A Placebo-Controlled Trial of Cannabinoid Treatment for Disruptive Behavior in Children and Adolescents with Autism Spectrum Disorder: Effects on Sleep Parameters as Measured by the CSHQ
Abstract
:1. Introduction
2. Materials and Methods
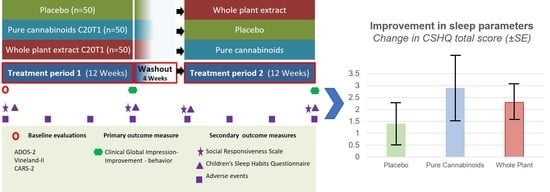
2.1. Study Design
2.2. Standard Protocol Approvals and Patient Consent
2.3. Study Population
2.4. Treatment Scheme
2.5. Baseline Evaluations
2.6. Outcomes
2.7. Statistical Analyses
3. Results
3.1. Participants
3.2. Baseline Sleep Disturbances
3.3. Impact of Cannabinoid Treatment on Sleep
3.4. Longitudinal Associations between Sleep, Behavior, and Autistic Core Symptoms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesfaye, R.; Wright, N.; Zaidman-Zait, A.; Bedford, R.; Zwaigenbaum, L.; Kerns, C.M.; Duku, E.; Mirenda, P.; Bennett, T.; Georgiades, S.; et al. Investigating longitudinal associations between parent reported sleep in early childhood and teacher reported executive functioning in school-aged children with autism. Sleep 2021, 44, zsab122. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.M.; Soke, G.N.; Sabourin, K.R.; Hepburn, S.; Katz, T.; Wiggins, L.D.; Schieve, L.A.; Levy, S.E. Sleep Problems in 2- to 5-Year-Olds with Autism Spectrum Disorder and Other Developmental Delays. Pediatrics 2019, 143, e20180492. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.P.; Zarrinnegar, P. Autism Spectrum Disorder and Sleep. Child Adolesc. Psychiatr. Clin. N. Am. 2021, 30, 195–208. [Google Scholar] [CrossRef]
- Lorsung, E.; Karthikeyan, R.; Cao, R. Biological Timing and Neurodevelopmental Disorders: A Role for Circadian Dysfunction in Autism Spectrum Disorders. Front. Neurosci. 2021, 15, 642745. [Google Scholar] [CrossRef]
- Pinato, L.; Galina Spilla, C.S.; Markus, R.P.; da Silveira Cruz-Machado, S. Dysregulation of Circadian Rhythms in Autism Spectrum Disorders. Curr. Pharm Des. 2019, 25, 4379–4393. [Google Scholar] [CrossRef]
- Williams Buckley, A.; Hirtz, D.; Oskoui, M.; Armstrong, M.J.; Batra, A.; Bridgemohan, C.; Coury, D.; Dawson, G.; Donley, D.; Findling, R.L.; et al. Practice guideline: Treatment for insomnia and disrupted sleep behavior in children and adolescents with autism spectrum disorder: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2020, 94, 392–404. [Google Scholar] [CrossRef] [Green Version]
- Kesner, A.J.; Lovinger, D.M. Cannabinoids, Endocannabinoids and Sleep. Front. Mol. Neurosci. 2020, 13, 125. [Google Scholar] [CrossRef]
- Guerrero-Alba, R.; Barragan-Iglesias, P.; Gonzalez-Hernandez, A.; Valdez-Morales, E.E.; Granados-Soto, V.; Condes-Lara, M.; Rodriguez, M.G.; Marichal-Cancino, B.A. Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol. 2018, 9, 1496. [Google Scholar] [CrossRef] [Green Version]
