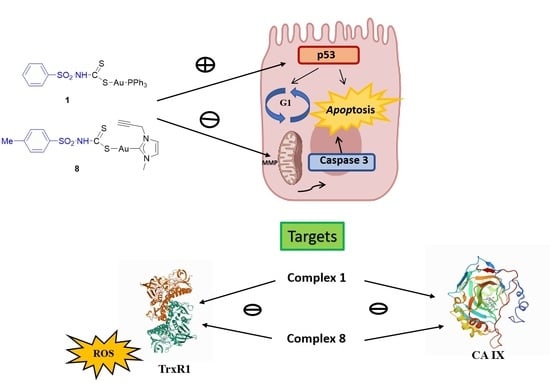
Sulfonamide-Derived Dithiocarbamate Gold(I) Complexes Induce the Apoptosis of Colon Cancer Cells by the Activation of Caspase 3 and Redox Imbalance
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of the Dithiocarbamates p-RC6H4SO2NHCS2Na (R = H, L1; Me, L2; Cl, L3)
2.3. Synthesis of the Complexes [Au(S2CNHSO2-p-RC6H4)(PPh3)] (R = H, 1; Me, 2; Cl, 3)
2.4. Synthesis of the Complexes [Au(S2CNHSO2-p-RC6H4)(TPPTS)] (R = H, 4; Me, 5; Cl, 6)
2.5. Synthesis of the Complexes [Au(S2CNHSO2-p-RC6H4)(IR″Propargyl)] (R = H, R″ = Me, 7, R″ = Bn, 9; R = Me, R″ = Me, 8; R″ = Bn, 10)
2.6. Distribution Coefficient (Log P7.4)
2.7. Solution Chemistry
2.8. Culture, Treatment and Cytotoxicity Determination in the Cells
2.9. Apoptosis Measurement
2.10. Propidium Iodide Staining of the DNA Content and Cell Cycle Analysis
2.11. Mitochondrial Membrane Potential Assay
2.12. Determination of the Caspase 3 and p53 Proteins
2.13. Thioredoxin Reductase 1 (TrxR1) Activity Assay
2.14. Carbonic Anhydrase (CA) Activity
2.15. Intracellular Levels of Reactive Oxygen Species (ROS)
2.16. Statistical Analysis
3. Results and Discussion
3.1. Chemical Studies
3.2. Biological Studies
3.2.1. Antiproliferative Activity of Gold Complexes
3.2.2. Type of Cell Death Produced by the Metal Complexes
3.2.3. Effect of the Gold Complex on the Cell Cycle
3.2.4. Thioredoxin Reductase and Carbonic Anhydrase IX as Potential Targets
3.2.5. Effect of Gold(I) Complexes on Intracellular ROS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kivelä, A.; Parkkila, S.; Saarnio, J.; Karttunen, T.J.; Kivelä, J.; Parkkila, A.K.; Waheed, A.; Sly, W.S.; Grubb, J.H.; Shah, G.; et al. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am. J. Patol. 2000, 156, 577–584. [Google Scholar] [CrossRef] [Green Version]
- McDonald, P.C.; Winum, J.-Y.; Supuran, C.T.; Dedhar, S. Recent developments in targeting carbonic anhydrase IX for Cancer Therapeutics. Oncotarget 2012, 3, 84–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supuran, C.T.; Alterio, V.; Di Fiore, A.; D’ Ambrosio, K.; Carta, F.; Monti, S.M.; De Simone, G. Inhibition of carbonic anhydrase IX targets primary tumors, metastases, and cancer stem cells: Three for the price of one. Med. Res. Rev. 2018, 38, 1799–1836. [Google Scholar] [CrossRef]
- Mboge, M.Y.; Mahon, B.P.; McKenna, R.; Frost, S.C. Carbonic anhydrases: Role in pH control and cancer. Metabolites 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gul, H.I.; Yamali, C.; Sakagami, H.; Angeli, A.; Leitans, J.; Kazaks, A.; Tars, K.; Ozgun, D.O.; Supuran, C.T. New anticancer drug candidates sulfonamides as selective hCA IX or hCA XII inhibitors. Bioorg. Chem. 2018, 77, 411–419. [Google Scholar] [CrossRef]
- Tülüce, Y.; Ahmed, B.A.; Koyuncu, İ.; Durgun, M. The cytotoxic, apoptotic and oxidative effects of carbonic anhydrase IX inhibitor on colorectal cancer cells. J. Bioenerg. Biomembr. 2018, 50, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Aldera, A.P.; Govender, D. Carbonic anhydrase IX: A regulator of pH and participant in carcinogenesis. J. Clin. Patol. 2021, 74, 350–354. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 2017, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Rulhania, S.; Jaswal, S.; Monga, V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2021, 209, 112923. [Google Scholar] [CrossRef]
