at least 3 months were included in a retrospective cohort study from 8 MS centers in Belgium. In 238 evaluable patients, 73% reported ≥20% decrease in spasticity NRS scores, experiencing reduced severity within 4 weeks. A total of 50% reported ≥30% improvement. Mean spasticity NRS scores improved from 8.1 (±1.08) at baseline to 5.2 (±1.85) at week 4, and 4.6 (±1.69) at week 8. A total of 80 patients discontinued within 8 weeks. By week 12, 171 patients reported a clinically meaningful response with a mean NRS score of 4.1 (±1.78) at week 12. More than 60% of the patients with MS who started add-on treatment with cannabinoid oromucosal spray reported a clinically relevant symptomatic effect and continued treatment after 12 weeks, and some continued to gain improved spasticity outcomes after 12 weeks. NRS improvement was maintained with mean scores of 4.3 (±1.77) at 6 months (n = 180) and 4.0 (±1.92) at 12 months (n = 113) [105]. | Sativex® oromucosal spray at 6 sprays/day. Sativex® was the add-on therapy to, at minimum, oral baclofen. |
| Sorosina et al. Observational Clinical Study [106] | 2016 2018 | A total of 93 nabiximols-treated MS patients across Italy were enrolled in an observational study. Whole blood was collected at baseline, 4 weeks (n = 93), and 14 weeks (n = 33, n = 19 responders and n = 14 non-responders) and analyzed by whole-genome microarray-based transcriptome profiling using Illumina® technology. Network analysis using the STRING interactome resource allowed comparisons between high responders and non-responders. Improvement in mean spasticity NRS scores of −2.9 at 4 weeks and −3.6 at 14 weeks in high responders to treatment (n = 19), as compared to +0.1 mean change in non-responders, as well as improvement in pain (mean change −3.6) scores, was associated with upregulation of ribosome pathway genes and downregulation of genes related to cell motility/migration, and immune and nervous systems including a genetic signature of 22 genes that differentiated responders from non-responders (p < 0.05) [106]. | Nabiximols (Sativex®) oromucosal spray 7 ± 2 (in responders) and 8 ± 3 (in non-responders) for 4 weeks. A total of 60 (81%) patients were treated with <10 mg/kg/d of CBD. Patients receiving other disease-modifying drugs were excluded. |
| Flachenecker et al. Prospective, Multicenter Cohort Study [107] | 2014a | A total of 276 patients across Germany with moderate to severe RRMS, SPMS, PPMS, and progressive and remitting MS were enrolled in an observational, prospective, multicenter study (MOVE2). The distribution of EDSS was ≤4.0 (17%), 4.5–6.5 (51%), and ≥7.0 (32%). After 1 month, nabiximols treatment provided relief of resistant MS in 74.6%, with a 12.3% reduction in mean spasticity NRS-11 score from 6.1 ± 1.7 to 5.2 ± 1.9, 41.7% experienced a ≥20% response, and 25.5% experienced ≥30% improvement from baseline (p < 0.0001; n = 216). After 3 months, 58.7% experienced ≥20% response, and 40.0% experienced ≥30% improvement from baseline (p < 0.0001; n = 95), reaching a mean NRS score of 4.7. Additionally, mean scores on the MSQoL-54 physical health composite (n = 47) improved by 25% from 39.2 ± 15.2 to 45.0 ± 15.0 (p = 0.0003), and on the MSQoL-54 mental health composite (n = 55), mean scores improved by 19% from 47.0 ± 17.1 to 53.1 ± 17.1 (p = 0.0012) [107]. | Nabiximols (THC/CBD 1:1, Sativex®) delivered using a pump action oromucosal spray, mean of 6.9 ± 2.8 sprays/day. A total of 89.5% of patients tried other antispastic drugs. A total of 72.8% of patients used nabiximol as an add-on to baclofen (50.0%), tolperisone (16.3%), tizanidine (13.8%), and gabapentin (9.1%). A total of 27.2% of patients had no concomitant antispastic medication. |
| Flachenecker et al. Prospective, Multicenter Cohort Study [108] | 2014b | A total of 51 MS patients participated in the 12-month prolongation of the prospective, multicenter cohort study in Germany (MOVE2 study). After 12 months of treatment with nabiximols, 52.9% (n = 27) had at least a 20% reduction in spasticity NRS score (p = 0.0004), and 41.2% (n = 21) had at least a reduction of 30% in spasticity NRS score (p = 0.0038). The mean NRS spasticity score decreased from 6.2 ± 1.8 to 4.6 ± 2.1 (p < 0.0001, n = 51) [108]. | Nabiximols (THC/CBD 1:1, Sativex®) delivered using a pump action oromucosal spray, mean of 6.9 ± 2.8 sprays/day. |
