Nano-Enabled Strategies for the Treatment of Lung Cancer: Potential Bottlenecks and Future Perspectives
Abstract
:1. Introduction
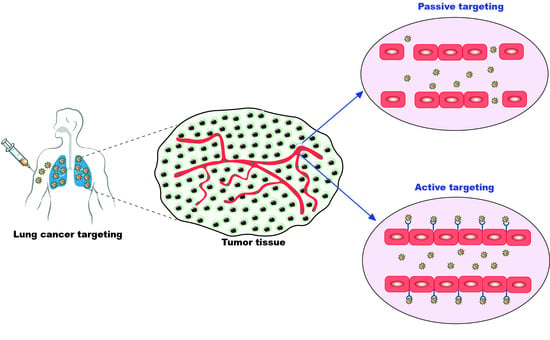
2. Targeting of Nano-Formulations for Lung Cancer
2.1. Passive Targeting of Nano-Formulations for Lung Cancer
2.2. Active Targeting of Nano-Formulations for Lung Cancer
3. Drug Resistance in Lung Cancer
4. Combinatorial Therapy for Lung Cancer Treatment
5. Clinical Studies of Nanocarriers in Lung Cancer
6. Challenges Associated with the Use of Nanomedicine in Cancer
6.1. Challenges Associated with Non-Targeted (Passively Targeted) Nano-Formulations
6.2. Challenges Associated with Targeted (Ligand Anchored/Active Targeted) Nano-Formulations
7. Recent Patent Literature: Innovative Approaches and Novel Formulations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrova, D.; Špacírová, Z.; Fernández-Martínez, N.F.; Ching-López, A.; Garrido, D.; Rodríguez-Barranco, M.; Pollán, M.; Redondo-Sánchez, D.; Espina, C.; Higueras-Callejón, C.; et al. The patient, diagnostic, and treatment intervals in adult patients with cancer from high-and lower-income countries: A systematic review and meta-analysis. PLoS Med. 2022, 19, e1004110. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Spiro, S.G.; Silvestri, G.A. One hundred years of lung cancer. Am. J. Respir. Crit. Care Med. 2005, 172, 523–529. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Y.; Li, M.; Liu, T.; Liu, J.; Xie, Y.; Xu, S.; Liu, H. A Dual-Functional HER2 Aptamer-Conjugated, PH-Activated Mesoporous Silica Nanocarrier-Based Drug Delivery System Provides in Vitro Synergistic Cytotoxicity in HER2-Positive Breast Cancer Cells. Int. J. Nanomed. 2019, 14, 4029. [Google Scholar]
- Carrasco-Esteban, E.; Antonio Domínguez-Rullán, J.; Barrionuevo-Castillo, P.; Pelari-Mici, L.; Leaman, O.; Sastre-Gallego, S.; López-Campos, F. Current Role of Nanoparticles in the Treatment of Lung Cancer. J. Clin. Transl. Res. 2021, 7, 140–155. [Google Scholar] [PubMed]
- Domagala-Kulawik, J.; Osinska, I.; Hoser, G. Mechanisms of immune response regulation in lung cancer. Transl. Lung Cancer Res. 2014, 3, 15. [Google Scholar]
- Liu, M.; Wang, X.; Li, W.; Yu, X.; Flores-Villanueva, P.; Xu-Monette, Z.Y.; Li, L.; Zhang, M.; Young, K.H.; Ma, X.; et al. Targeting PD-L1 in non-small cell lung cancer using CAR T cells. Oncogenesis 2020, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Liu, M.; Wang, L.; Zhu, K.; Cai, M.; Chen, X.; Feng, Y.; Yang, S.; Fu, S.; Zhi, C.; et al. Remodelling of tumour microenvironment by microwave ablation potentiates immunotherapy of AXL-specific CAR T cells against non-small cell lung cancer. Nat. Commun. 2022, 13, 6203. [Google Scholar] [CrossRef]
- Wang, M.; Herbst, R.S.; Boshoff, C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat. Med. 2021, 27, 1345–1356. [Google Scholar] [CrossRef]
- Perepelyuk, M.; Maher, C.; Lakshmikuttyamma, A.; Shoyele, S.A. Aptamer-Hybrid Nanoparticle Bioconjugate Efficiently Delivers MiRNA-29b to Non-Small-Cell Lung Cancer Cells and Inhibits Growth by Downregulating Essential Oncoproteins. Int. J. Nanomed. 2016, 11, 3533–3544. [Google Scholar]
- Wang, J.; Zhou, T.; Liu, Y.; Chen, S.; Yu, Z. Application of Nanoparticles in the Treatment of Lung Cancer with Emphasis on Receptors. Front. Pharmacol. 2022, 12, 781425. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Dash, M.; Behera, A. Nanophytochemicals for the Treatment of Type II Diabetes Mellitus: A Review. Environ. Chem. Lett. 2021, 19, 4349–4373. [Google Scholar] [CrossRef]
- Padhi, S.; Behera, A. Advanced Drug Delivery Systems in the Treatment of Ovarian Cancer. Adv. Drug Deliv. Syst. Manag. Cancer 2021, 127–139. [Google Scholar]
- Padhi, S.; Behera, A.; Hasnain, M.S.; Nayak, A.K. Chitosan-Based Drug Delivery Systems in Cancer Therapeutics. Chitosan Drug Deliv. 2022, 159–193. [Google Scholar]
- Patnaik, S.; Gorain, B.; Padhi, S.; Choudhury, H.; Gabr, G.A.; Md, S.; Kumar Mishra, D.; Kesharwani, P. Recent Update of Toxicity Aspects of Nanoparticulate Systems for Drug Delivery. Eur. J. Pharm. Biopharm. 2021, 161, 100–119. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Kapoor, R.; Verma, D.; Panda, A.K.; Iqbal, Z. Formulation and Optimization of Topotecan Nanoparticles: In Vitro Characterization, Cytotoxicity, Cellular Uptake and Pharmacokinetic Outcomes. J. Photochem. Photobiol. B Biol. 2018, 183, 222–232. [Google Scholar] [CrossRef]
- Behera, A.; Patra, N.; Mittu, B.; Padhi, S.; Singh, J. Bimetallic Nanoparticles: Green Synthesis, Applications, and Future Perspectives. Multifunct. Hybrid Nanomater. Sustain. Agri-Food Ecosyst. 2020, 639–682. [Google Scholar]
- Wang, H.; Zhang, F.; Wen, H.; Shi, W.; Huang, Q.; Huang, Y.; Xie, J.; Li, P.; Chen, J.; Qin, L.; et al. Tumor- and Mitochondria-Targeted Nanoparticles Eradicate Drug Resistant Lung Cancer through Mitochondrial Pathway of Apoptosis. J. Nanobiotechnol. 2020, 18, 8. [Google Scholar] [CrossRef]
