Anaemia and Congestion in Heart Failure: Correlations and Prognostic Role
Abstract
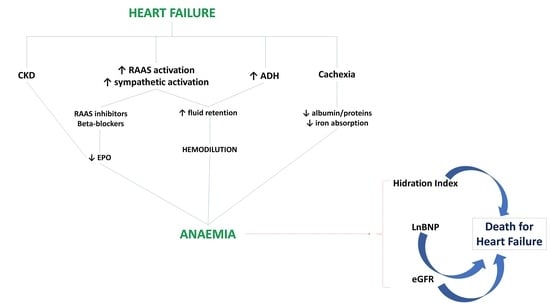
:1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Creatinine-Based Estimated Glomerular Filtration Rate (eGFR)
2.3. Hydration Status Assessed by Bioimpedance Vector Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anand, I.S.; Gupta, P. Anaemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.D.; Katz, S.D. The prevalence of anaemia in chronic heart failure and its impact on the clinical outcomes. Heart Fail. Rev. 2008, 13, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Groenveld, H.F.; Januzzi, J.L.; Damman, K.; van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J.; van der Meer, P. Anaemia and mortality in heart failure patients a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2008, 52, 818–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Shen, H.; Cha, W.; Lu, Q. The Prognostic Significance of Anaemia in Patients with Heart Failure: A Meta-Analysis of Studies from the Last Decade. Front. Cardiovasc. Med. 2021, 8, 632318. [Google Scholar] [CrossRef]
- Abebe, T.B.; Gebreyohannes, E.A.; Bhagavathula, A.S.; Tefera, Y.G.; Abegaz, T.M. Anaemia in severe heart failure patients: Does it predict prognosis? BMC Cardiovasc. Disord. 2017, 17, 248. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Jiang, C.; Lai, Y.; Li, L.; Zhao, X.; Wang, X.; Li, L.; Du, X.; Ma, C.; Dong, J. Association of On-Admission Anaemia with 1-Year Mortality in Patients Hospitalized with Acute Heart Failure: Results from the HERO Study. Front. Cardiovasc. Med. 2022, 9, 856246. [Google Scholar] [CrossRef]
- Van Veldhuisen, D.J.; Anker, S.D.; Ponikowski, P.; Macdougall, I.C. Anaemia and iron deficiency in heart failure: Mechanisms and therapeutic approaches. Nat. Rev. Cardiol. 2011, 8, 485–493. [Google Scholar] [CrossRef]
- Gheorghiade, M.; Follath, F.; Ponikowski, P.; Barsuk, J.H.; Blair, J.E.; Cleland, J.G.; Dickstein, K.; Drazner, M.H.; Fonarow, G.C.; Jaarsma, T.; et al. Assessing and grading congestion in acute heart failure: A scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur. J. Heart Fail. 2010, 12, 423–433. [Google Scholar] [CrossRef]
- Núñez, J.; de la Espriella, R.; Rossignol, P.; Voors, A.A.; Mullens, W.; Metra, M.; Chioncel, O.; Januzzi, J.L.; Mueller, C.; Richards, A.M.; et al. Congestion in heart failure: A circulating biomarker-based perspective. A review from the Biomarkers Working Group of the Heart Failure Association, European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1751–1766. [Google Scholar] [CrossRef]
- Guyton, A.C. Human Physiology and Mechanisms of Disease; W.B. Aunders: Philadelphia, PA, USA, 1982; Volume 240. [Google Scholar]
- Cotter, G.; Metra, M.; Milo-Cotter, O.; Dittrich, H.C.; Gheorghiade, M. Fluid overload in acute heart failure—Re-distribution and other mechanisms beyond fluid accumulation. Eur. J. Heart Fail. 2008, 10, 165–169. [Google Scholar] [CrossRef]
- Massari, F.; Iacoviello, M.; Scicchitano, P.; Mastropasqua, F.; Guida, P.; Riccioni, G.; Speziale, G.; Caldarola, P.; Ciccone, M.M.; Di Somma, S. Accuracy of bioimpedance vector analysis and brain natriuretic peptide in detection of peripheral edema in acute and chronic heart failure. Heart Lung. 2016, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A. Bioelectric impedance measurement for fluid status assessment. Contrib. Nephrol. 2010, 164, 143–152. [Google Scholar]