| Koehler et al. Retrospective Cohort Study [109] | 2016 | A total of 166 patients, 80% with secondary progressive MS, received treatment with THC/CBD spray (add-on n = 95; monotherapy n = 25) for a mean of 9 months. A clinical chart review was conducted over a 15-month period. The mean spasticity NRS score decreased from 7.0 to 3.0 in responders within 10 days, representing a 57% reduction [109]. | THC/CBD oromucosal spray in a 1:1 ratio. Mean 4.0 ± 2.6 sprays/day for add-on therapy and mean 3.0 ± 2.6 sprays/day for monotherapy. Other oral antispasticity medications used included baclofen, gabapentin, pregabalin, tolperisone, tetrazepam, and dantrolene. |
| Paolicelli et al. Prospective Cohort Study [110] | 2015 | See Table 3. | See Table 3. |
| Novotna et al. Randomized, Double-Blind (Phase B), Controlled Trial [111] | 2011 | A total of 241 MS patients with a mean duration in excess of 12 years, and a mean spasticity duration in excess of 7 years, were enrolled in a multicenter (51 sites in the United Kingdom, Italy, Poland, the Czech Republic), double-blind (Phase B), randomized, placebo-controlled, parallel-group study. After 12 weeks, the estimated difference between the nabiximol treatment (n = 124) and placebo (n = 117) groups in mean spasticity scores was 0.84 points (95% CI: −1.29 to −0.40) (p = 0.0002). The proportion of patients with at least a 30% improvement in spasticity in the active treatment group was significantly higher than in the placebo group (74% vs. 51%: odds ratio 2.73 (95% CI 1.59 to 4.69, p = 0.0003)) [111]. | Nabiximols add-on therapy delivered using a pump action oromucosal spray with each 100 μL actuation yielding 2.7 mg THC and 2.5 mg CBD. Subjects up-titrated to their optimal dose according to a predefined escalation scheme. Concomitant antispastic medications used were adamantane derivatives, benzodiazepine-related derivatives, dantrolene, antiepileptics, baclofen, tizanidine, and tolperisone. |
| Collin et al. Randomized, Double-Blind, Placebo-Controlled Parallel-Group Study [112] | 2010 | A total of 337 patients with MS spasticity not fully relieved with antispasticity therapy were enrolled in a 15-week (1-week baseline and 14-week treatment period), multicenter (15 centers in the UK and 8 in the Czech Republic), double-blind, randomized, placebo-controlled, parallel-group study. Sativex®-treated population (n = 150) showed a significant reduction in spasticity NRS scores with ≥30% improvement from baseline (−1.3 points) vs. placebo (n = 55, −0.8 points) (p = 0.035). In subjects who achieved ≥30% response, 98, 94, and 73% reported improvements of 10, 20, and 30%, respectively, at least once during the first 4 weeks of treatment [112]. | Sativex® pump action oromucosal spray. Each 100 mL actuation (maximum 8 in a 3 h period and 24 in a 24 h period) of active medication delivered a dose containing 2.7 mg THC and 2.5 mg CBD, self-titrated by patients to optimal dose. Concomitant medications included baclofen, azathioprine, and methylprednisolone. |
| Collin et al. Randomized, Double-Blind, Placebo-Controlled Clinical Trial [113] | 2007 | A total of 189 patients diagnosed with MS for 12–13 years from the UK were enrolled in a randomized, double-blind, placebo-controlled clinical trial. After 6 weeks, a greater proportion of the Sativex®-treated group (40%, n = 48) achieved a >30% reduction in spasticity as compared to the placebo group (22%, n = 14), a statistically significant difference (p = 0.014). Treatment group (n = 124) showed NRS reduction of 1.18 points from baseline, while placebo group (n = 65) showed reduction of 0.63 points from baseline. The estimated difference in mean spasticity score between the Sativex® treatment and placebo groups was 0.52 points (p = 0.048) [113]. | Sativex® oromucosal spray with each 100 μL actuation yielding 2.7 mg of Δ9-THC and 2.5 mg of CBD. Subjects up-titrated their daily dose over 2 weeks to a maximum of 48 sprays per day. Concomitant antispasticity medications were used and not listed. |