- Padhi, S.; Behera, A. Silver-Based Nanostructures as Antifungal Agents: Mechanisms and Applications. Silver Nanomater. Agri-Food Appl. 2021, 17–38. [Google Scholar]
- Behera, A.; Mittu, B.; Padhi, S.; Singh, A. Antimicrobial Efficacy of Essential Oil Nanoemulsions. In Nanotechnological Approaches in Food Microbiology; CRC Press: Boca Raton, FL, USA, 2020; pp. 293–309. [Google Scholar]
- Alshahrani, S.M. A Judicious Review on the Applications of Chemotherapeutic Loaded Nanoemulsions in Cancer Management. J. Drug Deliv. Sci. Technol. 2022, 68, 103085. [Google Scholar] [CrossRef]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nano-Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef]
- Padhi, S.; Behera, A. Cellular Internalization and Toxicity of Polymeric Nanoparticles. In Polymeric Nanoparticles for the Treatment of Solid Tumors; Padhi, S., Behera, A., Licht-fouse, E., Eds.; Springer Nature: Cham, Switzerland, 2022; Volume 71, pp. 473–488. [Google Scholar]
- Bazak, R.; Houri, M.; El Achy, S.; Hussein, W.; Refaat, T. Passive Targeting of Nanoparticles to Cancer: A Comprehensive Review of the Literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef]
- Kundu, A.; Padhi, S.; Behera, A.; Hasnain, M.S.; Nayak, A.K. Tumor Targeting Strategies by Chitosan-Based Nanocarriers. Chitosan Biomed. Appl. 2022, 163–188. [Google Scholar]
- Padhi, S.; Azharuddin, M.; Behera, A.; Zakir, F.; Mirza, M.A.; Chyad, A.A.; Iqbal, Z.; Mansoor, S. Nanocarriers as Delivery Tool for COVID-19 Drugs. Coronavirus Drug Discov. 2022, 2, 293–332. [Google Scholar]
- Behera, A.; Padhi, S. pH-Sensitive Polymeric Nanoparticles for Cancer Treatment. In Polymeric Nanoparticles for the Treatment of Solid Tumors; Padhi, S., Behera, A., Licht-fouse, E., Eds.; Springer Nature: Cham, Switzerland, 2022; Volume 71, pp. 401–425. [Google Scholar]
- Lee, H.Y.; Mohammed, K.A.; Nasreen, N. Nanoparticle-Based Targeted Gene Therapy for Lung Cancer. Am. J. Cancer Res. 2016, 6, 1118. [Google Scholar] [PubMed]
- Leighl, N.B.; Goss, G.D.; Lopez, P.G.; Burkes, R.L.; Dancey, J.E.; Rahim, Y.H.; Rudinskas, L.C.; Pouliot, J.F.; Rodgers, A.; Pond, G.R.; et al. Phase II Study of Pegylated Liposomal Doxorubicin HCl (Caelyx) in Combination with Cyclophosphamide and Vincristine as Second-Line Treatment of Patients with Small Cell Lung Cancer. Lung Cancer 2006, 52, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Guo, H.; Luan, X.; He, M.; Li, F.; Burnett, J.; Truchan, N.; Sun, D. Albumin nanoparticle of paclitaxel (Abraxane) decreases while taxol increases breast cancer stem cells in treatment of triple negative breast cancer. Mol. Pharm. 2020, 17, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Shrike Zhang, Y.; Bo, P.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy*. Nanomater. Neoplasms 2021, 31–142. [Google Scholar]
- Alphandéry, E. Biodistribution and Targeting Properties of Iron Oxide Nanoparticles for Treatments of Cancer and Iron Anemia Disease. Nanotoxicology 2019, 13, 573–596. [Google Scholar] [CrossRef]
- Behera, A.; Padhi, S. Passive and Active Targeting Strategies for the Delivery of the Camptothecin Anticancer Drug: A Review. Environ. Chem. Lett. 2020, 18, 1557–1567. [Google Scholar] [CrossRef]
- Adhipandito, C.F.; Cheung, S.H.; Lin, Y.H.; Wu, S.H. Atypical renal clearance of nanoparticles larger than the kidney filtration threshold. Int. J. Mol. Sci. 2021, 22, 11182. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, G.; Delivery, T.B. Lipoplatin Formulation Review Article. J. Drug Deliv. 2012, 2012, 581363. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Rivas-García, L.; Baptista, P.V.; Fernandes, A.R. Gene therapy in cancer treatment: Why go nano? Pharmaceutics 2020, 12, 233. [Google Scholar] [CrossRef] [PubMed]
- Amreddy, N.; Babu, A.; Muralidharan, R.; Munshi, A.; Ramesh, R. Polymeric nanoparticle-mediated gene delivery for lung cancer treatment. Top. Curr. Chem. 2018, 375, 35. [Google Scholar] [CrossRef]
- Bonnet, M.E.; Gossart, J.B.; Benoit, E.; Messmer, M.; Zounib, O.; Moreau, V.; Behr, J.P.; Lenne-Samuel, N.; Kedinger, V.; Meulle, A.; et al. Systemic Delivery of Sticky SiRNAs Targeting the Cell Cycle for Lung Tumor Metastasis Inhibition. J. Control. Release 2013, 170, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Cheng, F.Y.; Shieh, D.B.; Yeh, C.S.; Su, W.C. PLGA Nanoparticles Codeliver Paclitaxel and Stat3 SiRNA to Overcome Cellular Resistance in Lung Cancer Cells. Int. J. Nanomed. 2012, 7, 4269–4283. [Google Scholar] [CrossRef]
- Verma, D.; Thakur, P.S.; Padhi, S.; Khuroo, T.; Talegaonkar, S.; Iqbal, Z. Design Expert Assisted Nanoformulation Design for Co-Delivery of Topotecan and Thymoquinone: Optimization, in Vitro Characterization and Stability Assessment. J. Mol. Liq. 2017, 242, 382–394. [Google Scholar] [CrossRef]