- Szkudlarek, H.J.; Desai, S.J.; Renard, J.; Pereira, B.; Norris, C.; Jobson, C.E.L.; Rajakumar, N.; Allman, B.L.; Laviolette, S.R. Delta-9-Tetrahydrocannabinol and Cannabidiol produce dissociable effects on prefrontal cortical executive function and regulation of affective behaviors. Neuropsychopharmacology 2019, 44, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaca, M.V.; Scarante, F.F.; Joca, S.R.L.; Sales, A.J.; Gomes, F.V.; Sonego, A.B.; Rodrigues, N.S.; Galve-Roperh, I.; Guimaraes, F.S. Plastic and Neuroprotective Mechanisms Involved in the Therapeutic Effects of Cannabidiol in Psychiatric Disorders. Front. Pharmacol. 2017, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [Green Version]
- Iannotti, F.A.; Hill, C.L.; Leo, A.; Alhusaini, A.; Soubrane, C.; Mazzarella, E.; Russo, E.; Whalley, B.J.; Di Marzo, V.; Stephens, G.J. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: Potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci. 2014, 5, 1131–1141. [Google Scholar] [CrossRef] [Green Version]
- Cifelli, P.; Ruffolo, G.; De Felice, E.; Alfano, V.; van Vliet, E.A.; Aronica, E.; Palma, E. Phytocannabinoids in Neurological Diseases: Could They Restore a Physiological GABAergic Transmission? Int. J. Mol. Sci. 2020, 21, 723. [Google Scholar] [CrossRef] [Green Version]
- Zamberletti, E.; Gabaglio, M.; Parolaro, D. The Endocannabinoid System and Autism Spectrum Disorders: Insights from Animal Models. Int. J. Mol. Sci. 2017, 18, 1916. [Google Scholar] [CrossRef] [Green Version]
- Pietropaolo, S.; Bellocchio, L.; Bouzón-Arnáiz, I.; Yee, B.K. The role of the endocannabinoid system in autism spectrum disorders: Evidence from mouse studies. Prog. Mol. Biol. Transl. Sci. 2020, 173, 183–208. [Google Scholar] [CrossRef]
- Zou, M.; Liu, Y.; Xie, S.; Wang, L.; Li, D.; Li, L.; Wang, F.; Zhang, Y.; Xia, W.; Sun, C.; et al. Alterations of the endocannabinoid system and its therapeutic potential in autism spectrum disorder. Open Biol. 2021, 11, 200306. [Google Scholar] [CrossRef]
- Karhson, D.S.; Krasinska, K.M.; Dallaire, J.A.; Libove, R.A.; Phillips, J.M.; Chien, A.S.; Garner, J.P.; Hardan, A.Y.; Parker, K.J. Plasma anandamide concentrations are lower in children with autism spectrum disorder. Mol. Autism. 2018, 9, 18. [Google Scholar] [CrossRef]
- Aran, A.; Eylon, M.; Harel, M.; Polianski, L.; Nemirovski, A.; Tepper, S.; Schnapp, A.; Cassuto, H.; Wattad, N.; Tam, J. Lower circulating endocannabinoid levels in children with autism spectrum disorder. Mol. Autism. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Cassuto, H.; Lubotzky, A.; Wattad, N.; Hazan, E. Brief Report: Cannabidiol-Rich Cannabis in Children with Autism Spectrum Disorder and Severe Behavioral Problems-A Retrospective Feasibility Study. J. Autism. Dev. Disord. 2019, 49, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Harel, M.; Cassuto, H.; Polyansky, L.; Schnapp, A.; Wattad, N.; Shmueli, D.; Golan, D.; Castellanos, F.X. Cannabinoid treatment for autism: A proof-of-concept randomized trial. Mol. Autism. 2021, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Bar-Lev Schleider, L.; Mechoulam, R.; Saban, N.; Meiri, G.; Novack, V. Real life Experience of Medical Cannabis Treatment in Autism: Analysis of Safety and Efficacy. Sci. Rep. 2019, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Barchel, D.; Stolar, O.; De-Haan, T.; Ziv-Baran, T.; Saban, N.; Fuchs, D.O.; Koren, G.; Berkovitch, M. Oral Cannabidiol Use in Children With Autism Spectrum Disorder to Treat Related Symptoms and Co-morbidities. Front. Pharmacol. 2018, 9, 1521. [Google Scholar] [CrossRef]