| Author | Year | Findings | Formulations |
|---|---|---|---|
| Turri et al. Observational Clinical Study [114] | 2018 | A total of 28 MS patients were enrolled in an observational study to assess the effects of Sativex® oromucosal spray on pain. Of the 19 patients who completed the study, 8 presented with neuropathic pain, 6 had nociceptive pain, and 5 reported mixed pain. After receiving Sativex® for 1 month, the subjects reported a reduction in mean pain scores from 6.61 to 3.55 on the 11-NRS (p < 0.0001) [114]. | Sativex® oromucosal spray daily. All patients gradually increased their dose of oromucosal spray of Sativex® until they achieved a satisfactory number of administrations per day (mean puffs/day 6.9 ± 1.9, range 4–11). |
| Russo et al. Observational Clinical Study [115] | 2016 | A total of 20 MS patients in Italy were enrolled in an observational study that assessed clinical and neurophysiologic parameters before and after 4 weeks of treatment with Sativex®. Half of the patients had neuropathic pain. After one month of drug administration, those with neuropathic pain (n = 10) reported a reduction in pain on the VAS rating scale from 7 ± 1 down to 1 ± 1 (p = 0.001), and an improvement in quality of life on the MSQoL (p = 0.03) [115]. | Sativex® daily in a pump action sublingual spray, mean 8 sprays/day. THC (27 mg/mL) and CBD (25 mg/mL), with ethanol/propylene glycol (50:50) excipient. A pump delivers 100 mL of spray, containing THC 2.7 mg and CBD 2.5 mg. Concomitant baclofen. |
| Sorosina et al. Observational Clinical Study [106] | 2016 2018 | See Table 1. | See Table 1. |
| Paolicelli et al. Prospective Cohort Study [110] | 2015 | See Table 3. | See Table 3. |
| Langford et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial [116] | 2013 | A total of 339 MS patients were enrolled in a double-blind, placebo-controlled, multicenter (33 sites in the UK, Canada, Spain, France, the Czech Republic) study to assess the effects of Sativex® oromucosal spray on pain in patients who had failed to gain adequate analgesia from existing medication. A total of 58 patients entered Phase B which consisted of a 2-week re-titration period and a 12-week stable dose phase with THC/CBD spray, with an additional 4-week randomized withdrawal phase. A statistically significant treatment difference in favor of THC/CBD spray was observed at week 10 (p = 0.046). During the randomized withdrawal phase, the time to treatment failure was 57% of patients receiving placebo vs. 24% of patients receiving THC/CBD spray, a statistically significant difference (p = 0.04), showing a mean change from baseline in pain NRS (−0.79, p = 0.028) and sleep quality NRS (0.99, p = 0.015) scores [116]. | Sativex ® oromucosal spray as add-on treatment, each 100 μL actuation yielding THC 2.5 mg and CBD 2.5 mg. Concomitant analgesic medications included NSAIDs, anticonvulsant, tricyclic antidepressants, and opioids. |
| Author | Year | Findings | Formulations |
|---|---|---|---|
| Maniscalco et al. Pilot Prospective Study [117] | 2018 | A total of 15 MS patients with overactive bladder and spasticity NRS-11 ≥ 4 were enrolled in a pilot prospective study to assess the effects of Sativex® on resistant MS bladder symptoms. After 4 weeks of treatment with Sativex®, the median spasticity NRS improved from 8 to 6 (p < 0.001), the median OABSS (overactive bladder symptom score) total score decreased from 17 to 12 (p = 0.001), and the median PVR decreased from 80 mL to 30 mL (p = 0.016) [117]. | Nabiximols (Sativex®) THC/CBD oromucosal spray with a mean puffs per day of 3.8 ± 1.02. Concomitant medications used were interferon, teriflunomide, glatiramer acetate, diethyl fumarate, and natalizumab. |