- Padhi, S.; Mirza, M.A.; Verma, D.; Khuroo, T.; Panda, A.K.; Talegaonkar, S.; Khar, R.K.; Iqbal, Z. Revisiting the Nanoformulation Design Approach for Effective Delivery of Topotecan in Its Stable Form: An Appraisal of Its in Vitro Behavior and Tumor Amelioration Potential. Drug Deliv. 2015, 23, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Chang, S.; Ko, T.; Anker, J.; Agte, S.; Iams, W.; Choi, W.M.; Lee, K.; Cruz, M. Epithelial-mesenchymal transition (EMT) signature is inversely associated with T-cell infiltration in non-small cell lung cancer (NSCLC). Sci. Rep. 2018, 8, 2918. [Google Scholar]
- Karacosta, L.G.; Anchang, B.; Ignatiadis, N.; Kimmey, S.C.; Benson, J.A.; Shrager, J.B.; Tibshirani, R.; Bendall, S.C.; Plevritis, S.K. Mapping lung cancer epithelial-mesenchymal transition states and trajectories with single-cell resolution. Nat. Commun. 2019, 10, 5587. [Google Scholar] [CrossRef]
- Sousa, C.; Gouveia, L.F.; Kreutzer, B.; Silva-Lima, B.; Maphasa, R.E.; Dube, A.; Videira, M. Polymeric micellar formulation enhances antimicrobial and anticancer properties of salinomycin. Pharm. Res. 2019, 36, 83. [Google Scholar] [CrossRef]
- Gowda, S.S.; Rajasowmiya, S.; Vadivel, V.; Devi, S.B.; Jerald, A.C.; Marimuthu, S.; Devipriya, N. Gallic acid-coated sliver nanoparticle alters the expression of radiation-induced epithelial-mesenchymal transition in non-small lung cancer cells. Toxicol. In Vitro 2018, 52, 170–177. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, R.; Tang, N.; Lu, Z.; Wang, H.; Zhang, Z.; Xue, X.; Huang, Z.; Zhang, S.; Zhang, G.; et al. Active Targeting Theranostic Iron Oxide Nanoparticles for MRI and Magnetic Resonance-Guided Focused Ultrasound Ablation of Lung Cancer. Biomaterials 2017, 127, 25–35. [Google Scholar] [CrossRef]
- Padhi, S.; Behera, A. Nanotechnology Based Targeting Strategies for the Delivery of Camptothecin. In Sustainable Agriculture Reviews 44. Sustainable Agriculture Reviews; Saneja, A., Panda, A., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 243–272. [Google Scholar]
- Ulbrich, K.; Hola, I.; Bakandritsos, A.; Tuc, Í.; Zbor, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.; Amreddy, N.; Muralidharan, R.; Pathuri, G.; Gali, H.; Chen, A.; Zhao, Y.D.; Munshi, A.; Ramesh, R. Chemodrug Delivery Using Integrin-Targeted PLGA-Chitosan Nanoparticle for Lung Cancer Therapy. Sci. Rep. 2017, 7, 14674. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Sharma, G.; Sonali; Singh, S.; Bharti, S.; Pandey, B.L.; Koch, B.; Muthu, M.S. Chitosan-Folate Decorated Carbon Nanotubes for Site Specific Lung Cancer Delivery. Mater. Sci. Eng. C 2017, 77, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajaram, R. Target Delivery of Doxorubicin Tethered with PVP Stabilized Gold Nanoparticles for Effective Treatment of Lung Cancer. Sci. Rep. 2018, 8, 3815. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.D.; Shandilya, R.; Bhargava, A.; Kumar, R.; Tiwari, R.; Chaudhury, K.; Srivastava, R.K.; Goryacheva, I.Y.; Mishra, P.K. Quantum Dot Based Nano-Biosensors for Detection of Circulating Cell Free MiRNAs in Lung Carcinogenesis: From Biology to Clinical Translation. Front. Genet. 2018, 9, 616. [Google Scholar] [CrossRef]
- Mashinchian, O.; Johari-Ahar, M.; Ghaemi, B.; Rashidi, M.; Barar, J.; Omidi, Y. Impacts of Quantum Dots in Molecular Detection and Bioimaging of Cancer. Bioimpacts 2014, 4, 149. [Google Scholar] [CrossRef]
- Ranjbar-Navazi, Z.; Eskandani, M.; Johari-Ahar, M.; Nemati, A.; Akbari, H.; Davaran, S.; Omidi, Y. Doxorubicin-Conjugated D-Glucosamine- and Folate- Bi-Functionalised InP/ZnS Quantum Dots for Cancer Cells Imaging and Therapy. J. Drug Target 2017, 26, 267–277. [Google Scholar] [CrossRef]
- Zhao, T.; Qin, S.; Peng, L.; Li, P.; Feng, T.; Wan, J.; Yuan, P.; Zhang, L. Novel Hyaluronic Acid-Modified Temperature-Sensitive Nanoparticles for Synergistic Chemo-Photothermal Therapy. Carbohydr. Polym. 2019, 214, 221–233. [Google Scholar] [CrossRef]
- Haider, M.; Elsherbeny, A.; Pittalà, V.; Consoli, V.; Alghamdi, M.A.; Hussain, Z.; Khoder, G.; Greish, K. Nanomedicine Strategies for Management of Drug Resistance in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 1853. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer Development, Progression, and Therapy: An Epigenetic Overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.I.; Williams, R.T.; Henderson, M.J.; Norris, M.D.; Haber, M. ABC Transporters as Mediators of Drug Resistance and Contributors to Cancer Cell Biology. Drug Resist. Updat. 2016, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Bell, E.L.; Hughes, R.O.; Garfield, A.S. ABC transporters: Human disease and pharmacotherapeutic potential. Trends Mol. Med. 2023, 29, 152–172. [Google Scholar] [CrossRef]