- Kalinin, S.; Malkova, A.; Sharonova, T.; Sharoyko, V.; Bunev, A.; Supuran, C.T.; Krasavin, M. Carbonic anhydrase IX inhibitors as candidates for combination therapy of solid tumors. Int. J. Mol. Sci. 2021, 22, 13405. [Google Scholar] [CrossRef]
- Supuran, C.T. Multitargeting approaches involving carbonic anhydrase inhibitors: Hybrid drugs against a variety of disorders. J. Enzym. Inhib. Med. Chem. 2021, 36, 1702–1714. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Javed, T.; Alam, F.; Ali, A.; Ali, S.; Ullah, M.; Asad, H.B.; Hassham, M.; Hasan, F.; Muhammad, S. Therapeutic potential of carbonic anhydrase inhibitors. Pak. J. Pharma. Sci. 2019, 32, 709–720. [Google Scholar]
- Aspatwar, A.; Winum, J.-Y.; Carta, F.; Supuran, C.T.; Hammaren, M.; Parikka, M.; Parkkila, S. Carbonic anhydrase inhibitors as novel drugs against mycobacterial β-carbonic anhydrases: An update on in vitro and in vivo studies. Molecules 2018, 23, 2911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonardi, A.; Nocentini, A.; Bua, S.; Combs, J.; Lomelino, C.; Andring, J.; Lucarini, L.; Sgambellone, S.; Masini, E.; McKenna, R.; et al. Sulfonamide inhibitors of human carbonic anhydrases designed through a three-tails approach: Improving ligand/isoform matching and selectivity of action. J. Med. Chem. 2020, 63, 7422–7444. [Google Scholar] [CrossRef]
- Meşeli, T.; Doǧan, S.D.; Gündüz, M.G.; Kökbudak, Z.; Skaro Bogojevic, S.; Noonan, T.; Vojnovic, S.; Wolber, G.; Nikodinovic-Runic, J. Design, synthesis, antibacterial activity evaluation and molecular modeling studies of new sulfonamides containing a sulfathiazole moiety. New J. Chem. 2021, 45, 8166–8177. [Google Scholar] [CrossRef]
- Durgun, M.; Turkmen, H.; Zengin, G.; Zengin, H.; Koyunsever, M.; Koyuncu, I. Synthesis, characterization, in vitro cytotoxicity and antimicrobial investigation and evaluation of physicochemical properties of novel 4-(2-methylacetamide)benzenesulfonamide derivatives. Bioorg. Chem. 2017, 70, 163–172. [Google Scholar] [CrossRef]
- Petkar, P.A.; Jagtap, J.R. A review on antimicrobial potential of sulfonamide scaffold. Int. J. Pharma. Sci. Res. 2021, 12, 2535–2547. [Google Scholar]
- Lal, J.; Gupta, S.K.; Thavaselvam, D.; Agarwal, D.D. Biological activity, design, synthesis and structure activity relationship of some novel derivatives of curcumin containing sulfonamides. Eur. J. Med. Chem. 2013, 64, 579–588. [Google Scholar] [CrossRef]
- Abbas, A.; Murtaza, S.; Tahir, M.N.; Shamim, S.; Sirajuddin, M.; Rana, U.A.; Naseem, K.; Rafique, H. Synthesis, antioxidant, enzyme inhibition and DNA binding studies of novel N-benzylated derivatives of sulfonamide. J. Mol. Struct. 2016, 1117, 269–275. [Google Scholar] [CrossRef]
- Chandna, N.; Kumar, S.; Kaushik, P.; Kaushik, D.; Roy, S.K.; Gupta, G.K.; Jachak, S.M.; Kapoor, J.K.; Sharma, P.K. Synthesis of novel celecoxib analogues by bioisosteric replacement of sulfonamide as potent anti-inflammatory agents and cyclooxygenase inhibitors. Bioorg. Med. Chem. 2013, 21, 4581–4590. [Google Scholar] [CrossRef]
- Wan, Y.; Fang, G.; Chen, H.; Deng, X.; Tang, Z. Sulfonamide derivatives as potential anti-cancer agents and their SARs elucidation. Eur. J. Med. Chem. 2021, 226, 113837. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, Z.; Cheng, X.-L.; Zhang, K.; Zhang, X.; Qin, Y.; Fan, J.; Yan, T.; Han, T.; Shiu, K.K.; et al. A red-light-activated sulfonamide porphycene for highly efficient photodynamic therapy against hypoxic tumor. Eur. J. Med. Chem. 2021, 209, 112867. [Google Scholar] [CrossRef] [PubMed]