| Paolicelli et al. Prospective Cohort Study [110] | 2015 | A total of 102 MS patients (58% secondary progressive, 10% primary progressive, and 25% remitting-relapsing) enrolled in a prospective cohort study and administered THC/CBD spray as add-on therapy. After 1 month, there was a reduction in the mean spasticity NRS score from 8.7 ± 1.3 to 6.2 ± 1.8 with stabilization at this level at 3-, 6-, and 12-month follow-up (p < 0.001). A total of 57% of subjects who had pain refractory to gabapentinoids (n = 33) reported improvement in pain NRS scores at the first month (6.1 ± 2.5 vs. 3.4 ± 2) that remained stable at the sixth-month follow-up (p = 0.011). Bladder dysfunction IPSS score decreased from 15 ± 8.2 to 9.9 ± 7.4 at the first month (p = 0.011) and 9.3 ± 9.6 at the 6-month (p = 0.008) follow-up. Bladder-related QoL score decreased from 2.1 ± 0.5 to 1.5 ± 0.6 at the first month (p = 0.001) and 1.8 ± 0.7 at the 6-month (p = 0.041) follow-up [110]. | Sativex® THC/CBD oromucosal spray with an average of 6.5 ± 1.6 sprays each day. Concomitant drugs used were interferon-β, glatiramer acetate, azathioprine, fingolimod, and natalizumab. |
| Kavia et al. Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Trial [118] | 2010 | A total of 118 (56 Sativex®, 62 placebo) MS patients with overactive bladder symptoms refractory to other treatments completed per protocol a randomized, double-blind, placebo-controlled, parallel-group trial. At 8 weeks after treatment, the difference between the treatment and control groups in adjusted mean change was 0.85 in total number of voids per 24 h, 0.28 in the number of nocturia episodes per day and the number of daytime voids, and 0.76 in the number of void urgency episodes per day in favor of the Sativex-treated group (n = 60) as compared to the placebo group (n = 64), and the differences were statistically significant at the level of p < 0.05. The difference in adjusted mean change was 1.16 for overall bladder condition, with OBS NRS score showing improvement to a greater extent in the Sativex®-treated group (n = 61) as compared to the placebo group (n = 66) (p = 0.001) [118]. | Nabiximols (Sativex®) add-on treatment, pump action oromucosal spray 2.7 mg THC:2.5 mg CBD. Maximum permitted dose 8 puffs in any 3 h period and 48 puffs in any 24 h period. Concomitant anticholinergic medication. |
| Author | Year | Findings | Formulations |
|---|---|---|---|
| Braley et al. Cross-Sectional Survey-Based Study [119] | 2020 | Of 427 individuals who reported using cannabis in the past year either recreationally or for medical reasons through a University of Michigan national survey of MS patients, 70% reported benefits for pain, 56% reported benefits for sleep concerns, and 49% reported benefits for spasticity. The self-reported benefits in ability to fall asleep were significant (n = 180, p < 0.001) in the sub-groups who reported using low THC/CBD (n = 6), high THC/CBD (n = 27), CBD monotherapy (n = 46), and THC monotherapy (n = 15). The sleep benefits strongly correlated with relief in pain that interferes with sleep (r = 0.65, p < 0.0001). For those who expressed a preference for specific THC/CBD ratios, CBD-predominant formulations were favored [119]. | Self-reported formulations used daily for a year included cannabis, smoking, edibles, vaping, topical lotions/patches, and capsules, classified into the following 4 categories: low THC/low CBD, high THC/high CBD, CBD monotherapy or predominant therapy, and THC monotherapy or predominant therapy. |
| Flachenecker et al. Prospective, Multicenter Cohort Study [108] | 2014b | A total of 50 MS patients participated in the 12-month prolongation of the prospective, multicenter cohort study in Germany (MOVE2 study). After 12 months of treatment with nabiximols, the mean sleep NRS score decreased from 5.1 ± 2.9 down to 3.2 ± 2.5 points (p < 0.001) [108]. | Nabiximols (THC/CBD 1:1, Sativex®) delivered using a pump action oromucosal spray, mean of 6.9 ± 2.8 sprays/day. |
| Langford et al. Randomized, Double-Blind, Placebo-Controlled, Multicenter Clinical Trial [116] | 2013 | See Table 3. | See Table 3. |
| Author | Year | Findings | Formulations |
|---|---|---|---|
