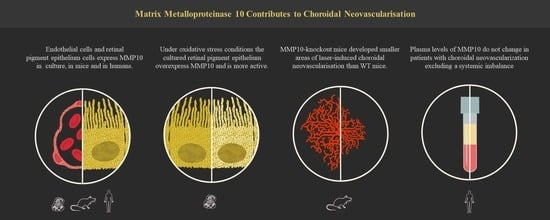
Matrix Metalloproteinase 10 Contributes to Choroidal Neovascularisation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.1.1. ARPE-19 Cell Culture
2.1.2. Primary Human RPE Cell Culture
2.1.3. Induced Oxidative Stress Conditions
2.1.4. Western Blotting for MMP10
2.1.5. RQ-PCR of MMP10 mRNA
2.2. Laser-Induced Choroidal Neovascularisation Model in Mice
2.3. Neovascularisation Staining and MMP10 Immunofluorescence
2.4. Human Retina Immunofluorescence
2.4.1. Human Tissue Processing for MMP10 Immunofluorescence
2.4.2. Immunofluorescence in Human Flatmounted Eyes
2.5. WetAMD Patients
2.5.1. Patient Sample
2.5.2. Plasma Analysis
2.5.3. MMP10 Measurements by ELISA
2.6. Statistical Analysis
3. Results
3.1. MMP10 Expression Pattern and Oxidative Stress Effect on Transcription, Synthesis and Activation of MMP10
3.1.1. MMP10 Is Expressed in ARPE-19 and hRPE Cells
3.1.2. Mice and Human RPE and Ganglion Cell Layer Expressed MMP10
3.1.3. MMP10 Is Overexpressed in ARPE-19 Cells under Oxidative Stress Conditions
3.1.4. MMP10 Is Activated upon Exposure to H2O2
3.2. Laser-Induced Choroidal Neovascularisation in Mice
3.2.1. MMP10-/- Mice Develop Smaller CNV Areas after Laser Injury
3.2.2. Endothelial Cells from Laser-Induced CNV Express MMP10
3.3. MMP10 Plasma Levels Case-Control Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kauppinen, A. Introduction to the multi-author review on macular degeneration. Cell. Mol. Life Sci. 2020, 77, 779–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Age-Related Macular Degeneration; National Institute for Health and Care Excellence (NICE): London, UK, 2018.
- Luibl, V.; Isas, J.M.; Kayed, R.; Glabe, C.G.; Langen, R.; Chen, J. Drusen deposits associated with aging and age-related macular degeneration contain nonfibrillar amyloid oligomers. J. Clin. Investig. 2006, 116, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, N.; Giannotti, R.; Buomprisco, G. Metalloproteinases and eye diseases. Biomed. Aging Pathol. 2013, 3, 97–105. [Google Scholar] [CrossRef]
- Bilbao-Malavé, V.; González-Zamora, J.; de la Puente, M.; Recalde, S.; Fernandez-Robredo, P.; Hernandez, M.; Layana, A.G.; de Viteri, M.S.; de la Puente, M.; Recalde, S.; et al. Mitochondrial Dysfunction and Endoplasmic Reticulum Stress in Age Related Macular Degeneration, Role in Pathophysiology, and Possible New Therapeutic Strategies. Antioxidants 2021, 10, 1170. [Google Scholar] [CrossRef]
- García-Onrubia, L.; Valentín-Bravo, F.J.; Coco-Martin, R.M.; González-Sarmiento, R.; Pastor, J.C.; Usategui-Martín, R.; Pastor-Idoate, S. Matrix metalloproteinases in age-related macular degeneration (Amd). Int. J. Mol. Sci. 2020, 21, 5934. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. In Advances in Pharmacology; Academic Press Inc.: Cambridge, MA, USA, 2018; Volume 81, pp. 241–330. [Google Scholar]
- Ra, H.J.; Parks, W.C. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007, 26, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. In Progress in Molecular Biology and Translational Science; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 147, pp. 1–73. [Google Scholar]
- Krogh Nielsen, M.; Subhi, Y.; Rue Molbech, C.; Nilsson, L.L.; Nissen, M.H.; Sørensen, T.L. Imbalances in tissue inhibitors of metalloproteinases differentiate choroidal neovascularization from geographic atrophy. Acta Ophthalmol. 2019, 97, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Raza, S.L.; Cornelius, L.A. Matrix metalloproteinases: Pro- and anti-angiogenic activities. J. Investig. Dermatol. Symp. Proc. 2000, 5, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Rohani, M.G.; McMahan, R.S.; Razumova, M.V.; Hertz, A.L.; Cieslewicz, M.; Pun, S.H.; Regnier, M.; Wang, Y.; Birkland, T.P.; Parks, W.C. MMP-10 Regulates Collagenolytic Activity of Alternatively Activated Resident Macrophages. J. Investig. Dermatol. 2015, 135, 2377–2384. [Google Scholar] [CrossRef] [Green Version]