- Peng, S.; Wang, J.; Lu, C.; Xu, Z.; Chai, J.J.; Ke, Q.; Deng, X.Z. Emodin Enhances Cisplatin Sensitivity in Non-Small Cell Lung Cancer through Pgp Downregulation. Oncol. Lett. 2021, 21, 230. [Google Scholar] [CrossRef]
- Morgillo, F.; Della Corte, C.M.; Fasano, M.; Ciardiello, F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open 2016, 1, e000060. [Google Scholar] [CrossRef]
- Schaufler, D.; Ast, D.F.; Tumbrink, H.L.; Abedpour, N.; Maas, L.; Schwäbe, A.E.; Spille, I.; Lennartz, S.; Fassunke, J.; Aldea, M.; et al. Clonal dynamics of BRAF-driven drug resistance in EGFR-mutant lung cancer. NPJ Precis. Oncol. 2021, 5, 102. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, P.; Zhao, M.; Cao, H.; Zhao, Y.; Ji, M.; Hou, P.; Chen, M. EGFL7 drives the evolution of resistance to EGFR inhibitors in lung cancer by activating NOTCH signaling. Cell Death Discov. 2022, 13, 910. [Google Scholar] [CrossRef]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef]
- La Montagna, M.; Ginn, L.; Garofalo, M. Mechanisms of drug resistance mediated by long non-coding RNAs in non-small-cell lung cancer. Cancer Gene Ther. 2021, 28, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Heiden, M.G.V.; Kroemer, G. Metabolic Targets for Cancer Therapy. Nat. Rev. Drug Discov. 2013, 12, 829–846. [Google Scholar] [CrossRef]
- Wangpaichitr, M.; Wu, C.; Li, Y.Y.; Nguyen, D.J.M.; Kandemir, H.; Shah, S.; Chen, S.; Feun, L.G.; Prince, J.S.; Kuo, M.T.; et al. Exploiting ROS and Metabolic Differences to Kill Cisplatin Resistant Lung Cancer. Oncotarget 2017, 8, 49275–49292. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Cabral, H.; Matsumoto, Y.; Wu, S.; Kano, M.R.; Yamori, T.; Nishiyama, N.; Kataoka, K. Improving Drug Potency and Efficacy by Nanocarrier-Mediated Subcellular Targeting. Sci. Transl. Med. 2011, 3, 64ra2. [Google Scholar] [CrossRef]
- Tang, D.; Zhao, X.; Zhang, L.; Wang, Z.; Wang, C. Identification of Hub Genes to Regulate Breast Cancer Metastasis to Brain by Bioinformatics Analyses. J. Cell. Biochem. 2019, 120, 9522–9531. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Li, X.; Ran, M.; Ji, M.; Gou, J.; Yin, T.; He, H.; Wang, Y.; Zhang, Y.; Tang, X. Fabricating Nanoparticles Co-Loaded with Survivin SiRNA and Pt(IV) Prodrug for the Treatment of Platinum-Resistant Lung Cancer. Int. J. Pharm. 2021, 601, 120577. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, S.; Ming, Y.; Wang, L.; Li, C.; Luo, M.; Li, Z.; Li, B.; Chen, J. Specific Cancer Stem Cell-Therapy by Albumin Nanoparticles Functionalized with CD44-Mediated Targeting. J. Nanobiotechnol. 2018, 16, 99. [Google Scholar] [CrossRef]
- Yabuki, N.; Sakata, K.; Yamasaki, T.; Terashima, H.; Mio, T.; Miyazaki, Y.; Fujii, T.; Kitada, K. Gene Amplification and Expression in Lung Cancer Cells with Acquired Paclitaxel Resistance. Cancer Genet. Cytogenet. 2007, 173, 1–9. [Google Scholar] [CrossRef]
- Saad, M.; Garbuzenko, O.B.; Minko, T. Co-Delivery of SiRNA and an Anticancer Drug for Treatment of Multidrug-Resistant Cancer. Nanomedicine 2008, 3, 761–776. [Google Scholar] [CrossRef]
- Lu, Z.; Su, J.; Li, Z.; Zhan, Y.; Ye, D. Hyaluronic Acid-Coated, Prodrug-Based Nanostructured Lipid Carriers for Enhanced Pancreatic Cancer Therapy. Drug Dev. Ind. Pharm. 2016, 43, 160–170. [Google Scholar] [CrossRef]
- Sharma, A.; Shambhwani, D.; Pandey, S.; Singh, J.; Lalhlenmawia, H.; Kumarasamy, M.; Singh, S.K.; Chellappan, D.K.; Gupta, G.; Prasher, P.; et al. Advances in Lung Cancer Treatment Using Nanomedicines. ACS Omega 2023, 8, 10–41. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Raposo, L.R.; Valente, R.; Fernandes, A.R.; Baptista, P.V. Combined cancer therapeutics-Tackling the complexity of the tumor microenvironment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1704. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, Y.; Wu, Z.; Zhang, L.; He, D.; Li, X.; Wang, Z. Synergistic Combination Therapy of Lung Cancer: Cetuximab Functionalized Nanostructured Lipid Carriers for the Co-Delivery of Paclitaxel and 5-Demethylnobiletin. Biomed. Pharmacother. 2019, 118, 109225. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Leng, D.; Cun, D.; Foged, C.; Yang, M. Advances in Combination Therapy of Lung Cancer: Rationales, Delivery Technologies and Dosage Regimens. J. Control. Release 2017, 260, 78–91. [Google Scholar] [CrossRef]
- Jeannot, V.; Gauche, C.; Mazzaferro, S.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Didier, C.; Vollaire, J.; Josserand, V.; Coll, J.L.; et al. Anti-Tumor Efficacy of Hyaluronan-Based Nanoparticles for the Co-Delivery of Drugs in Lung Cancer. J. Control. Release 2018, 275, 117–128. [Google Scholar] [CrossRef]
- Taratula, O.; Garbuzenko, O.B.; Chen, A.M.; Minko, T. Innovative Strategy for Treatment of Lung Cancer: Targeted Nanotechnology-Based Inhalation Co-Delivery of Anticancer Drugs and SiRNA. J. Drug Target. 2011, 19, 900–914. [Google Scholar] [CrossRef]