- AminiLari, M.; Wang, L.; Neumark, S.; Adli, T.; Couban, R.J.; Giangregorio, A.; Carney, C.E.; Busse, J.W. Medical Cannabis and Cannabinoids for Impaired Sleep: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Sleep 2021, 45, zsab234. [Google Scholar] [CrossRef]
- Suraev, A.S.; Marshall, N.S.; Vandrey, R.; McCartney, D.; Benson, M.J.; McGregor, I.S.; Grunstein, R.R.; Hoyos, C.M. Cannabinoid therapies in the management of sleep disorders: A systematic review of preclinical and clinical studies. Sleep Med. Rev. 2020, 53, 101339. [Google Scholar] [CrossRef]
- Kaul, M.; Zee, P.C.; Sahni, A.S. Effects of Cannabinoids on Sleep and their Therapeutic Potential for Sleep Disorders. Neurotherapeutics 2021, 18, 217–227. [Google Scholar] [CrossRef]
- Spanagel, R.; Bilbao, A. Approved cannabinoids for medical purposes—Comparative systematic review and meta-analysis for sleep and appetite. Neuropharmacology 2021, 196, 108680. [Google Scholar] [CrossRef]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [Green Version]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H., Jr.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism. Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, S.S.; Balla, D.A.; Cicchetti, D.V. Vineland Adaptive Behavior Scales: Survey Form Manual; American Guidance Service: Circle Pines, MN, USA, 1984. [Google Scholar]
- Schopler, E.; Reichler, R.J.; DeVellis, R.F.; Daly, K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). J. Autism. Dev. Disord. 1980, 10, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A.; Spirito, A.; McGuinn, M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 2000, 23, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; DeMand, A.; Lecavalier, L.; Smith, T.; Aman, M.; Foldes, E.; Scahill, L. Psychometric properties of the children’s sleep habits questionnaire in children with autism spectrum disorder. Sleep Med. 2016, 20, 5–11. [Google Scholar] [CrossRef]
- Hatch, B.; Nordahl, C.W.; Schwichtenberg, A.J.; Ozonoff, S.; Miller, M. Factor Structure of the Children’s Sleep Habits Questionnaire in Young Children with and Without Autism. J. Autism. Dev. Disord. 2021, 51, 3126–3137. [Google Scholar] [CrossRef] [PubMed]
- Zaidman-Zait, A.; Zwaigenbaum, L.; Duku, E.; Bennett, T.; Szatmari, P.; Mirenda, P.; Smith, I.; Vaillancourt, T.; Volden, J.; Waddell, C.; et al. Factor analysis of the children’s sleep habits questionnaire among preschool children with autism spectrum disorder. Res. Dev. Disabil. 2020, 97, 103548. [Google Scholar] [CrossRef] [PubMed]