- McMahan, R.S.; Birkland, T.P.; Smigiel, K.S.; Vandivort, T.C.; Rohani, M.G.; Manicone, A.M.; McGuire, J.K.; Gharib, S.A.; Parks, W.C. Stromelysin-2 (MMP10) Moderates Inflammation by Controlling Macrophage Activation. J. Immunol. 2016, 197, 899–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matilla, L.; Roncal, C.; Ibarrola, J.; Arrieta, V.; Garciá-Penã, A.; Fernández-Celis, A.; Navarro, A.; Álvarez, V.; Gainza, A.; Orbe, J.; et al. A Role for MMP-10 (Matrix Metalloproteinase-10) in Calcific Aortic Valve Stenosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1370–1382. [Google Scholar] [CrossRef] [PubMed]
- Toni, M.; Hermida, J.; Goñi, M.J.; Fernández, P.; Parks, W.C.; Toledo, E.; Montes, R.; Díez, N. Matrix metalloproteinase-10 plays an active role in microvascular complications in type 1 diabetic patients. Diabetologia 2013, 56, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Orbe, J.; Montero, I.; Rodríguez, J.A.; Beloqui, O.; Roncal, C.; Páramo, J.A. Independent association of matrix metalloproteinase-10, cardiovascular risk factors and subclinical atherosclerosis. J. Thromb. Haemost. 2007, 5, 91–97. [Google Scholar] [CrossRef]
- Ehlken, C.; Grundel, B.; Michels, D.; Junker, B.; Stahl, A.; Schlunck, G.; Hansen, L.L.; Feltgen, N.; Martin, G.; Agostini, H.T.; et al. Increased expression of angiogenic and inflammatory proteins in the vitreous of patients with ischemic central retinal vein occlusion. PLoS ONE 2015, 10, e0126859. [Google Scholar] [CrossRef] [Green Version]
- Boucher, B.J. Matrix metalloproteinase-10 and microvascular complications of type 1 diabetes: Might vitamin D status be relevant? Diabetologia 2014, 57, 1081. [Google Scholar] [CrossRef] [Green Version]
- Yeo, N.J.Y.; Chan, E.J.J.; Cheung, C. Choroidal Neovascularization: Mechanisms of Endothelial Dysfunction. Front. Pharmacol. 2019, 10, 1363. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.; Zou, J.; Yoshida, S.; Jiang, B.; Zhou, Y. The role of inflammation in age-related macular degeneration. Int. J. Biol. Sci. 2020, 16, 2989–3001. [Google Scholar] [CrossRef]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: Molecular pathways and therapeutic targets. Nat. Med. 2011, 17, 1359–1370. [Google Scholar] [CrossRef]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Ghajar, C.M.; George, S.C.; Putnam, A.J. Matrix Metalloproteinase Control of Capillary Morphogenesis. Crit. Rev. Eukaryot. Gene Expr. 2008, 18, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betsholtz, C. Cell–cell signaling in blood vessel development and function. EMBO Mol. Med. 2018, 10, e8610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, S.-H.; Choi, Y.-J.; Ryoo, H.-M.; Cho, J.-Y. Expression profiling of ETS and MMP factors in VEGF-activated endothelial cells: Role of MMP-10 in VEGF-induced angiogenesis. J. Cell. Physiol. 2010, 224, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Bong, S.J.; Kao, H.Y.; Jin, Z.G. VEGF stimulates HDAC7 phosphorylation and cytoplasmic accumulation modulating matrix metalloproteinase expression and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1782–1788. [Google Scholar] [CrossRef] [Green Version]
- Hangai, M.; Kitaya, N.; Xu, J.; Chan, C.K.; Kim, J.J.; Werb, Z.; Ryan, S.J.; Brooks, P.C. Matrix metalloproteinase-9-dependent exposure of a cryptic migratory control site in collagen is required before retinal angiogenesis. Am. J. Pathol. 2002, 161, 1429–1437. [Google Scholar] [CrossRef] [Green Version]