- Krymov, S.K.; Scherbakov, A.M.; Salnikova, D.I.; Sorokin, D.V.; Dezhenkova, L.G.; Ivanov, I.V.; Vullo, D.; De Luca, V.; Capasso, C.; Supuran, C.T.; et al. Synthesis, biological evaluation, and in silico studies of potential activators of apoptosis and carbonic anhydrase inhibitors on isatin-5-sulfonamide scaffold. Eur. J. Med. Chem. 2022, 228, 113997. [Google Scholar] [CrossRef]
- Shinde, S.D.; Sakla, A.P.; Shankaraiah, N. An insight into medicinal attributes of dithiocarbamates: Bird’s eye view. Bioorg. Chem. 2020, 105, 104346. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, D.; Zaidi, S. The role of organic dithiocarbamates in drug discovery research. Res. Rev. J. Chem. 2016, 5, 10. [Google Scholar]
- Akinboye, E.S.; Bamji, Z.D.; Kwabi-Addo, B.; Ejeh, D.; Copeland, R.L.; Denmeade, S.R.; Bakare, O. Design, synthesis and cytotoxicity studies of dithiocarbamate ester derivatives of emetine in prostate cancer cell lines. Bioorg. Med. Chem. 2015, 23, 5839–5845. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.-J.; Zhang, S.-Y.; Liu, Y.-C.; Zhang, L.; Liu, J.-J.; Song, J.; Zhao, R.-H.; Li, F.; Sun, H.-H.; Liu, H.-M.; et al. Design, synthesis and antiproliferative activity studies of novel dithiocarbamate–chalcone derivates. Bioorg. Med. Chem. Lett. 2016, 26, 3918–3922. [Google Scholar] [CrossRef]
- Ding, P.-P.; Gao, M.; Mao, B.-B.; Cao, S.-L.; Liu, C.-H.; Yang, C.-R.; Li, Z.-F.; Liao, J.; Zhao, H.; Li, Z.; et al. Synthesis and biological evaluation of quinazolin-4(3H)-one derivatives bearing dithiocarbamate side chain at C2-position as potential antitumor agents. Eur. J. Med. Chem. 2016, 108, 364–373. [Google Scholar] [CrossRef]
- Li, R.-D.; Wang, H.-L.; Li, Y.-B.; Wang, Z.-Q.; Wang, X.; Wang, Y.-T.; Ge, Z.-M.; Li, R.-T. Discovery and optimization of novel dual dithiocarbamates as potent anticancer agents. Eur. J. Med. Chem. 2015, 93, 381–391. [Google Scholar] [CrossRef]
- Xie, R.; Li, Y.; Tang, P.; Yuan, Q. Design, synthesis and biological evaluation of novel 2-aminobenzamides containing dithiocarbamate moiety as histone deacetylase inhibitors and potent antitumor agents. Eur. J. Med. Chem. 2018, 143, 320–333. [Google Scholar] [CrossRef]
- Wang, M.-M.; Chu, W.-C.; Yang, Y.; Yang, Q.-Q.; Qin, S.-S.; Zhang, E. Dithiocarbamates: Efficient metallo-β-lactamase inhibitors with good antibacterial activity when combined with meropenem. Bioorg. Med. Chem. Lett. 2018, 28, 3436–3440. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Xu, L.-W.; Liu, Y.; Sun, L.-Y.; Gao, H.; Li, J.-Q.; Yang, K. Dithiocarbamate as a Valuable Scaffold for the Inhibition of Metallo-β-Lactmases. Biomolecules 2019, 9, 699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aspatwar, A.; Hammarén, M.; Koskinen, S.; Luukinen, B.; Barker, H.; Carta, F.; Supuran, C.T.; Parikka, M.; Parkkila, S. β-CA-specific inhibitor dithiocarbamate Fc14–584B: A novel antimycobacterial agent with potential to treat drug-resistant tuberculosis. J. Enzym. Inhib. Med. Chem. 2017, 32, 832–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, K.; Sharma, M.; Singh, P.; Kumar, V.; Shukla, P.K.; Siddiqi, M.I.; Chauhan, P.M.S. Discovery of a new class of dithiocarbamates and rhodanine scaffolds as potent antifungal agents: Synthesis, biology and molecular docking. Med. Chem. Commun. 