- Petruzzelli, M.G.; Matera, E.; Giambersio, D.; Marzulli, L.; Gabellone, A.; Legrottaglie, A.R.; Margari, A.; Margari, L. Subjective and Electroencephalographic Sleep Parameters in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. J. Clin. Med. 2021, 10, 3893. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, T.; Chen, J.; Chen, L.; Dai, Y.; Zhang, J.; Li, L.; Jia, F.; Wu, L.; Hao, Y.; et al. Sleep problems in children with autism spectrum disorder: A multicenter survey. BMC Psychiatry 2021, 21, 406. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.T.; McGough, J.; Shah, B.; Cronin, P.; Hong, D.; Aman, M.G.; Arnold, L.E.; Lindsay, R.; Nash, P.; Hollway, J.; et al. Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med. 2002, 347, 314–321. [Google Scholar] [CrossRef]
- Constantino, J.N.; Gruber, C.P. The Social Responsiveness Scale (SRS) (Manual); Western Psychological Services: Los Angeles, CA, USA, 2005. [Google Scholar]
- Friedman, D.; French, J.A.; Maccarrone, M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019, 18, 504–512. [Google Scholar] [CrossRef]
- Kirkland, A.E.; Fadus, M.C.; Gruber, S.A.; Gray, K.M.; Wilens, T.E.; Squeglia, L.M. A scoping review of the use of cannabidiol in psychiatric disorders. Psychiatry Res. 2022, 308, 114347. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Burke, S.L.; Maddux, M. Current state of evidence of cannabis utilization for treatment of autism spectrum disorders. BMC Psychiatry 2019, 19, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [Green Version]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D.; et al. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021, 78, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Stockings, E.; Campbell, G.; Hall, W.D.; Nielsen, S.; Zagic, D.; Rahman, R.; Murnion, B.; Farrell, M.; Weier, M.; Degenhardt, L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain 2018, 159, 1932–1954. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hong, P.J.; May, C.; Rehman, Y.; Oparin, Y.; Hong, C.J.; Hong, B.Y.; AminiLari, M.; Gallo, L.; Kaushal, A.; et al. Medical cannabis or cannabinoids for chronic non-cancer and cancer related pain: A systematic review and meta-analysis of randomised clinical trials. BMJ 2021, 374, n1034. [Google Scholar] [CrossRef]
- Häuser, W.; Welsch, P.; Klose, P.; Radbruch, L.; Fitzcharles, M.A. Efficacy, tolerability and safety of cannabis-based medicines for cancer pain: A systematic review with meta-analysis of randomised controlled trials. Schmerz 2019, 33, 424–436. [Google Scholar] [CrossRef]
- Grimison, P.; Mersiades, A.; Kirby, A.; Lintzeris, N.; Morton, R.; Haber, P.; Olver, I.; Walsh, A.; McGregor, I.; Cheung, Y.; et al. Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: A randomised, placebo-controlled, phase II crossover trial. Ann. Oncol. 2020, 31, 1553–1560. [Google Scholar] [CrossRef]
- Chow, R.; Valdez, C.; Chow, N.; Zhang, D.; Im, J.; Sodhi, E.; Lock, M. Oral cannabinoid for the prophylaxis of chemotherapy-induced nausea and vomiting-a systematic review and meta-analysis. Support Care Cancer 2020, 28, 2095–2103. [Google Scholar] [CrossRef]
- Black, N.; Stockings, E.; Campbell, G.; Tran, L.T.; Zagic, D.; Hall, W.D.; Farrell, M.; Degenhardt, L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 995–1010. [Google Scholar] [CrossRef]