- Lo, Y.L.; Huang, X.S.; Chen, H.Y.; Huang, Y.C.; Liao, Z.X.; Wang, L.F. ROP and ATRP Fabricated Redox Sensitive Micelles Based on PCL-SS-PMAA Diblock Copolymers to Co-Deliver PTX and CDDP for Lung Cancer Therapy. Colloids Surf. B Biointerfaces 2021, 198, 111443. [Google Scholar] [CrossRef]
- Menon, J.U.; Kuriakose, A.; Iyer, R.; Hernandez, E.; Gandee, L.; Zhang, S.; Takahashi, M.; Zhang, Z.; Saha, D.; Nguyen, K.T. Dual-Drug Containing Core-Shell Nanoparticles for Lung Cancer Therapy. Sci. Rep. 2017, 7, 13249. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Z.; Li, C.; Duan, G.; Wang, K.; Li, Q.; Tao, T. RGD Peptide-Modified, Paclitaxel Prodrug-Based, Dual-Drugs Loaded, and Redox-Sensitive Lipid-Polymer Nanoparticles for the Enhanced Lung Cancer Therapy. Biomed. Pharmacother. 2018, 106, 275–284. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, Z.; Wang, B.; Chen, G.; Zhang, Y.; Deng, H.; Tang, Z.; Mao, J.; Wang, L. Combination Chemotherapy of Lung Cancer—Co-Delivery of Docetaxel Prodrug and Cisplatin Using Aptamer-Decorated Lipid–Polymer Hybrid Nanoparticles. Drug Des. Devel. Ther. 2020, 14, 2249–2261. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, H.; Han, L.; Qiang, Z.; Zhang, X.; Gao, W.; Zhao, K.; Song, Y. Synergistic Combination Therapy of Lung Cancer Using Paclitaxel- and Triptolide-Coloaded Lipid–Polymer Hybrid Nanoparticles. Drug Des. Devel. Ther. 2018, 12, 3199–3209. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Wang, Y.; Guo, Z.; Chen, J.; Lin, L.; Wu, J.; Tian, H.; Chen, X. Pulmonary Delivery by Exploiting Doxorubicin and Cisplatin Co-Loaded Nanoparticles for Metastatic Lung Cancer Therapy. J. Control. Release 2019, 295, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kumari, R.M.; Gupta, N.; Syed, A.; Bahkali, A.H.; Nimesh, S. Poly-(Lactic-Co-Glycolic) Acid Nanoparticles for Synergistic Delivery of Epirubicin and Paclitaxel to Human Lung Cancer Cells. Molecules 2020, 25, 4243. [Google Scholar] [CrossRef]
- Reda, M.; Ngamcherdtrakul, W.; Nelson, M.A.; Siriwon, N.; Wang, R.; Zaidan, H.Y.; Bejan, D.S.; Reda, S.; Hoang, N.H.; Crumrine, N.A.; et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. 2022, 13, 4261. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B.; He, Z.; Tu, B.; Zhao, P.; Wang, H.; Asrorov, A.; Muhitdinov, B.; Jiang, J.; Huang, Y. Nanotherapeutic macrophage-based immunotherapy for the peritoneal carcinomatosis of lung cancer. Nanoscale 2022, 14, 2304–2315. [Google Scholar] [CrossRef]
- Lafuente-Gómez, N.; Wang, S.; Fontana, F.; Dhanjani, M.; García-Soriano, D.; Correia, A.; Castellanos, M.; Diaz, C.R.; Salas, G.; Santos, H.A.; et al. Synergistic immunomodulatory effect in macrophages mediated by magnetic nanoparticles modified with miRNAs. Nanoscale 2022, 14, 11129–11138. [Google Scholar] [CrossRef] [PubMed]
- Hrkach, J.; Von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T.; De Witt, D.; Figa, M.; Figueiredo, M.; Horhota, A.; et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012, 4, 128ra39. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Riely, G.J.; Azzoli, C.G.; Miller, V.A.; Ng, K.K.; Fiore, J.; Chia, G.; Brower, M.; Heelan, R.; Hawkins, M.J.; et al. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non–small-cell lung cancer. J. Clin. Oncol. 2008, 26, 639–643. [Google Scholar] [CrossRef]
- Landen, C.N.; Kinch, M.S.; Sood, A.K. EphA2 as a Target for Ovarian Cancer Therapy. Expert Opin Ther Targets 2005, 9, 1179–1187. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Petros, R.A.; Napier, M.E.; Desimone, J.M. The Complex Role of Multivalency in Nanoparticles Targeting the Transferrin Receptor for Cancer Therapies. J. Am. Chem. Soc. 2010, 132, 11306–11313. [Google Scholar] [CrossRef] [PubMed]
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenblum, D.; Peer, D. Omics-Based Nanomedicine: The Future of Personalized Oncology. Cancer Lett. 2014, 352, 126–136. [Google Scholar] [CrossRef]
- Meacham, C.E.; Morrison, S.J. Tumour Heterogeneity and Cancer Cell Plasticity. Nature 2013, 501, 328–337. [Google Scholar] [CrossRef]
- Ryan, M.B.; Der, C.J.; Wang-Gillam, A.; Cox, A.D. Targeting RAS-Mutant Cancers: Is ERK the Key? Trends Cancer 2015, 1, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Kedmi, R.; Veiga, N.; Ramishetti, S.; Goldsmith, M.; Rosenblum, D.; Dammes, N.; Hazan-Halevy, I.; Nahary, L.; Leviatan-Ben-Arye, S.; Harlev, M.; et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 2018, 13, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.; Malik, A.; Waquar, S.; Arooj, M.; Zahid, S.; Asif, M.; Shaheen, S.; Hussain, A.; Ullah, H.; Gan, S.H. New Challenges in the Use of Nanomedicine in Cancer Therapy. Bioengineered 2022, 13, 759–773. [Google Scholar] [CrossRef]