2012, 3, 1104–1110. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, Y.; Zhang, W.; Zhan, L.; Yu, Y.; Chen, Y.; Jia, W.; Liu, Z.; Qian, J.; Zhang, Y.; et al. Base promoted synthesis of novel indole-dithiocarbamate compounds as potential anti-inflammatory therapeutic agents for treatment of acute lung injury. Eur. J. Med. Chem. 2019, 171, 54–65. [Google Scholar] [CrossRef]
- Jiang, N.; Huang, Q.; Liu, J.; Liang, N.; Li, Q.; Li, Q.; Xie, S.-S. Design, synthesis and biological evaluation of new coumarin-dithiocarbamate hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 146, 287–298. [Google Scholar] [CrossRef]
- Fu, J.; Bao, F.; Gu, M.; Liu, J.; Zhang, Z.; Ding, J.; Xie, S.-S.; Ding, J. Design, synthesis and evaluation of quinolinone derivatives containing dithiocarbamate moiety as multifunctional AChE inhibitors for the treatment of Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2020, 35, 118–128. [Google Scholar] [CrossRef]
- Adokoh, C.K. Therapeutic potential of dithiocarbamate supported gold compounds. RSC Adv. 2020, 10, 2975–2988. [Google Scholar] [CrossRef] [Green Version]
- Hogarth, G. Metal-dithiocarbamate complexes: Chemistry and biological activity. Mini-Rev. Med. Chem. 2012, 12, 1202–1215. [Google Scholar] [CrossRef]
- Nardon, C.; Fregona, D. Gold(III) Complexes in the oncological preclinical arena: From aminoderivatives to peptidomimetics. Curr. Top. Med. Chem. 2016, 16, 360–380. [Google Scholar] [CrossRef]
- Saggioro, D.; Rigobello, M.P.; Paloschi, L.; Folda, A.; Moggach, S.A.; Parsons, S.; Ronconi, L.; Fregona, D.; Bindoli, A. Gold(III)-dithiocarbamato complexes induce cancer cell death triggered by thioredoxin redox system inhibition and activation of ERK pathway. Chem. Biol. 2007, 14, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Giovagnini, L.; Marzano, C.; Bettio, F.; Graziani, R.; Pilloni, G.; Fregona, D. Gold dithiocarbamate derivatives as potential antineoplastic agents: Design, spectroscopic properties, and in vitro antitumor activity. Inorg. Chem. 2005, 44, 1867–1881. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, L.; Aldinucci, D.; Dou, Q.P.; Fregona, D. Latest insights into the anticancer activity of gold(III)-dithiocarbamato complexes. Anti-Cancer Agents Med. Chem. 2010, 10, 283–292. [Google Scholar] [CrossRef]
- Kouodom, M.N.; Ronconi, L.; Celegato, M.; Nardon, C.; Marchio, L.; Dou, Q.P.; Aldinucci, D.; Formaggio, F.; Fregona, D. Toward the Selective Delivery of Chemotherapeutics into Tumor Cells by Targeting Peptide Transporters: Tailored Gold-Based Anticancer Peptidomimetics. J. Med. Chem. 2012, 55, 2212–2226. [Google Scholar] [CrossRef] [PubMed]
- Milacic, V.; Chen, D.; Ronconi, L.; Landis-Piwowar, K.R.; Fregona, D.; Dou, Q.P. A novel anticancer gold(III) dithiocarbamate compound inhibits the activity of a purified 20S proteasome and 26S proteasome in human breast cancer cell cultures and xenografts. Cancer Res. 2006, 66, 10478–10486. [Google Scholar] [CrossRef] [Green Version]