- Bonn-Miller, M.O.; Sisley, S.; Riggs, P.; Yazar-Klosinski, B.; Wang, J.B.; Loflin, M.J.E.; Shechet, B.; Hennigan, C.; Matthews, R.; Emerson, A.; et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: A randomized cross-over clinical trial. PLoS ONE 2021, 16, e0246990. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Reis, R.; Almeida, K.J.; da Silva Lopes, L.; de Melo Mendes, C.M.; Bor-Seng-Shu, E. Efficacy and adverse event profile of cannabidiol and medicinal cannabis for treatment-resistant epilepsy: Systematic review and meta-analysis. Epilepsy Behav. 2019, 102, 106635. [Google Scholar] [CrossRef] [PubMed]
- Fleury-Teixeira, P.; Caixeta, F.V.; Ramires da Silva, L.C.; Brasil-Neto, J.P.; Malcher-Lopes, R. Effects of CBD-Enriched Cannabis sativa Extract on Autism Spectrum Disorder Symptoms: An Observational Study of 18 Participants Undergoing Compassionate Use. Front. Neurol. 2019, 10, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdman, R.; Vigil, D.; Robinson, K.; Shah, P.; Contreras, A.E. Safety and Efficacy of Medical Cannabis in Autism Spectrum Disorder Compared with Commonly Used Medications. Cannabis Cannabinoid Res. 2021. ahead of print. [Google Scholar] [CrossRef]
- McVige, J.; Headd, V.; Alwahaidy, M.; Lis, D.; Kaur, D.; Albert, B.; Mechtler, L. Medical Cannabis in the Treatment of Patients with Autism Spectrum Disorder (1648); AAN Enterprises: Apex, NC, USA, 2020. [Google Scholar]
- Yau, J.C.; Yu, S.M.; Panenka, W.J.; Pearce, H.; Gicas, K.M.; Procyshyn, R.M.; MacCallum, C.; Honer, W.G.; Barr, A.M. Characterization of mental health in cannabis dispensary users, using structured clinical interviews and standardized assessment instruments. BMC Psychiatry 2019, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Ferber, S.G.; Namdar, D.; Hen-Shoval, D.; Eger, G.; Koltai, H.; Shoval, G.; Shbiro, L.; Weller, A. “The Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020, 18, 87–96. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain”, No Gain. Front. Plant Sci. 2018, 9, 1969. [Google Scholar] [CrossRef]
- Linares, I.M.P.; Guimaraes, F.S.; Eckeli, A.; Crippa, A.C.S.; Zuardi, A.W.; Souza, J.D.S.; Hallak, J.E.; Crippa, J.A.S. No Acute Effects of Cannabidiol on the Sleep-Wake Cycle of Healthy Subjects: A Randomized, Double-Blind, Placebo-Controlled, Crossover Study. Front. Pharmacol. 2018, 9, 315. [Google Scholar] [CrossRef]
- Morgan, C.J.; Freeman, T.P.; Schafer, G.L.; Curran, H.V. Cannabidiol attenuates the appetitive effects of Delta 9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2010, 35, 1879–1885. [Google Scholar] [CrossRef] [Green Version]
- Morgan, C.J.; Gardener, C.; Schafer, G.; Swan, S.; Demarchi, C.; Freeman, T.P.; Warrington, P.; Rupasinghe, I.; Ramoutar, A.; Tan, N.; et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol. Med. 2012, 42, 391–400. [Google Scholar] [CrossRef]