- Massari, F.; Scicchitano, P.; Iacoviello, M.; Valle, R.; Sanasi, M.; Piscopo, A.; Guida, P.; Mastropasqua, F.; Caldarola, P.; Ciccone, M.M. Serum biochemical determinants of peripheral congestion assessed by bioimpedance vector analysis in acute heart failure. Heart Lung. 2019, 48, 395–399. [Google Scholar] [CrossRef]
- Abramov, D.; Cohen, R.S.; Katz, S.D.; Mancini, D.; Maurer, M.S. Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am. J. Cardiol. 2008, 102, 1069–1072. [Google Scholar] [CrossRef] [Green Version]
- Desai, A.S.; Bibbins-Domingo, K.; Shlipak, M.G.; Wu, A.H.; Ali, S.; Whooley, M.A. Association between anaemia and N-terminal pro-B-type natriuretic peptide (NT-proBNP): Findings from the Heart and Soul Study. Eur. J. Heart Fail. 2007, 9, 886–891. [Google Scholar] [CrossRef] [Green Version]
- Anand, I.S.; Chandrashekhar, Y.; Ferrari, R.; Poole-Wilson, P.A.; Harris, P.C. Pathogenesis of oedema in chronic severe anaemia: Studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br. Heart J. 1993, 70, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.H.; Omland, T.; Wold Knudsen, C.; McCord, J.; Nowak, R.M.; Hollander, J.E.; Duc, P.; Storrow, A.B.; Abraham, W.T.; Clopton, P.; et al. Relationship of B-type natriuretic peptide and anaemia in patients with and without heart failure: A substudy from the Breathing Not Properly (BNP) Multinational Study. Am. J. Hematol. 2005, 80, 174–180. [Google Scholar] [CrossRef]
- Hogenhuis, J.; Voors, A.A.; Jaarsma, T.; Hoes, A.W.; Hillege, H.L.; Kragten, J.A.; van Veldhuisen, D.J. Anaemia and renal dysfunction are independently associated with BNP and NT-proBNP levels in patients with heart failure. Eur. J. Heart Fail. 2007, 9, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Sîrbu, O.; Floria, M.; Dascalita, P.; Stoica, A.; Adascalitei, P.; Sorodoc, V.; Sorodoc, L. Anemia in heart failure—From guidelines to controversies and challenges. Anatol. J. Cardiol. 2018, 20, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, K.; Young, J.B.; Anand, I.S.; Cheng, S.; Desai, A.S.; Diaz, R.; Maggioni, A.P.; McMurray, J.J.; O’Connor, C.; Pfeffer, M.A.; et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N. Engl. J. Med. 2013, 368, 1210–1219. [Google Scholar] [CrossRef] [Green Version]
- Westenbrink, B.D.; Visser, F.W.; Voors, A.A.; Smilde, T.D.; Lipsic, E.; Navis, G.; Hillege, H.L.; van Gilst, W.H.; van Veldhuisen, D.J. Anaemia in chronic heart failure is not only related to impaired renal perfusion and blunted erythropoietin production, but to fluid retention as well. Eur. Heart J. 2007, 28, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adlbrecht, C.; Kommata, S.; Hulsmann, M.; Szekeres, T.; Bieglmayer, C.; Strunk, G.; Karanikas, G.; Berger, R.; Mortl, D.; Kletter, K.; et al. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body’s red cell volume. Eur. Heart J. 2008, 29, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Gault, M.H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Damman, K.; Testani, J.M.; Martens, P.; Mueller, C.; Lassus, J.; Tang, W.H.W.; Skouri, H.; Verbrugge, F.H.; Orso, F.; et al. Evaluation of kidney function throughout the heart failure trajectory—A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 584–603. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.; Viklund, I.; Jonsson, A.; Valham, F.; Bergdahl, E.; Lindmark, K.; Norberg, H. Comparison of creatinine-based methods for estimating glomerular filtration rate in patients with heart failure. ESC Heart Fail. 2020, 7, 1150–1160. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Q.; Li, J.; Wang, X.; Ju, J.; Xu, H.; Shi, D. Estimated Glomerular Filtration Rate Is Associated with an Increased Risk of Death in Heart Failure Patients with Preserved Ejection Fraction. Front. Cardiovasc. Med. 2021, 8, 643358. [Google Scholar] [CrossRef]
- Szummer, K.; Evans, M.; Carrero, J.J.; Alehagen, U.; Dahlström, U.; Benson, L.; Lund, L.H. Comparison of the Chronic Kidney Disease Epidemiology Collaboration, the Modification of Diet in Renal Disease study and the Cockcroft-Gault equation in patients with heart failure. Open Heart 2017, 4, e000568. [Google Scholar] [CrossRef]
- Massari, F.; Mastropasqua, F.; Guida, P.; De Tommasi, E.; Rizzon, B.; Pontraldolfo, G.; Pitzalis, M.V.; Rizzon, P. Whole-body bioelectrical impedance analysis in patients with chronic heart failure: Reproducibility of the method and effects of body side. Ital. Heart J 2001, 2, 594–598. [Google Scholar]
- Sharma, R.; Francis, D.P.; Pitt, B.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Haemoglobin predicts survival in patients with chronic heart failure: A substudy of the ELITE II trial. Eur. Heart J. 2004, 25, 1021–1028. [Google Scholar] [CrossRef] [Green Version]
- Kajimoto, K.; Sato, N.; Takano, T.; Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) registry. Association between anaemia, clinical features and outcome in patients hospitalized for acute heart failure syndromes. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 568–576. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; McAlister, F.A.; Armstrong, P.W. Anaemia is common in heart failure and is associated with poor outcomes: Insights from a cohort of 12 065 patients with new-onset heart failure. Circulation 2003, 107, 223–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggioni, A.P.; Opasich, C.; Anand, I.; Barlera, S.; Carbonieri, E.; Gonzini, L.; Tavazzi, L.; Latini, R.; Cohn, J. Anaemia in patients with heart failure: Prevalence and prognostic role in a controlled trial and in clinical practice. J. Card. Fail. 2005, 11, 91–98. [Google Scholar] [CrossRef]
- Pintér, A.; Behon, A.; Veres, B.; Merkel, E.D.; Schwertner, W.R.; Kuthi, L.K.; Masszi, R.; Lakatos, B.K.; Kovács, A.; Becker, D.; et al. The Prognostic Value of Anaemia in Patients with Preserved, Mildly Reduced and Recovered Ejection Fraction. Diagnostics 2022, 12, 517. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.W.; Ashok, T.; Patni, N.; Fatima, M.; Lamis, A.; Anne, K.K. Anaemia and Heart Failure: A Narrative Review. Cureus 2022, 14, e27167. [Google Scholar]