- Cattaruzza, L.; Fregona, D.; Mongiat, M.; Ronconi, L.; Fassina, A.; Colombatti, A.; Aldinucci, D. Antitumor activity of gold( III)-dithiocarbamato derivatives on prostate cancer cells and xenografts. Int. J. Cancer 2011, 128, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celegato, M.; Fregona, D.; Mongiat, M.; Ronconi, L.; Borghese, C.; Canzonieri, V.; Casagrande, N.; Nardon, C.; Colombatti, A.; Aldinucci, D. Preclinical activity of multiple-target gold(III)-dithiocarbamato peptidomimetics in prostate cancer cells and xenografts. Future Med. Chem. 2014, 6, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Frezza, M.; Milacic, V.; Ronconi, L.; Fan, Y.H.; Bi, C.F.; Fregona, D.; Dou, Q.P. Inhibition of tumor proteasome activity by gold-dithiocarbamato complexes via both redox-dependent and -independent processes. J. Cell. Biochem. 2010, 109, 162. [Google Scholar] [CrossRef] [Green Version]
- Bozdag, M.; Carta, F.; Vullo, D.; Isik, S.; AlOthman, Z.; Osman, S.M.; Scozzafava, A.; Supuran, C.T. Dithiocarbamates with potent inhibitory activity against the Saccharomyces cerevisiae β-carbonic anhydrase. J. Enzym. Inhib. Med. Chem. 2016, 31, 132–136. [Google Scholar] [CrossRef]
- Bozdag, M.; Carta, F.; Vullo, D.; Akdemir, A.; Isik, S.; Lanzi, C.; Scozzafava, A.; Masini, E.; Supuran, C.T. Synthesis of a new series of dithiocarbamates with effective human carbonic anhydrase inhibitory activity and antiglaucoma action. Bioorg. Med. Chem. 2015, 23, 2368–2376. [Google Scholar] [CrossRef]
- Carta, F.; Aggarwal, M.; Maresca, A.; Scozzafava, A.; McKenna, R.; Supuran, C.T. Dithiocarbamates: A new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chem. Commun. 2012, 48, 1868–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altıntop, M.D.; Sever, B.; Akalın Çiftçi, G.; Kucukoglu, K.; Özdemir, A.; Soleimani, S.S.; Nadaroglu, H.; Kaplancıklı, Z.A. Synthesis and evaluation of new benzodioxole-based dithiocarbamate derivatives as potential anticancer agents and hCA-I and hCA-II inhibitors. Eur. J. Med. Chem. 2017, 125, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Aggarwal, M.; Maresca, A.; Scozzafava, A.; McKenna, R.; Masini, E.; Supuran, C.T. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. J. Med. Chem. 2012, 55, 1721–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sağlık, B.N.; Osmaniye, D.; Çevik, U.A.; Levent, S.; Çavuşoğlu, B.K.; Büyükemir, O.; Nezir, D.; Karaduman, A.B.; Özkay, Y.; Koparal, A.S.; et al. Synthesis, characterization and carbonic anhydrase I and II inhibitory evaluation of new sulfonamide derivatives bearing dithiocarbamate. Eur. J. Med. Chem. 2020, 198, 112392. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.; Zhang, X.; Yu, L.; Gong, X.; Wang, P. Anticancer sulfonamide hybrids that inhibit bladder cancer cells growth and migration as tubulin polymerisation inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 1380–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, S.; Jones, L.A.; Mohr, F.; Laguna, M. Homogenous catalysis with gold: Efficient hydration of phenylacetylene in aqueous media. Organometallics 2007, 26, 952–957. [Google Scholar] [CrossRef]
- Johnson, A.; Gimeno, M.C. Synthesis of propargyl-functionalized NHC gold complexes. Organometallics 2017, 36, 1278–1286. [Google Scholar] [CrossRef]
- Atrian-Blasco, E.; Gascon, S.; Rodriguez-Yoldi, M.J.; Laguna, M.; Cerrada, E. Novel gold(I) thiolate derivatives synergistic with 5-fluorouracil as potential selective anticancer agents in colon cancer. Inorg. Chem. 2017, 56, 8562–8579. [Google Scholar] [CrossRef]