| Nichols et al. Observational Experimental Animal Study [87] | 2021 | EAE was induced in C57BL/6 mice, and oral CBD treatment was started 5 days later. At day 10, there was a significant decrease in the proportion of MOG35–55 specific, IFN-γ producing CD8+ T cells in the spleen (p < 0.05). At day 18, clinical EAE scores (on a scale of 1–5) were significantly reduced (p < 0.05). Trends were noted for moderate reductions in the number of T cells (p = 0.098) and the size of infiltrated and inflammatory lesions (p = 0.13) within spinal cord tissues [87]. | Oral CBD 75 mg/kg for 5 days after initiation of EAE. |
| Elliott et al. Observational Experimental Animal Study [120] | 2018 | C57BL/6 mice with MOG35–55 peptide-induced EAE treated with CBD exhibited delayed onset and decreased severity of clinical EAE (maximum score of 2.2 ± 0.16). The CBD treatment significantly reduced the proportions of CD4+ and CD8+ T-cell infiltrates in brain and spinal cord tissues and reduced levels of IL-17 and IFN-γ inflammatory cytokines as compared to controls (p < 0.0001) [120]. | CBD (20 mg/kg; 16% DMSO/PBS) was injected daily intraperitoneally, starting at day 9 through day 25. |
| González-García et al. Observational Experimental Animal Study [121] | 2017 | C57BL/6J mice with adoptively transferred EAE, induced by MOG35–55-specific T cells, were treated daily with 50 mg/kg of CBD intravenously. CBD markedly improved the clinical signs of EAE and reduced infiltration, demyelination, and axonal damage (p < 0.01). The CBD-mediated decrease in the viability of encephalitogenic T cells involved ROS generation, apoptosis, and a decrease in IL-6 production [121]. | CBD was administered intravenously at 50 mg/kg daily. |
| Kozela et al. Observational Experimental Study [122] | 2016 | Encephalitogenic TMOG cells were stimulated with MOG35–55 in the presence of spleen-derived antigen-presenting cells (APCs) with or without CBD. Gene expression profiling showed that the CBD treatment inhibited transcription of proinflammatory cytokines, cytokine receptors, MOG35–55-induced TMOG cell proliferation and Th17 activity, transcription factors, and TNF superfamily signaling molecules, while increasing antiproliferative interferon-dependent transcripts (p < 0.005). Furthermore, CBD enhanced the transcription of oxidative stress modulation [122]. | Incubation of T cells in vitro for 8 h with 5 μM CBD. |
| Kong et al. Observational Experimental Study [123] | 2014 | MOG35–55 EAE C57BL/6 mouse treatment with a selective CB2 receptor agonist, Gp1a, reduced severity and facilitated clinical recovery (p < 0.05), decreased accumulation of total mononuclear cells (p < 0.05), CD3+ and CD4+ T cells (p < 0.05), and Iba-1+ (activated) microglia/macrophages in CNS tissues, decreased production of INF-γ (p < 0.05) and IL-17 (p < 0.01) in splenocytes (in contrast to wildtype controls and CB2R knockout mice), decreased demyelination and axonal degeneration, decreased gene expression of chemokine receptors, chemokines, and adhesion molecules in vivo, and inhibited CD4+ Th1/Th17 differentiation of splenocytes in vitro (p < 0.05) [123]. | Gp1a-selective CB2 receptor agonist (N-(piperidin-1-yl)- 1-(2,4-dichlorophenyl)-1,4-dihydro-6-methylindeno[1,2-c]pyra zole-3-carboxamide) 5 mg/kg via tail vein injection, twice per week starting either day 0 or day 7. Splenocytes cultured from transgenic EAE (MOG35–55-specific TCR) in either CB2 receptor (Cnr2) +/+ or +/− knockout mice on C57BL/6 background were treated with 5 and 10 μM Gp1a in vitro. |
| Hilliard et al. Observational Experimental Study [86] | 2012 | Active remitting-relapsing EAE was induced in adult Biozzi ABH mice by subcutaneous injection on days 0 and 7 of mouse spinal cord homogenate. Intravenous injections with either cannabinoids alone or baclofen alone or vehicle were administered to mice in post-relapse remission, 7–8 months after inoculation. Spasticity, “stiffness”, and measurements of force needed to bend hindlimb to full flexion were assessed 10 min after intravenous treatment. Low dose (5 mg/kg THC + 5 mg/kg CBD) caused a 20% reduction in hindlimb stiffness (p = 0.007). Higher dose (10 mg/kg THC + 10 mg/kg CBD) caused a 40% reduction in hindlimb stiffness (p = 0.002). Baclofen (5 mg/kg) also provided a 40% reduction in hindlimb stiffness; however, cannabinoid treatment appeared to be better tolerated [86]. | A 0.1 mL solution containing a 1:1 ratio of THC/CBD was administered by tail vein injection of either low dose, 5 mg/kg, or high dose, 10 mg/kg. Positive control group received 5 mg/kg baclofen alone. |