- Keller, J.G.; Graham, U.M.; Koltermann-Jülly, J.; Gelein, R.; Ma-Hock, L.; Landsiedel, R.; Wiemann, M.; Oberdörster, G.; Elder, A.; Wohlleben, W. Predicting dissolution and transformation of inhaled nanoparticles in the lung using abiotic flow cells: The case of barium sulfate. Sci. Rep. 2020, 10, 458. [Google Scholar] [CrossRef]
- Thai, L.P.A.; Mousseau, F.; Oikonomou, E.; Radiom, M.; Berret, J.F. Effect of Nanoparticles on the Bulk Shear Viscosity of a Lung Surfactant Fluid. ACS Nano 2019, 14, 466–475. [Google Scholar] [CrossRef]
- Radiom, M.; Sarkis, M.; Brookes, O.; Oikonomou, E.K.; Baeza-Squiban, A.; Berret, J.F. Pulmonary surfactant inhibition of nanoparticle uptake by alveolar epithelial cells. Sci. Rep. 2020, 10, 19436. [Google Scholar] [CrossRef]
- Schultheis, B.; Strumberg, D.; Santel, A.; Vank, C.; Gebhardt, F.; Keil, O.; Lange, C.; Giese, K.; Kaufmann, J.; Khan, M.; et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014, 32, 4141–4148. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef]
- Imran, M.; Kumar Arora, M.; Asdaq, S.M.B.; Khan, S.A.; Alaqel, S.I.; Alshammari, M.K.; Alshehri, M.M.; Alshrari, A.S.; Mateq Ali, A.; Al-Shammeri, A.M.; et al. Discovery, Development, and Patent Trends on Molnupiravir: A Prospective Oral Treatment for COVID-19. Molecules 2021, 26, 5795. [Google Scholar] [CrossRef]
- Imran, M.; Alshrari, A.S.; Tauseef, M.; Khan, S.A.; Hudu Abida, S.A. Mucormycosis Medications: A Patent Review. Expert Opin. Ther. Pat. 2021, 31, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Alshrari, A.S.; Asdaq, S.M.B. Abida Trends in the Development of Remdesivir Based Inventions against COVID-19 and Other Disorders: A Patent Review. J. Infect. Public Health 2021, 14, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Zale, S.E.; Troiano, G.; Ali, M.M.; Hrkach, J.; Wright, J. Therapeutic Polymeric Nanoparticle Compositions with High Glass Transition Temperature or High Molecular Weight Copolymers. United State Patent Number US8912212B2, 16 December 2014. [Google Scholar]
- Zale, S.E.; Ali, M.M. Cancer Cell Targeting Using Nanoparticles. United State Patent Number US8236330B2, 7 August 2012. [Google Scholar]
- Zale, S.E.; Ali, M.M. Cancer Cell Targeting Using Nanoparticles. United State Patent Number US8603500B2, 10 December 2013. [Google Scholar]
- Desai, N.P.; Soon-Shiong, P. Methods of Treating Cancer. United State Patent Number US9393318B2, 19 July 2016. [Google Scholar]
- Adiwijaya, B.; Fitzgerald, J.B.; Lee, H. Nanoliposomal Irinotecan for Use in Treating Small Cell Lung Cancer. United State Patent Number US11318131B2, 3 May 2022. [Google Scholar]
- Blanchette, S.F.; Drummond, D.C.; Fitzgerald, J.B.; Moyo, V. Combination Therapy for Cancer Treatment. United State Patent Number US9895365B2, 20 February 2018. [Google Scholar]
- Zuo, J.; Liu, Z.; Xiao, X. A New Der p1 Nano Vaccine for the Treatment of Lung Cancer and Its Preparation Method and Application. Chinese Patent Number CN104524565B, 10 April 2018. [Google Scholar]
- Yan, H.; Du, Z.; Zheng, X.; Ma, S.; Emori, T.G.; Kun, Z.; Iwao, F.; Zhao, S.; Wang, H.; Lu, Y.; et al. Silver Nanoparticle Composition for Control of Lung Cancer and Prostate Cancer. Chinese Patent Application Publicatio Number CN105640986A, 8 June 2016. [Google Scholar]
- Yin, L.; Chen, W.; Wei, H. A Kind of Hesperidin Nanoparticle and Preparation Method Thereof. Chinese Patent Application Publication Number CN114557979A, 31 May 2022. [Google Scholar]
- Lu, K.; He, L.; Zhang, M. Anti-Lung Cancer Active Targeting Liposome and Its Preparation Method and Application. Chinese Patent Application Publication Number CN105726483A, 6 July 2016. [Google Scholar]
- Zhang, Y.; Zhang, X. Triptolide Liposome Preparation for Treatment of Small Cell Lung Cancer and Preparation Method Thereof. Chinese Patent Application Publication Number CN103393598A, 20 November 2013. [Google Scholar]




| Nanocarrier | Drug | Targeting Ligand | Result | Ref. |
|---|---|---|---|---|
| Polymeric nanoparticles | Paclitaxel; transcription-3 (Stat-3) siRNA | — | A combinatorial formulation inhibited Stat-3 expression and increased cellular death by accumulating paclitaxel in A549 cells. | [39] |
| Polymeric nanoparticles | Topotecan | — | The drug was stabilized to stay in its lactone form and displayed a release pattern for 15 days due to the maintenance of a low pH inside the nanoparticles. Additionally, in vivo antitumor effects and in vitro cytotoxicity testing (using the LLC cell line) showed considerable potential for greater proliferation inhibition as contrasted with the pure drug TPT. | [41] |
| Micelle | Salinomycin | — | Inhibited EMT in lung cancer, resulting in a decrease in the ability of A459 lung cancer cells to migrate without impairing cell growth. | [44] |
| Silver nanoparticles | Gallic acid | — | A459 lung cancer cells’ ability to spread through EMT was decreased. | [45] |