- Zlebnik, N.E.; Cheer, J.F. Beyond the CB1 Receptor: Is Cannabidiol the Answer for Disorders of Motivation? Annu. Rev. Neurosci. 2016, 39, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurd, Y.L.; Yoon, M.; Manini, A.F.; Hernandez, S.; Olmedo, R.; Ostman, M.; Jutras-Aswad, D. Early Phase in the Development of Cannabidiol as a Treatment for Addiction: Opioid Relapse Takes Initial Center Stage. Neurother. J. Am. Soc. Exp. NeuroTherapeutics 2015, 12, 807–815. [Google Scholar] [CrossRef]
- Morgan, C.J.; Schafer, G.; Freeman, T.P.; Curran, H.V. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: Naturalistic study. Br. J. Psychiatry J. Ment. Sci. 2010, 197, 285–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzetti, V.; Solowij, N.; Yucel, M. The Role of Cannabinoids in Neuroanatomic Alterations in Cannabis Users. Biol. Psychiatry 2016, 79, e17–e31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, M.O.; Kebir, O.; Jay, T.M. Exposure to cannabinoids can lead to persistent cognitive and psychiatric disorders. Eur. J. Pain 2019, 23, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iseger, T.A.; Bossong, M.G. A systematic review of the antipsychotic properties of cannabidiol in humans. Schizophr. Res. 2015, 162, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.B.; Balbino, C.Q.; Weiber, A.F. The relationship between cannabidiol and psychosis: A review. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr. 2015, 27, 134–141. [Google Scholar]
- Ortiz-Medina, M.B.; Perea, M.; Torales, J.; Ventriglio, A.; Vitrani, G.; Aguilar, L.; Roncero, C. Cannabis consumption and psychosis or schizophrenia development. Int. J. Soc. Psychiatry 2018, 64, 690–704. [Google Scholar] [CrossRef]
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef] [Green Version]
- Malow, B.A.; Katz, T.; Reynolds, A.M.; Shui, A.; Carno, M.; Connolly, H.V.; Coury, D.; Bennett, A.E. Sleep Difficulties and Medications in Children With Autism Spectrum Disorders: A Registry Study. Pediatrics 2016, 137 (Suppl. S2), S98–S104. [Google Scholar] [CrossRef] [Green Version]
- Bangerter, A.; Chatterjee, M.; Manyakov, N.V.; Ness, S.; Lewin, D.; Skalkin, A.; Boice, M.; Goodwin, M.S.; Dawson, G.; Hendren, R.; et al. Relationship Between Sleep and Behavior in Autism Spectrum Disorder: Exploring the Impact of Sleep Variability. Front. Neurosci. 2020, 14, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| All (n = 150) | Group A (n = 50) | Group B (n = 50) | Group C (n = 50) | Sig. | |
|---|---|---|---|---|---|
| Treatment 1st period | Placebo | Pure cannabinoids | Whole plant | ||
| Treatment 2nd period | Whole plant | Placebo | Pure cannabinoids | ||
| Sex | |||||
| Males n (%) | 120 (80%) | 42 (84%) | 42 (84%) | 36 (72%) | 0.22 * |
| Age | |||||
| Mean ± SD [median, range] | 11.8 ± 4.1 [11.3, 5.1–20.8] | 11.7 ± 3.8 [10.7, 5.8–20.0] | 11.6 ± 4.3 [10.3, 5.1–20.4] | 12.1 ± 4.3 [12.6, 5.1–20.8] | 0.79 # |
| BMI | |||||
| Mean ± SD [median, range] | 20.8 ± 5.7 [19.0, 12.3–39.6] | 20.5 ± 5.2 [19.1, 12.8–34.0] | 20.5 ± 6.0 [19.1, 12.3–39.6] | 21.3 ± 6.1 [19.0, 13.9–39.5] | 0.72 # |
| ADOS comparison score | |||||
| Mean ± SD [median, range] | 8.8 ± 1.5 [10.0, 4.0–10.0] | 8.6 ± 1.6 [9.0, 4.0–10.0] | 9.2 ± 1.3 [10.0, 6.0–10.0] | 8.6 ± 1.6 [9.0, 4.0–10.0] | 0.07 # |