- Quero, J.; Ruighi, F.; Osada, J.; Gimeno, M.C.; Cerrada, E.; Rodriguez-Yoldi, M.J. Gold(I) complexes bearing alkylated 1,3,5-triaza-7-phosphaadamantane ligands as thermoresponsive anticancer agents in human colon cells. Biomedicines 2021, 9, 1848. [Google Scholar] [CrossRef]
- Marmol, I.; Virumbrales-Munoz, M.; Quero, J.; Sanchez-De-Diego, C.; Fernandez, L.; Ochoa, I.; Cerrada, E.; Yoldi, M.J.R. Alkynyl gold(I) complex triggers necroptosis via ROS generation in colorectal carcinoma cells. J. Inorg. Biochem. 2017, 176, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-de-Diego, C.; Marmol, I.; Perez, R.; Gascon, S.; Rodriguez-Yoldi, M.J.; Cerrada, E. The anticancer effect related to disturbances in redox balance on Caco-2 cells caused by an alkynyl gold(I) complex. J. Inorg. Biochem. 2017, 166, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Leal, M.; George, S. An in vitro procedure for evaluation of early stage oxidative stress in an established fish cell line applied to investigation of PHAH and pesticide toxicity. Mar. Environ. Res. 2004, 58, 631–635. [Google Scholar] [CrossRef]
- Brown, D.A.; Glass, W.K.; Burke, M.A. General use of ir spectral criteria in discussions of bonding and structure of metal dithiocarbamates. Spectrochim. Acta 1976, 32, 137–143. [Google Scholar] [CrossRef]
- Kellner, R.; Stnikolov, G.; Trendafilova, N. Detecting the bonding type of dithiocarbamate ligands in their complexes as inferred from the asymmetric cs mode. Inorg. Chim. Acta 1984, 84, 233–239. [Google Scholar] [CrossRef]
- Giovagnini, L.; Ronconi, L.; Aldinucci, D.; Lorenzon, D.; Sitran, S.; Fregona, D. Synthesis, characterization, and comparative in vitro cytotoxicity studies of platinum(II), palladium(II), and gold(III) methylsarcosinedithiocarbamate complexes. J. Med. Chem. 2005, 48, 1588–1595. [Google Scholar] [CrossRef]
- Vergara, E.; Miranda, S.; Mohr, F.; Cerrada, E.; Tiekink, E.R.T.; Romero, P.; Mendia, A.; Laguna, M. Gold(I) and Palladium(II) thiolato complexes containing water-soluble phosphane ligands. Eur. J. Inorg. Chem. 2007, 2007, 2926–2933. [Google Scholar] [CrossRef]
- Erxleben, A. Mitochondria-targeting anticancer metal complexes. Curr. Med. Chem. 2019, 26, 694–728. [Google Scholar] [CrossRef]
- Stenger-Smith, J.R.; Mascharak, P.K. Gold drugs with {Au(PPh3)}+ Moiety: Advantages and medicinal applications. ChemMedChem 2020, 15, 2136–2145. [Google Scholar] [CrossRef]
- Rubbiani, R.; Salassa, L.; de Almeida, A.; Casini, A.; Ott, I. Cytotoxic Gold(I) N-heterocyclic carbene complexes with phosphane ligands as potent enzyme inhibitors. ChemMedChem 2014, 9, 1205–1210. [Google Scholar] [CrossRef]
- Abás, E.; Bellés, A.; Rodríguez-Diéguez, A.; Laguna, M.; Grasa, L. Selective cytotoxicity of cyclometalated gold(III) complexes on Caco-2 cells is mediated by G2/M cell cycle arrest. Met. Integr. Biometal Sci. 2021, 13, mfab034. [Google Scholar] [CrossRef]
- Marmol, I.; Castellnou, P.; Alvarez, R.; Gimeno, M.C.; Rodriguez-Yoldi, M.J.; Cerrada, E. Alkynyl Gold(I) complexes derived from 3-hydroxyflavones as multi-targeted drugs against colon cancer. Eur. J. Med. Chem. 2019, 183, 111661. [Google Scholar] [CrossRef]
- Tu, S.P.; Sun, R.W.Y.; Lin, M.C.M.; Cui, J.T.; Zou, B.; Gu, Q.; Kung, H.F.; Che, C.M.; Wong, B.C.Y. Gold (III) porphyrin complexes induce apoptosis and cell cycle arrest and inhibit tumor growth in colon cancer. Cancer 2009, 115, 4459–4469. [Google Scholar] [CrossRef]