| Polymeric nanoparticles | Paclitaxel | RGD peptide | In vivo experiments showcased that GRGDSP inhibited tumor growth and minimized detrimental implications. | [49] |
| Carbon nanotube | Digitoxin | Folate | The cytotoxicity assay revealed substantial intracellular levels and enhanced cellular internalization of the nanocarrier. By incubation in A549 cells, the nano-formulation reached 89 times the therapeutic efficacy in IC50 measures when compared with the commercial product DOCELTM. | [50] |
| Drug | Nanocarrier System | Targeting Ligand | Tumor Model | Therapeutic Effectiveness | Ref. |
|---|---|---|---|---|---|
| Gefitinib and vorinostat | Polymeric nanoparticles | Hyaluronic acid | Female NMRI nude mice | Targeted delivery to subcutaneous CD44-overexpressing tumors. Co-delivery of the drugs employing the nanoparticles decreased the cytotoxic effects of the native drugs. Compared to free drugs, intrapulmonary delivery of dual drug-loaded nanoparticles exhibited a better orthotopic lung tumor growth reduction. | [79] |
| Doxorubicin and cisplatin combined with siRNA | Mesoporous silica nanoparticles | LHRH peptide | NCR nude mice | By limiting their buildup in other non-target organs and preventing their egress into the systemic circulation, the local biodistribution of the formulation by inhalation contributed to the predominant deposition of NPs at the target site. | [80] |
| Paclitaxel + Cisplatin | Micelle | — | When exposed to an acidic reducing environment (pH 5.5 + dithiothreitol), about 100% of both medications were generated within 192 h. Compared to the free drugs, the dual drug-loaded micelles caused 1.77 times more cellular killing when evaluated in NCI-H520 LC cells. | [81] | |
| NU7441 - a potent radiosensitizer and gemcitabine | Polymeric nanoparticles | — | H460 tumor-bearing mice | Biphasic release of NU7441, as well as pH-dependent gemcitabine release, was noted. Superior hemocompatibility, along with remarkable dose-dependent caveolae-mediated in vitro internalization, was observed in lung cancer cells. | [82] |
| Paclitaxel and cisplatin | Lipid-polymer nanoparticles | RGD peptide | lung tumor xenografts (A549 cells injected into female BALB/c mice). | IC50 values of 26.7 and 75.3 g/mL for dual drug-loaded nanoformulation and free drugs, respectively, indicated much stronger anticancer activity. Regression of tumor size from 1486 mm3 to 263 mm3. | [83] |
| Docetaxel prodrug (DTXp) and cisplatin (DDP) | Lipid–polymer hybrid nanoparticles | Aptamer | lung tumor xenografts (A549 cells injected into female BALB/c mice). | Comparing APT-DTXp/DDP-LPHNs to non-aptamer-functionalized LPHNs and single drug-entrapped LPHNs, these LPHNs demonstrated significantly improved cytotoxicity with synergistic antitumor impact with a combination index of 0.62 and substantial tumor-inhibition capability. | [84] |
| Paclitaxel (PTX) and triptolide (TL) | Lipid–polymer hybrid nanoparticles (LPNs) | — | lung tumor xenografts (A549 cells injected into female BALB/c mice). | When the PTX: TL weight ratio was 5:3, the combinatorial therapy showcased synergistic benefits. The experimental group’s in vivo tumor development curve was less pronounced than that of the control group, and the tumor volumes in the P/T-LPNs and control groups were observed to be 392 and 1737 mm3, respectively. | [85] |
| Doxorubicin and cis-platinum | Polymeric nanoparticles | — | B16F10 tumor-bearing mouse models | The co-delivery system showed more cytotoxic potential than patients treated with either of the neat drugs alone, according to in vitro cytotoxicity studies performed on the B16F10 cell line. Local delivery of combinatorial drug therapy by pulmonary injection in B16F10 tumor-bearing mouse models showed that dual drug-loaded NPs had highly effective deposition in the lungs but infrequently in normal lung tissues. | [86] |
| Epirubicin and paclitaxel | Polymeric nanoparticles | — | — | Compared to the native drugs, the MTT assessment revealed that PLGA-PEI-EPI-PTX NPs had a substantial amount of antitumor effects. Additionally, a Western blot analysis of the expression of the p53 protein revealed increased expression. | [87] |
| Intervention | Nanocarrier | Trial Phase | Identifier No. | Primary Endpoint | Status |
|---|---|---|---|---|---|
| BIND-014 | Prostate-specific membrane antigen targeted nanoparticles | II | NCT01792479 | Number of patients with either a complete or partial response | Completed |
| Drug: HLX10 Drug: carboplatin and nab-paclitaxel Drug: Placebo | Nanoparticle albumin bound (Nab) Paclitaxel | III | NCT04033354 | Tumor assessment Progression-free survival (PFS) | Active, not recruiting |
| Drug: carboplatin Drug: erlotinib hydrochloride Drug: paclitaxel albumin-stabilized nanoparticle formulation Radiation: radiation therapy | Paclitaxel albumin-stabilized nanoparticle | II | NCT00553462 | Determination of therapeutic activity | Completed |