| VABS composite score | |||||
| Mean ± SD [median, range] | 52.3 ± 14.5 [51.0, 20.0–102.0] | 52.0 ± 15.0 [49.0, 26.0–102.0] | 52.4 ± 15.2 [54.0, 25.0–89.0] | 52.3 ± 13.6 [52.0, 20.0–78.0] | 0.99 # |
| CARS total score | |||||
| Mean ± SD [median, range] | 45.4 ± 8.4 [47.5, 29.5–59.0] | 46.0 ± 8.5 [47.3, 30.5–59.0] | 45.5 ± 8.9 [48.5, 29.5–57.5] | 44.6 ± 7.8 [46.5, 31.0–56.5] | 0.68 # |
| SRS | |||||
| Mean ± SD [median, range] | 119 ± 27 [121, 53–180] | 122 ± 23 [124, 53–159] | 118 ± 31 [118, 64–178] | 117 ± 27 [117, 66–180] | 0.37 # |
| Concomitant medications | |||||
| Atypical antipsychotics n (%) | 76 (50.7%) | 28 (56.0%) | 20 (40.0%) | 28 (56.0%) | 0.18 * |
| Typical antipsychotics n (%) | 13 (8.7%) | 5 (10.0%) | 3 (6.0%) | 5 (10.0%) | 0.82 * |
| Anticonvulsants n (%) | 18 (8.7%) | 6 (12.0%) | 4 (8.0%) | 8 (16.0%) | 0.47 * |
| Stimulants n (%) | 20 (13.3%) | 5 (8.0%) | 11 (22.0%) | 5 (10.0%) | 0.08 * |
| Benzodiazepines n (%) | 5 (3.3%) | 1 (2.0%) | 2 (4.0%) | 2 (4.0%) | 1.00 * |
| Melatonin n (%) | 12 (8.0%) | 6 (12.0%) | 4 (8.0%) | 2 (4.0%) | 0.39 * |
| SSRIs n (%) | 21 (14.0%) | 6 (12.0%) | 8 (16.0%) | 7 (4.0%) | 0.84 * |
| Total CSHQ score | |||||
| Mean ± SD [median, range] | 49.9 ± 9.2 [48.5, 34.0–73.5] | 49.7 ± 8.7 [49.0, 34.0–69.2] | 50.1 ± 9.4 [47.5, 36.0–72.0] | 49.7 ± 9.6 [35.0, 34.0–73.5] | 0.97 # |
| Bedtime Resistance | |||||
| Mean ± SD [median, range] | 9.4 ± 3.3 [8.0, 6.0–17.0] | 9.7 ± 3.2 [9.0, 6.0–17.0] | 9.4 ± 3.4 [6.0–16.0] | 9.1 ± 3.3 [8.0, 6.0–17.0] | 0.63 # |
| Sleep Onset Delay | |||||
| Mean ± SD [median, range] | 1.9 ± 0.8 [2.0, 1.0–3.0] | 1.9 ± 0.8 [2.0, 1.0–3.0] | 1.9 ± 0.9 [2.0, 1.0–3.0] | 2.0 ± 0.8 [2.0, 1.0–3.0] | 0.99 # |
| Sleep Duration | |||||
| Mean ± SD [median, range] | 4.6 ± 1.8 [4.0, 3.0–9.0] | 4.6 ± 1.8 [4.0, 3.0–9.0] | 4.6 ± 1.8 [4.0, 3.0–9.0] | 4.5 ± 1.8 [4.0, 3.0–9.0] | 0.96 # |
| Sleep Anxiety | |||||
| Mean ± SD [median, range] | 6.3 ± 2.3 [6.0, 4.0–12.0] | 6.4 ± 2.1 [6.0, 4.0–11.0] | 6.5 ± 2.4 [6.0, 4.0–12.0] | 6.1 ± 2.3 [5.0, 4.0–12.0] | 0.64 # |
| Night Wakings | |||||
| Mean ± SD [median, range] | 4.9 ± 1.9 [4.0, 3.0–9.0] | 4.8 ± 1.7 [4.5, 3.0–9.0] | 5.13 ± 2.0 [5.0, 3.0–9.0] | 4.7 ± 2.0 [4.0, 3.0–9.0] | 0.52 # |
| Parasomnias | |||||
| Mean ± SD [median, range] | 9.2 ± 2.0 [9.0, 7.0–18.2] | 8.6 ± 1.7 [9.0, 7.0–12.0] | 9.5 ± 2.0 [9.0, 7.0–14.0] | 9.3 ± 2.3 [8.8, 7.0–18.2] | 0.44 # |
| Sleep Disordered Breathing | |||||
| Mean ± SD [median, range] | 3.9 ± 1.4 [3.0, 3.0–9.0] | 3.9 ± 1.2 [3.5, 3.0–7.0] | 3.7 ± 1.3 [3.0, 3.0–9.0] | 4.1 ± 1.6 [3.0, 3.0–7.0] | 0.40 # |
| Daytime Sleepiness | |||||
| Mean ± SD [median, range] | 14.4 ± 3.6 [14.0, 9.0–24.0] | 14.2 ± 3.7 [14.0, 9.0–23.0] | 14.3 ± 3.7 [13.1, 9.0–24.0] | 14.7 ± 3.5 [15.0, 9.0–24.0] | 0.79 # |
| Placebo n = 45 [Change in Points] | Pure Cannabinoids n = 42 [Change in Points] | Whole Plant n = 44 [Change in Points] | Total n = 131 [Change in Points] | Sig ^ | |
|---|---|---|---|---|---|
| Total CSHQ score | |||||