- Barnum, K.J.; O′Connell, M.J. Cell Cycle Regulation by Checkpoints. In Cell Cycle Control: Mechanisms and Protocols, 2nd ed.; Noguchi, E., Gadaleta, M.C., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2014; Volume 1170, pp. 29–40. [Google Scholar]
- Mirgayazova, R.; Khadiullina, R.; Mingaleeva, R.; Chasov, V.; Gomzikova, M.; Garanina, E.; Rizvanov, A.; Bulatov, E. Novel Isatin-based activator of p53 transcriptional functions in tumor cells. Mol. Biol. Res. Commun. 2019, 8, 119–128. [Google Scholar] [CrossRef]
- Chen, J.D. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. CSH Perspect Med. 2016, 6, a026104. [Google Scholar] [CrossRef]
- Carvajal, L.A.; Manfredi, J.J. Another fork in the road-life or death decisions by the tumour suppressor p53. Embo Rep. 2013, 14, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, B.; Li, X.; Han, X.; Liu, R.; Fang, J. Small molecule inhibitors of mammalian thioredoxin reductase as potential anticancer agents: An update. Med. Res. Rev. 2019, 39, 5–39. [Google Scholar] [CrossRef] [Green Version]
- Bindoli, A.; Rigobello, M.P.; Scutari, G.; Gabbiani, C.; Casini, A.; Messori, L. Thioredoxin reductase: A target for gold compounds acting as potential anticancer drugs. Coord. Chem. Rev. 2009, 253, 1692–1707. [Google Scholar] [CrossRef]
- Liu, L.; Wang, W.Q.; Huang, J.; Zhao, Z.J.; Li, H.L.; Xu, Y.F. Novel benzoyl thioureido benzene sulfonamides as highly potent and selective inhibitors of carbonic anhydrase IX: Optimization and bioactive studies. MedChemComm 2018, 9, 2100–2105. [Google Scholar] [CrossRef]
- Vullo, D.; Franchi, M.; Gallori, E.; Pastorek, J.; Scozzafava, A.; Pastorekova, S.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. Bioor. Med. Chem. Lett. 2003, 13, 1005–1009. [Google Scholar] [CrossRef]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med. Chem. 2014, 6, 1149–1165. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.T.; Villani, R.M.; Wang, H.L.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X.W. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef] [PubMed]
- Marmol, I.; Montanel-Perez, S.; Royo, J.C.; Gimeno, M.C.; Villacampa, M.D.; Rodriguez-Yoldi, M.J.; Cerrada, E. Gold(I) and Silver(I) Complexes with 2-anilinopyridine-based heterocycles as multitarget drugs against colon cancer. Inorg. Chem. 2020, 59, 17732–17745. [Google Scholar] [CrossRef] [PubMed]
- Vergara, E.; Casini, A.; Sorrentino, F.; Zava, O.; Cerrada, E.; Rigobello, M.P.; Bindoli, A.; Laguna, M.; Dyson, P.J. Anticancer therapeutics that target selenoenzymes: Synthesis, characterization, in vitro cytotoxicity, and thioredoxin reductase inhibition of a series of gold(I) complexes containing hydrophilic phosphine ligands. ChemMedChem 2010, 5, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.L.; Sun, Y.; Liu, Y.H.; Xu, Z.R.; Fan, R.; Liu, Z.W.; Liu, W.K. A Gold(I) Complex containing an oleanolic acid derivative as a potential anti-ovarian-cancer agent by inhibiting trxr and activating ROS-mediated ERS. Chem. Eur. J. 2020, 26, 7092–7108. [Google Scholar] [CrossRef] [PubMed]