- Tanner, H.; Moschovitis, G.; Kuster, G.M.; Hullin, R.; Pfiiffner, D.; Hess, O.M.; Mohacsi, P. The prevalence of anemia in chronic heart failure. Int. J. Cardiol. 2002, 86, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.C.; Dev, M.; Paul, P.; Pal Singh, D.; Rathoure, S.; Kumar, R.; Dhiman, A.; Kandoria, A.; Ganju, N.; Sharma, R.; et al. Prevalence, risk factors, and significance of iron deficiency and anemia in nonischemic heart failure patients with reduced ejection fraction from a Himachal Pradesh heart failure registry. Indian Heart J. 2018, 70, S182–S188. [Google Scholar] [CrossRef]
- Efstratiadis, G.; Konstantinou, D.; Chytas, I.; Vergoulas, G. Cardio-renal anemia syndrome. Hippokratia 2008, 12, 11–16. [Google Scholar]
- Krzesiński, P.; Galas, A.; Gielerak, G.; Uziębło-Życzkowska, B. Haemodynamic Effects of Anaemia in Patients with Acute Decompensated Heart Failure. Cardiol. Res. Pract. 2020, 2020, 9371967. [Google Scholar] [CrossRef] [PubMed]
- González-Islas, D.; Arámbula-Garza, E.; Orea-Tejeda, A.; Castillo-Martínez, L.; Keirns-Davies, C.; Salgado-Fernández, F.; Hernández-Urquieta, L.; Hernández-López, S.; Pilotzi-Montiel, Y. Body composition changes assessment by bioelectrical impedance vectorial analysis in right heart failure and left heart failure. Heart Lung 2020, 49, 42–47. [Google Scholar] [CrossRef]
- Santarelli, S.; Russo, V.; Lalle, I.; De Berardinis, B.; Navarin, S.; Magrini, L.; Piccoli, A.; Codognotto, M.; Castello, L.M.; Avanzi, G.C.; et al. Usefulness of combining admission brain natriuretic peptide (BNP) plus hospital discharge bioelectrical impedance vector analysis (BIVA) in predicting 90 days cardiovascular mortality in patients with acute heart failure. Intern. Emerg. Med. 2017, 12, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Scicchitano, P.; Massari, F. The role of bioelectrical phase angle in patients with heart failure. Rev. Endocr. Metab. Disord. 2022, 1–13, epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.; Ludman, A.J.; Dworzynski, K.; Al-Mohammad, A.; Cowie, M.R.; McMurray, J.J.; Mant, J.; NICE Guideline Development Group for Acute Heart Failure. The diagnostic accuracy of the natriuretic peptides in heart failure: Systematic review and diagnostic meta-analysis in the acute care setting. BMJ 2015, 350, h910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, W.; Yang, D.; Yu, P.; Yu, H. Comparison of predictive value of NT-proBNP, sST2 and MMPs in heart failure patients with different ejection fractions. BMC Cardiovasc. Disord. 2020, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Abassi, Z.; Khoury, E.E.; Karram, T.; Aronson, D. Edema formation in congestive heart failure and the underlying mechanisms. Front. Cardiovasc. Med. 2022, 9, 933215. [Google Scholar] [CrossRef] [PubMed]
- Harrison-Bernard, L.M. The renal renin-angiotensin system. Adv. Physiol. Educ. 2009, 33, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Jonaitienė, N.; Ramantauskaitė, G.; Laukaitienė, J. Anaemia in Heart Failure Patients, Associated with Angiotensin–Renin–Aldosterone System Altering Medications. Heart Views 2021, 22, 196–200. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tsujino, T.; Naito, Y.; Lee-Kawabata, M.; Ezumi, A.; Yamamoto, K.; Mano, T.; Masuyama, T. Anemia as a factor that elevates plasma brain natriuretic peptide concentration in apparently healthy subjects. Int. Heart J. 2008, 49, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Lelli, D.; Antonelli Incalzi, R.; Pedone, C. Hemoglobin Concentration Influences N-Terminal Pro B-Type Natriuretic Peptide Levels in Hospitalized Older Adults with and without Heart Failure. J. Am. Geriatr. Soc. 2017, 65, 2369–2373. [Google Scholar] [CrossRef]