| Mean ± SD [median, range] | −1.4 ± 6.6 [−1.9, −20.3–13.0] | −2.9 ± 9.2 [−1.5, −27.9–18.0] | −2.3 ± 5.6 [−1.5, −18.0–7.3] | −2.2 ± 7.2 [−1.9, −27.9–18.0] | 0.63 |
| Bedtime Resistance | |||||
| Mean ± SD [median, range] | −0.6 ± 1.6 [0.0, −4.0–3.0] | −0.5 ± 2.7 [0.0, −9.0–5.7] | −0.3 ± 1.6 [0.0, −6.0–3.0] | −0.4 ± 2.0 [0.0, −9.0–5.7] | 0.79 |
| Sleep Onset Delay | |||||
| Mean ± SD [median, range] | −0.1 ± 0.6 [0.0, −1.0–2.0] | −0.1 ± 0.8 [0.0, −2.0–2.0] | −0.2 ± 0.8 [0.0, −2.0–1.0] | −0.2 ± 0.7 [0.0, −2.0–2.0] | 0.98 |
| Sleep Duration | |||||
| Mean ± SD [median, range] | −0.1 ± 1.6 [0.0, −4.0–4.0] | 0.0 ± 2.0 [0.0, −5.0–4.0] | −0.5 ± 1.9 [0.0, −5.0–4.0] | −0.2 ± 1.8 [0.0, −5.0–4.0] | 0.38 |
| Sleep Anxiety | |||||
| Mean ± SD [median, range] | −0.4 ± 1.2 [0.0, −4.0–2.0] | −0.6 ± 1.3 [0.0, −4.0–1.7] | −0.2 ± 1.5 [0.0, −4.0–2.0] | −0.4 ± 1.3 [0.0, −4.0–2.0] | 0.59 |
| Night Wakings | |||||
| Mean ± SD [median, range] | −0.2 ± 1.3 [0.0, −3.0–3.0] | −0.8 ± 1.5 [−0.5, −4.0–1.0] | −0.6 ± 1.2 [0.0, −4.0–1.0] | −0.5 ± 1.4 [0.0, −4.0–3.0] | 0.11 |
| Parasomnias | |||||
| Mean ± SD [median, range] | −0.2 ± 1.6 [0.0, −4.0–4.0] | −0.6 ± 1.9 [−0.9, −7.0–4.0] | −0.5 ± 1.4 [0.0, −4.5–2.3] | −0.5 ± 1.6 [0.0, −7.0–4.0] | 0.53 |
| Sleep Disordered Breathing | |||||
| Mean ± SD [median, range] | −0.0 ± 0.9 [0.0, −2.0–3.0] | −0.3 ± 1.0 [−0.0, −4.0–1.0] | −0.1 ± 0.8 [0.0, −2.0–1.0] | −0.2 ± 0.9 [0.0, −4.0–3.0] | 0.36 |
| Daytime Sleepiness | |||||
| Mean ± SD [median, range] | 0.1 ± 3.0 [0.0, −9.0–7.8] | 0.2 ± 3.5 [0.0, −7.0–7.0] | 0.0 ± 2.7 [0.0, −5.0–5.0] | 0.1 ± 3.1 [0.0, −9.0–7.8] | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnapp, A.; Harel, M.; Cayam-Rand, D.; Cassuto, H.; Polyansky, L.; Aran, A. A Placebo-Controlled Trial of Cannabinoid Treatment for Disruptive Behavior in Children and Adolescents with Autism Spectrum Disorder: Effects on Sleep Parameters as Measured by the CSHQ. Biomedicines 2022, 10, 1685. https://doi.org/10.3390/biomedicines10071685
Schnapp A, Harel M, Cayam-Rand D, Cassuto H, Polyansky L, Aran A. A Placebo-Controlled Trial of Cannabinoid Treatment for Disruptive Behavior in Children and Adolescents with Autism Spectrum Disorder: Effects on Sleep Parameters as Measured by the CSHQ. Biomedicines. 2022; 10(7):1685. https://doi.org/10.3390/biomedicines10071685
Chicago/Turabian StyleSchnapp, Aviad, Moria Harel, Dalit Cayam-Rand, Hanoch Cassuto, Lola Polyansky, and Adi Aran. 2022. "A Placebo-Controlled Trial of Cannabinoid Treatment for Disruptive Behavior in Children and Adolescents with Autism Spectrum Disorder: Effects on Sleep Parameters as Measured by the CSHQ" Biomedicines 10, no. 7: 1685. https://doi.org/10.3390/biomedicines10071685
APA StyleSchnapp, A., Harel, M., Cayam-Rand, D., Cassuto, H., Polyansky, L., & Aran, A. (2022). A Placebo-Controlled Trial of Cannabinoid Treatment for Disruptive Behavior in Children and Adolescents with Autism Spectrum Disorder: Effects on Sleep Parameters as Measured by the CSHQ. Biomedicines, 10(7), 1685. https://doi.org/10.3390/biomedicines10071685