| Kozela et al. Observational Experimental Study [85] | 2011 | Active EAE was induced in C57BL/6 mice by subcutaneous injections on day 1 and day 8 of 300 µg of MOG35–55 peptide. Mice were treated intravenously with either CBD (5 mg/kg) or vehicle on days 19, 20, and 21. The mean clinical EAE scores were lower in the CBD-treated group beginning on day 19 and remained significantly lower over the next 11 days, markedly delaying disease progression as compared to controls. On day 21, the mean EAE scores were 0.13 ± 0.09 in CBD-treated mice as compared to 1.03 ± 0.29 in controls (p < 0.05). Furthermore, treatment with CBD significantly reduced CD3+ T-cell infiltration (p < 0.0001), Iba-1+ microglial/macrophage presence (p < 0.001) and activation (p < 0.001), and axonal damage in the spinal cord as compared to controls [85]. | CBD 5 mg/kg was administered intravenously. |
| Zhang et al. Observational Experimental Study [124] | 2009 | Treatment of mice in either chronic EAE (C57BL/6/MOG35–55), remitting-relapsing EAE (SJL/J/PLP139–151), or adoptively transferred EAE with selective CB2 agonist, O-1966, significantly reduced disease severity (p < 0.05), leukocyte rolling (p < 0.05), and adhesion to cerebral microvessels in vivo (p < 0.05) [124]. | Selective CB2 agonist O-1966 administered at 1 mg/kg on day 7 and every 4th day thereafter up to day 28. |
| Maresz et al. Observational Experimental Study [125] | 2007 | Treatment with 25 mg/kg of THC significantly reduced disease severity in actively induced EAE in ABH. Induction of active EAE in homozygous CB2 receptor knockout mice resulted in increased disease severity. Using CB2−/− encephalitogenic T cells for induction of adoptive EAE enhanced disease severity (p < 0.05), increased T-cell proliferation in the CNS (p < 0.05), decreased apoptosis (p < 0.05), and increased production of IL-2 and IFN-γ (p < 0.05), demonstrating that CB2 receptor expression by T cells was critical for controlling inflammation in EAE [125]. | THC 25 mg/kg delivered by intraperitoneal injections on days 10–22. |
| Author | Year | Findings | Formulations |
|---|---|---|---|
| Aguado et al. Observational Experimental Study [126] | 2021 | Cuprizone-fed mice treated with 3 mg/kg of THC for 5 days after discontinuation of Cpz diet showed improved motor functional recovery (p = 0.05) in conjunction with increased axonal myelination in the corpus callosum (CC) by electron microscopy on day 5, enhanced levels of MAG, MOG, and MBP myelin-associated proteins in CC extract, and enhanced oligodendrocyte regeneration 5 and 10 days after start of THC treatment via CB1 receptor-mediated effects as compared to vehicle controls (p < 0.05). Addition of 1 μM THC for 4 days to cerebellar organotypic slice cultures that had been subjected to lysolecithin (LPC)-induced demyelination in vitro enhanced MBP+ areas by confocal microscopy as compared to vehicle controls (p < 0.05) [126]. | THC 3 mg/kg delivered by intraperitoneal injection for 5 consecutive days starting at 24 h after Cpz removal from the diet of mice that had been fed a Cpz diet for 6 weeks. THC 1μM added for 4 days to cerebellar organotypic slice cultures starting 15–17 h after incubation with lysolecithin (lysophosphatidylcholine). |
| Tomas-Roig et al. Observational Experimental Study [127] | 2020 | Cuprizone-fed mice treated with 0.5 mg/Kg cannabinoid agonist WIN-55,212-2 showed greater numbers of myelinated axons in the corpus callosum at 3 weeks and 6 weeks (p < 0.05) as compared to untreated Cpz-fed mice. However, CNS myelination was impaired by treatment with 1 mg/Kg WIN-55,212-2 [127]. | 0.5 mg/Kg cannabinoid agonist WIN-55,212-2 delivered by intraperitoneal injections to mice being fed a Cpz diet for 3 to 6 weeks. 1 mg/Kg WIN-55,212-2 had deleterious effects on remyelination. |