| Drug: CRLX101 Other: Best Supportive Care | Camptothecin-conjugated polymeric nanoparticle | II | NCT01380769 | To compare the overall survival of patients treated with CRLX101 + BSC to those patients treated with BSC only Comparison of survival among patients treated with CRLX101 + best supportive care vs. patients treated with best supportive care only | Completed |
| Drug: Paclitaxel (Genexol®) Drug: Paclitaxel loaded polymeric micelle (Genexol-PM®)C | Micelle | II | NCT01023347 | The response rate to therapy | Completed |
| Drug: LY01610 (Irinotecan hydrochloride liposome injection) | Liposome | II | NCT04381910 | Objective response rate (ORR) Duration of response (DoR) | Recruiting |
| Drug: Irinotecan liposome injection Drug: Topotecan | Liposome | II/III | NCT03088813 | Overall survival (OS) | Active; not yet recruiting |
| Drug: paclitaxel albumin-stabilized nanoparticle formulation | Albumin stabilized nanoparticles | I/II | NCT00077246 | Maximum-tolerated dose (MTD) and dose-limiting toxicity (DLT) of ABI-007 Objective target lesion response | Completed |
| Biological: quaratusugene ozeplasmid Drug: osimertinib | Lipid nanoparticles | I/II | NCT04486833 | Recommended phase 2 dose (RP2D) | Recruiting |
| Patent/Application Number (Applicant) | Type of Nanocarriers | Drugs | Targets | Ref. |
| US8912212B2 (Bind Therapeutics) | Polymeric nanoparticle | Docetaxel | Non-Small-Cell Lung Cancer | [110] |
| US8236330B2 (Bind Biosciences) | Polymeric nanoparticle | Docetaxel | Non-Small-Cell Lung Cancer | [111] |
| US8603500B2 (Bind Therapeutics) | Polymeric nanoparticle | Paclitaxel/Docetaxel/Doxorubicin/gemcitabine/5-Fluorouracil/Daunorubicin/9-Dihydrotaxol | Non-Small-Cell Lung Cancer | [112] |
| US9393318B2 (Abraxis Bioscience) | Paclitaxel albumin-bound nanoparticles | Paclitaxel/Carboplatin | Non-Small-Cell Lung Cancer | [113] |
| US11318131B2 (Ipsen Biopharm) | Irinotecan-Loaded Liposome | Irinotecan | Non-Small-Cell Lung Cancer | [114] |
| US9895365B2 (Ipsen Biopharm) | Parenteral Liposomal Irinotecan | Irinotecan with other chemotherapeutics, e.g., Poly(ADP-ribose) Polymerase (PARP) inhibitor (niraparib, olaparib, veliparib, rucaparib, and talazoparib | Small-Cell Lung Cancer and Non-Small-Cell Lung Cancer | [115] |
| CN104524565B (Nanhua University) | PLGA-based polymeric nanoparticles | Dermatophagoides pteronyssinus-1 (Der p1) albumen | Epithelial Barrier of Lung | [116] |
| CN105640986A (Guangdong University of Technology) | Silver nanoparticles | Nano-silver and pharmaceutically acceptable Injection excipients (a suspending aid, a dispersing agent, a surfactant, an analgesic, a pH adjustment buffer agent, an osmotic pressure regulator, and water). | H1299 cells | [117] |
| CN114557979A (Medium-Mountain University) | Hesperidin-Lecithin Liposome | Hesperidin | A549 lung cancer cells | [118] |
| CN105726483A (Lu Kaihua) | Sunitinib and Smallanthus sonchifolius diterpene acid-Loaded Liposome | Sunitinib and Smallanthus sonchifolius diterpene acid | Pleural Mesothelioma | [119] |
| CN103393598A (Nanjing University of Chinese Medicine) | Triptolide liposome | Triptolide | Non-Small-Cell Lung Cancer | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, M.K.; Almomen, E.Y.; Alshahrani, K.F.; Altwalah, S.F.; Kamal, M.; Al-Twallah, M.F.; Alsanad, S.H.; Al-Batti, M.H.; Al-Rasheed, F.J.; Alsalamah, A.Y.; et al. Nano-Enabled Strategies for the Treatment of Lung Cancer: Potential Bottlenecks and Future Perspectives. Biomedicines 2023, 11, 473. https://doi.org/10.3390/biomedicines11020473
Alshammari MK, Almomen EY, Alshahrani KF, Altwalah SF, Kamal M, Al-Twallah MF, Alsanad SH, Al-Batti MH, Al-Rasheed FJ, Alsalamah AY, et al. Nano-Enabled Strategies for the Treatment of Lung Cancer: Potential Bottlenecks and Future Perspectives. Biomedicines. 2023; 11(2):473. https://doi.org/10.3390/biomedicines11020473
Chicago/Turabian StyleAlshammari, Mohammed Kanan, Eman Yaser Almomen, Kholoud Falah Alshahrani, Shroog Farhan Altwalah, Mehnaz Kamal, May Faiz Al-Twallah, Suheir Hassan Alsanad, Mariam Hassan Al-Batti, Faisal Jarallah Al-Rasheed, Abdulaziz Yousef Alsalamah, and et al. 2023. "Nano-Enabled Strategies for the Treatment of Lung Cancer: Potential Bottlenecks and Future Perspectives" Biomedicines 11, no. 2: 473. https://doi.org/10.3390/biomedicines11020473
APA StyleAlshammari, M. K., Almomen, E. Y., Alshahrani, K. F., Altwalah, S. F., Kamal, M., Al-Twallah, M. F., Alsanad, S. H., Al-Batti, M. H., Al-Rasheed, F. J., Alsalamah, A. Y., Alhazza, M. B., Alasmari, F. A., Abida, & Imran, M. (2023). Nano-Enabled Strategies for the Treatment of Lung Cancer: Potential Bottlenecks and Future Perspectives. Biomedicines, 11(2), 473. https://doi.org/10.3390/biomedicines11020473