- Vempati, P.; Popel, A.S.; Mac Gabhann, F. Extracellular regulation of VEGF: Isoforms, proteolysis, and vascular patterning. Cytokine Growth Factor Rev. 2014, 25, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Han, X.H.; Caron, J.M.; Brooks, P.C. Cryptic collagen elements as signaling hubs in the regulation of tumor growth and metastasis. J. Cell. Physiol. 2020, 235, 9005–9020. [Google Scholar] [CrossRef]
- Hussain, A.A.; Lee, Y.; Marshall, J. Understanding the complexity of the matrix metalloproteinase system and its relevance to age-related diseases: Age-related macular degeneration and Alzheimer’s disease. Prog. Retin. Eye Res. 2020, 74, 100775. [Google Scholar] [CrossRef]
- Kumar, S.; Nakashizuka, H.; Jones, A.; Lambert, A.; Zhao, X.; Shen, M.; Parker, M.; Wang, S.; Berriochoa, Z.; Fnu, A.; et al. Proteolytic Degradation and Inflammation Play Critical Roles in Polypoidal Choroidal Vasculopathy. Am. J. Pathol. 2017, 187, 2841–2857. [Google Scholar] [CrossRef] [Green Version]
- Batra, J.; Soares, A.S.; Mehner, C.; Radisky, E.S. Matrix metalloproteinase-10/TIMP-2 structure and analyses define conserved core interactions and diverse exosite interactions in MMP/TIMP complexes. PLoS ONE 2013, 8, e75836. [Google Scholar] [CrossRef] [Green Version]
- Savaraj, N.; Wei, Y.; Unate, H.; Liu, P.M.; Wu, C.J.; Wangpaichitr, M.; Xia, D.; Xu, H.J.; Hu, S.X.; Kuo, M.T. Redox regulation of matrix metalloproteinase gene family in small cell lung cancer cells. Free Radic. Res. 2005, 39, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lim, J.W.; Kim, H. β-Carotene Inhibits Expression of Matrix Metalloproteinase-10 and Invasion in Helicobacter pylori-Infected Gastric Epithelial Cells. Molecules 2021, 26, 1567. [Google Scholar] [CrossRef] [PubMed]
- Orbe, J.; Rodríguez, J.A.; Calvayrac, O.; Rodríguez-Calvo, R.; Rodrȷguez, C.; Roncal, C.; Martínez de Lizarrondo, S.; Barrenetxe, J.; Reverter, J.C.; Martínez-González, J.; et al. Matrix Metalloproteinase-10 Is Upregulated by Thrombin in Endothelial Cells and Increased in Patients With Enhanced Thrombin Generation. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2109–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coll, B.; Rodríguez, J.A.; Craver, L.; Orbe, J.; Martínez-Alonso, M.; Ortiz, A.; Díez, J.; Beloqui, O.; Borras, M.; Valdivielso, J.M.; et al. Serum levels of matrix metalloproteinase-10 are associated with the severity of atherosclerosis in patients with chronic kidney disease. Kidney Int. 2010, 78, 1275–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, R.; Yang, J.; Moses, M.A. Matrix Metalloproteinases As Novel Biomarkers and Potential Therapeutic Targets in Human Cancer. J. Clin. Oncol. 2009, 27, 5287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vira, H.J.; Pradhan, V.D.; Umare, V.D.; Chaudhary, A.K.; Rajadhyksha, A.G.; Nadkar, M.Y.; Ghosh, K.; Nadkarni, A.H. Role of MMP-2 and its inhibitor TIMP-2 as biomarkers for susceptibility to systemic lupus erythematosus. Biomarkers Med. 2020, 14, 1109–1119. [Google Scholar] [CrossRef]
- Medeiros, N.I.; Gomes, J.A.S.; Fiuza, J.A.; Sousa, G.R.; Almeida, E.F.; Novaes, R.O.; Rocha, V.L.S.; Chaves, A.T.; Dutra, W.O.; Rocha, M.O.C.; et al. MMP-2 and MMP-9 plasma levels are potential biomarkers for indeterminate and cardiac clinical forms progression in chronic Chagas disease. Sci. Rep. 2019, 9, 14170. [Google Scholar] [CrossRef]
- Chau, K.Y.; Sivaprasad, S.; Patel, N.; Donaldson, T.A.; Luthert, P.J.; Chong, N.V. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye 2007, 21, 1511–1515. [Google Scholar] [CrossRef]