| Mecha et al. Observational Experimental Study [128] | 2018 | TMEV-infected SJL/J mice treated with either 5 mg/kg of 2-AG or a reversible inhibitor of 2-AG hydrolysis intraperitoneally for 7 days reduced the total number of microglia in the cerebral cortex (p ≤ 0.001), diminished the percentage area of Iba-1+ reactive microglia (2-AG p ≤ 0.05; UCM p ≤ 0.001), and hampered the development of jellyfish-like morphology, by confocal microscopy, characteristic of microglial activation. Treatment effects were not seen in Sham mice. 2-AG decreased expression of NOS-II in microglia (p ≤ 0.01), IL-1β (p ≤ 0.01), TNF-α, ICAM-1, chemokines (p ≤ 0.05), inflammatory infiltrates (p ≤ 0.001), and numbers of cells in lymph nodes (p ≤ 0.001); increased anti-inflammatory IL-10 (p ≤ 0.05), antiviral IFN-γ (p ≤ 0.05), proapoptotic bax and caspase 9 (p ≤ 0.05), numbers of cells in the spleen (p ≤ 0.001), numbers of immunosuppressive Arg-1+/CD11b+ MDSCs (p ≤ 0.05), and numbers of apoptotic CD4+ T cells in the brain (p ≤ 0.05); and improved long-term motor function (p ≤ 0.05). The AG-2 effects were mediated by the CB2 receptor [128]. | Treatment of 5 mg/kg of endocannabinoid 2-AG or reversible inhibitor of 2-AG degradation UCM-03025 delivered by intraperitoneal injections daily for 7 days. |
| Arevalo-Martin et al. Observational Experimental Study [129] | 2012 | Treatment of TMEV-infected SJL/J mice with CB1/CB2 agonist WIN-55,212-2 for 10 days restored self-tolerance, decreased delayed-type hypersensitivity responses to myelin PLP139–151 peptide ~60 days after infection (dpi) (p < 0.001), induced long-lasting motor function recovery on rotarod, and vertical and horizontal activity measures (p < 0.001), decreased the activation of CD4+CD25+Foxp3− T cells (p < 0.05), and increased regulatory CD4+CD25+Foxp3+ T cells (p < 0.01) in the CNS. Treatment reduced expression of IL-6 and CXCL1 in the CNS (p < 0.01) and upregulated production of IL-5, MCP-1, and IFN-γ (p < 0.05), and IL-9 and VEGF (p < 0.01) at 110 dpi as compared to vehicle-treated controls. Splenocytes transferred from WIN-treated mice at the onset of TMEV-IDD (30 dpi) inhibited the autoimmune inflammatory DTH response to PLP139–151 peptide 50 days later (80 dpi) and improved performance on motor function tests. Cyclophosphamide repressed WIN-induced self-tolerance and overrode WIN-induced recovery [129]. | CB1/CB2 agonist WIN-55,212-2 by daily intraperitoneal injection; doses were progressively increased 2.5 mg/kg on days 1–3, 3.75 mg/kg on days 4–6, and 5 mg/kg on days 7–10 to avoid potential habituation. |
| Mestre et al. Observational Experimental Study [130] | 2009 | TMEV-IDD mouse treatment with 1.5 mg/kg CB1/CB2 receptor agonist WIN-55,212-2 for 3 days restricted CD11b+ microglial activation (p < 0.01), limited perivascular CD4+ T-cell infiltrates (p < 0.01), downregulated adhesion molecule-1 (ICAM-1, p < 0.01) and vascular cell adhesion molecule-1 (VCAM-1) in the brain endothelium, and improved motor coordination by rotarod and by horizontal and vertical activity by activity cage (p < 0.05) [130]. | Treatment of 1.5 mg/kg CB1/CB2 agonist WIN55,212-2 administered for 3 consecutive days immediately after TMEV infection. |
| Study | Risk of Bias ‡ | Inconsistency | Indirectness | Imprecision | Dose–Response Relationship | Size of Effect | Confounding | GRADE |
|---|---|---|---|---|---|---|---|---|
| Spasticity | ||||||||
| D’hooghe [105] | Not serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Not serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Sorosina [106] | Not serious | Not Serious | Not Serious | Very Serious | Very Serious ⁕ | Not Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Flachenecker [107] | Not serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Serious | Serious † | ⊕⊕〇〇 (Low) |
| Flachenecker [108] | Not serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Serious | Serious † | ⊕⊕〇〇 (Low) |
| Koehler [109] | Not Serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Not Serious | Serious † | ⊕⊕〇〇 (Low) |