- McCall, R.; Miles, M.; Lascuna, P.; Burney, B.; Patel, Z.; Sidoran, K.J.; Sittaramane, V.; Kocerha, J.; Grossie, D.A.; Sessler, J.L.; et al. Dual targeting of the cancer antioxidant network with 1,4-naphthoquinone fused Gold(I) N-heterocyclic carbene complexes. Chem. Sci. 2017, 8, 5918–5929. [Google Scholar] [CrossRef] [Green Version]
- Reddy, T.S.; Priver, S.H.; Mirzadeh, N.; Bhargava, S.K. Synthesis of gold(I) phosphine complexes containing the 2-BrC6F4PPh2 ligand: Evaluation of anticancer activity in 2D and 3D spheroidal models of HeLa cancer cells. Eur. J. Med. Chem. 2018, 145, 291–301. [Google Scholar] [CrossRef]
- Vousden, K.H.; Ryan, K.M. p53 and metabolism. Nat. Rev. Cancer 2009, 9, 691–700. [Google Scholar] [CrossRef]








| Complex | IC50 (μM) | Log P7.4 |
|---|---|---|
| [Au(S2CNHSO2C6H5)(PPh3)] (1) | 1.14 ± 0.25 | 0.75 |
| [Au(S2CNHSO2-p-Me-C6H4)(PPh3)] (2) | 1.25 ± 0.35 | 0.71 |
| [Au(S2CNHSO2-p-Cl-C6H4)(PPh3)] (3) | 1.70 ± 0.15 | 0.91 |
| [Au(S2CNHSO2C6H5)(TPPTS)] (4) | 46.71 ± 5.61 | −0.40 |
| [Au(S2CNHSO2-p-Me-C6H4)(TPPTS)] (5) | 31.81 ± 6.05 | −0.78 |
| [Au(S2CNHSO2-p-Cl-C6H4)(TPPTS)] (6) | 33.68 ± 2.74 | −0.83 |
| [Au(S2CNHSO2C6H5)(IMePropargyl)] (7) | 33.27 ± 5.01 | 0.35 |
| [Au(S2CNHSO2-p-Me-C6H4)(IMePropargyl)] (8) | 7.22 ± 0.38 | −0.13 |
| [Au(S2CNHSO2C6H5)(IBnPropargyl)] (9) | 8.56 ± 1.68 | 0.42 |
| [Au(S2CNHSO2-p-Me-C6H4)(IBnPropargyl)] (10) | 13.15 ± 3.34 | 0.36 |
| Complex | IC50 (μM) | Selectivity Index |
|---|---|---|
| [Au(S2CNHSO2C6H5)(PPh3)] (1) | 12.72 ± 0.47 | 11.2 |
| [Au(S2CNHSO2-p-Cl-C6H4)(PPh3)] (3) | 14.28 ± 0.19 | 8.05 |
| [Au(S2CNHSO2-p-Me-C6H4)(IMePropargyl)] (8) | 46.62 ± 3.26 | 6.46 |
| [Au(S2CNHSO2C6H5)(IBnPropargyl)] (9) | 19.54 ± 2.64 | 2.28 |
| Complex | IC50 24 h (μM) | IC50 48 h (μM) | IC50 72 h (μM) |
|---|---|---|---|
| [Au(S2CNHSO2C6H5)(PPh3)] (1) | 1.92 ± 0.13 | 1.38 ± 0.21 | 1.14 ± 0.25 |
| [Au(S2CNHSO2-p-Cl-C6H4)(PPh3)] (3) | 2.48 ± 0.14 | 1.51 ± 0.10 | 1.70 ± 0.15 |
| [Au(S2CNHSO2-p-Me-C6H4)(IMePropargyl)] (8) | >50 | 37.41 ± 7.48 | 7.22 ± 0.38 |
| [Au(S2CNHSO2C6H5)(IBnPropargyl)] (9) | 31.25 ± 6.85 | 15.48 ± 1.89 | 8.56 ± 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quero, J.; Royo, J.C.; Fodor, B.; Gimeno, M.C.; Osada, J.; Rodríguez-Yoldi, M.J.; Cerrada, E. Sulfonamide-Derived Dithiocarbamate Gold(I) Complexes Induce the Apoptosis of Colon Cancer Cells by the Activation of Caspase 3 and Redox Imbalance. Biomedicines 2022, 10, 1437. https://doi.org/10.3390/biomedicines10061437
Quero J, Royo JC, Fodor B, Gimeno MC, Osada J, Rodríguez-Yoldi MJ, Cerrada E. Sulfonamide-Derived Dithiocarbamate Gold(I) Complexes Induce the Apoptosis of Colon Cancer Cells by the Activation of Caspase 3 and Redox Imbalance. Biomedicines. 2022; 10(6):1437. https://doi.org/10.3390/biomedicines10061437
Chicago/Turabian StyleQuero, Javier, José Carlos Royo, Beatrice Fodor, María Concepción Gimeno, Jesús Osada, María Jesús Rodríguez-Yoldi, and Elena Cerrada. 2022. "Sulfonamide-Derived Dithiocarbamate Gold(I) Complexes Induce the Apoptosis of Colon Cancer Cells by the Activation of Caspase 3 and Redox Imbalance" Biomedicines 10, no. 6: 1437. https://doi.org/10.3390/biomedicines10061437
APA StyleQuero, J., Royo, J. C., Fodor, B., Gimeno, M. C., Osada, J., Rodríguez-Yoldi, M. J., & Cerrada, E. (2022). Sulfonamide-Derived Dithiocarbamate Gold(I) Complexes Induce the Apoptosis of Colon Cancer Cells by the Activation of Caspase 3 and Redox Imbalance. Biomedicines, 10(6), 1437. https://doi.org/10.3390/biomedicines10061437