- Zeng, R.; Wen, F.; Zhang, X.; Su, Y. Serum levels of matrix metalloproteinase 2 and matrix metalloproteinase 9 elevated in polypoidal choroidal vasculopathy but not in age-related macular degeneration. Mol. Vis. 2013, 19, 729. [Google Scholar]
- Subhi, Y.; Nielsen, M.K.; Molbech, C.R.; Liisborg, C.; Søndergaard, H.B.; Sellebjerg, F.; Sørensen, T.L. The transcriptome of peripheral blood mononuclear cells in patients with clinical subtypes of late age-related macular degeneration. Immun. Ageing 2019, 16, 1–16. [Google Scholar] [CrossRef]
- Niazi, S.; Krogh Nielsen, M.; Sørensen, T.L.; Subhi, Y. Neutrophil-to-lymphocyte ratio in age-related macular degeneration: A systematic review and meta-analysis. Acta Ophthalmol. 2019, 97, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.G.; Dimitrova, E.; Beppu, A.; Wang, Y.; Jefferies, C.A.; Parks, W.C. Macrophage MMP10 Regulates TLR7-Mediated Tolerance. Front. Immunol. 2018, 9, 2817. [Google Scholar] [CrossRef] [PubMed]
- Sokai, A.; Handa, T.; Tanizawa, K.; Oga, T.; Uno, K.; Tsuruyama, T.; Kubo, T.; Ikezoe, K.; Nakatsuka, Y.; Tanimura, K.; et al. Matrix metalloproteinase-10: A novel biomarker for idiopathic pulmonary fibrosis. Respir. Res. 2015, 16, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozing, M.P.; Durhuus, J.A.; Krogh Nielsen, M.; Subhi, Y.; Kirkwood, T.B.; Westendorp, R.G.; Sørensen, T.L. Age-related macular degeneration: A two-level model hypothesis. Prog. Retin. Eye Res. 2020, 76, 100825. [Google Scholar] [CrossRef]
- Zhang, G.; Miyake, M.; Lawton, A.; Goodison, S.; Rosser, C.J. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer 2014, 14, 310. [Google Scholar] [CrossRef] [Green Version]
- García-Irigoyen, O.; Latasa, M.U.; Carotti, S.; Uriarte, I.; Elizalde, M.; Urtasun, R.; Vespasiani-Gentilucci, U.; Morini, S.; Benito, P.; Ladero, J.M.; et al. Matrix metalloproteinase 10 contributes to hepatocarcinogenesis in a novel crosstalk with the stromal derived factor 1/C-X-C chemokine receptor 4 axis. Hepatology 2015, 62, 166–178. [Google Scholar] [CrossRef]






| Control (n = 52) | AMD (n = 52) | p-Value | |
|---|---|---|---|
| Age (years) | 72.70 ± 0.99 | 72.61 ± 0.98 | 0.951 |
| Female (%) | 71% | 71% | 1.0 |
| HBP (%) | 33% | 44% | 0.314 |
| Smokers (%) | 17% | 25% | 0.326 |
| Dyslipidemia (%) | 27% | 34% | 0.524 |
| Cardiovascular diseases (%) | 19% | 19% | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Zamora, J.; Hernandez, M.; Recalde, S.; Bezunartea, J.; Montoliu, A.; Bilbao-Malavé, V.; Orbe, J.; Rodríguez, J.A.; Llorente-González, S.; Fernández-Robredo, P.; et al. Matrix Metalloproteinase 10 Contributes to Choroidal Neovascularisation. Biomedicines 2022, 10, 1557. https://doi.org/10.3390/biomedicines10071557
González-Zamora J, Hernandez M, Recalde S, Bezunartea J, Montoliu A, Bilbao-Malavé V, Orbe J, Rodríguez JA, Llorente-González S, Fernández-Robredo P, et al. Matrix Metalloproteinase 10 Contributes to Choroidal Neovascularisation. Biomedicines. 2022; 10(7):1557. https://doi.org/10.3390/biomedicines10071557
Chicago/Turabian StyleGonzález-Zamora, Jorge, María Hernandez, Sergio Recalde, Jaione Bezunartea, Ana Montoliu, Valentina Bilbao-Malavé, Josune Orbe, José A. Rodríguez, Sara Llorente-González, Patricia Fernández-Robredo, and et al. 2022. "Matrix Metalloproteinase 10 Contributes to Choroidal Neovascularisation" Biomedicines 10, no. 7: 1557. https://doi.org/10.3390/biomedicines10071557
APA StyleGonzález-Zamora, J., Hernandez, M., Recalde, S., Bezunartea, J., Montoliu, A., Bilbao-Malavé, V., Orbe, J., Rodríguez, J. A., Llorente-González, S., Fernández-Robredo, P., & García-Layana, A. (2022). Matrix Metalloproteinase 10 Contributes to Choroidal Neovascularisation. Biomedicines, 10(7), 1557. https://doi.org/10.3390/biomedicines10071557