| Paolicelli [110] | Not Serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Not Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Novotna [111] | Not Serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Collin [112] | Not Serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Collin [113] | Not Serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Pain | ||||||||
| Turri [114] | Serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Not Serious | Unknown† | ⊕⊕〇〇 (Low) |
| Russo [115] | Serious | Not Serious | Not Serious | Very Serious | Very Serious ⁕ | Not Serious | Serious † | ⊕〇〇〇 (Very Low) |
| Sorosina [106] | Not Serious | Not Serious | Not Serious | Very Serious | Very Serious ⁕ | Not Serious | Serious † | ⊕⊕〇〇 (Low) |
| Langford [116] | Not Serious | Not Serious | Not Serious | Serious | Not Serious | Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Paolicelli [110] | Not Serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Not Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Lower Urinary Tract Dysfunction | ||||||||
| Maniscalco [117] | Not Serious | Not Serious | Not Serious | Very Serious | Very Serious ⁕ | Serious | Serious † | ⊕⊕〇〇 (Low) |
| Paolicelli [110] | Not Serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Not Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Kavia [118] | Not Serious | Not Serious | Not Serious | Serious | Not Serious | Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Sleep Disturbance | ||||||||
| Braley [119] | Serious | Not Serious | Serious | Not serious | Serious | Unknown | Very Serious † | ⊕〇〇〇 (Very Low) |
| Flachenecker [108] | Not serious | Not Serious | Not Serious | Serious | Very Serious ⁕ | Serious | Serious † | ⊕⊕〇〇 (Low) |
| Langford [116] | Not Serious | Not Serious | Not Serious | Serious | Not Serious | Serious | Serious † | ⊕⊕⊕〇 (Moderate) |
| Outcomes | Illustrative Comparative Risks (95% CI) | No. of Participants (Studies) | Quality of Evidence (GRADE) | Comments | |
|---|---|---|---|---|---|
| Assessment Risk (Usual Care/Control) | Corresponding Risk (Cannabinoid Treatment) | ||||
| Spasticity | Mean change in spasticity NRS score from baseline was −0.20. |
| 1582 (9 studies) | 6 ⊕⊕⊕〇 (Moderate) 3 ⊕⊕〇〇 (Low) |
|
| Pain |
| 573 (5 studies) | 2 ⊕⊕⊕〇 (Moderate) 2 ⊕⊕〇〇 (Low) 1 ⊕〇〇〇 (Very Low) | Randomized withdrawal phase confirmed effect in clinical trial. | |
| Lower Urinary Tract Dysfunction | -Adjusted mean change from baseline in total no. voids −0.9, urgency −1.12, nocturia episodes per day −0.24, and OBS NRS score −1.05 at 8 wks. |
| 235 (3 studies) | 2 ⊕⊕⊕〇 (Moderate) 1⊕⊕〇〇 (Low) | Bladder-related QoL improved. |
| Sleep Disturbance |
| 816 (3 studies) | 1 ⊕⊕⊕〇 (Moderate) 1 ⊕⊕〇〇 (Low) | MSQoL-54 improved by 25%. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longoria, V.; Parcel, H.; Toma, B.; Minhas, A.; Zeine, R. Neurological Benefits, Clinical Challenges, and Neuropathologic Promise of Medical Marijuana: A Systematic Review of Cannabinoid Effects in Multiple Sclerosis and Experimental Models of Demyelination. Biomedicines 2022, 10, 539. https://doi.org/10.3390/biomedicines10030539
Longoria V, Parcel H, Toma B, Minhas A, Zeine R. Neurological Benefits, Clinical Challenges, and Neuropathologic Promise of Medical Marijuana: A Systematic Review of Cannabinoid Effects in Multiple Sclerosis and Experimental Models of Demyelination. Biomedicines. 2022; 10(3):539. https://doi.org/10.3390/biomedicines10030539
Chicago/Turabian StyleLongoria, Victor, Hannah Parcel, Bameelia Toma, Annu Minhas, and Rana Zeine. 2022. "Neurological Benefits, Clinical Challenges, and Neuropathologic Promise of Medical Marijuana: A Systematic Review of Cannabinoid Effects in Multiple Sclerosis and Experimental Models of Demyelination" Biomedicines 10, no. 3: 539. https://doi.org/10.3390/biomedicines10030539
APA StyleLongoria, V., Parcel, H., Toma, B., Minhas, A., & Zeine, R. (2022). Neurological Benefits, Clinical Challenges, and Neuropathologic Promise of Medical Marijuana: A Systematic Review of Cannabinoid Effects in Multiple Sclerosis and Experimental Models of Demyelination. Biomedicines, 10(3), 539. https://doi.org/10.3390/biomedicines10030539