- Ueno, H.; Nakayama, M.; Kojima, S.; Kusuhara, K.; Nagayoshi, Y.; Yamamuro, M.; Nishijima, T.; Usuku, H.; Kaikita, K.; Sumida, H.; et al. The synergistic combined effect of anemia with high plasma levels of B-type natriuretic peptide significantly predicts an enhanced risk for major adverse cardiac events. Heart Vessels 2008, 23, 243–248. [Google Scholar] [CrossRef]
- Díez, J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: Implications for therapy. Eur. J. Heart Fail. 2017, 19, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majmundar, M.; Doshi, R.; Zala, H.; Shah, P.; Adalja, D.; Shariff, M.; Kumar, A. Prognostic role of anaemia in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Indian Heart J. 2021, 73, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Kato, T.; Morimoto, T.; Yaku, H.; Inuzuka, Y.; Tamaki, Y.; Ozasa, N.; Shiba, M.; Yamamoto, E.; Yoshikawa, Y.; et al. Improved and new-onset anaemia during follow-up in patients with acute decompensated heart failure: Characteristics and outcomes. Medicine 2021, 100, e26892. [Google Scholar] [CrossRef] [PubMed]



| Clinical Characteristics | Overall (n = 434) |
|---|---|
| Age, yrs | 75 ± 11 |
| Male/Female, % | 52/48 |
| BMI, kg/mq | 28 ± 5 |
| NYHA class II/III/IV, % | 43/30/27 |
| Peripheral edema, % | 30 |
| Medical history, % | |
| Coronary artery disease | 31 |
| Diabetes | 24 |
| Atrial fibrillation | 43 |
| CKD | 33 |
| COPD | 21 |
| AHF | 42 |
| ICD | 11 |
| Instrumental Evaluations | |
| LVEF | 42 ± 12% |
| Preserved LVEF, % | 48 |
| Mid-range LVEF, % | 10 |
| Reduced LVEF, % | 42 |
| BIVA, hydration index, % | 76 ± 5 |
| Laboratory values | |
| BNP, pg/mL, median (CI) | 516 (423–582) |
| Hemoglobin, g/dL | 13 ± 2 |
| Anaemia, % | 45 |
| BUN, mg/dL | 30 ± 17 |
| Uric acid, mg/dL | 6.2 ± 2 |
| Creatinine, mg/dL | 1.4 ± 0.8 |
| Creatinine > 1.5 mg/dL, % | 20 |
| eCrCl, mL/min per 1.73 m2 | 57 ± 29 |
| eCrCl, <60 mL/min per 1.73 m2, % | 59 |
| eCrCl, <30 mL/min per 1.73 m2,% | 18 |
| Sodium, mmol/L | 139 ± 4 |
| Potassium, mmol/L | 4.0 ± 0.6 |
| Therapies, % | |
| Furosemide | 70 |
| Beta-blockers | 50 |
| ACE inhibitors | 39 |
| ARBs | 21 |
| MRAs | 69 |
| Digitalis | 21 |
| Ivabradine | 5 |
| IV inotropes | 5 |
| Variables | Odds Ratio (95% CI) | p | B Coefficient | SE | Wald |
|---|---|---|---|---|---|
| AHF vs. CHF | 1.25 (0.74–2.11) | 0.4 | |||
| NYHA class | 0.83 (0.62–1.10) | 0.2 | |||
| Hydration index, % | 1.09 (1.03–1.15) | =0.0008 | 0.09 | 0.03 | 11.2 |
| LnBNP, pg/mL | 1.43 (1.14–1.79) | =0.002 | 0.36 | 0.11 | 9.7 |
| eGFR, mL/min | 1.25 (1.01–1.44) | =0.001 | 0.22 | 0.071 | 10.3 |
| Univariate Cox Regression Analysis | Adjusted Cox Regression Analysis | ||||
|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | Wald | |
| AHF vs. CHF | 2.70 (1.77–4.14) | <0.0001 | |||
| Age, year | 1.07 (1.05–1.10) | <0.0001 | |||
| NYHA class | 1.80 (1.40–2.30) | <0.0001 | |||
| LVEF, % | 0.99 (0.97–1.01) | =0.2 | |||
| Anaemia, yes vs. no | 2.55 (1.66–3.91) | <0.0001 | |||
| Hemoglobin, g/dL | 0.78 (0.70–0.85) | <0.0001 | |||
| Hydration Index, % | 1.11 (1.07–1.15) | <0.0001 | 1.05 (1.005–1.08) | =0.03 | 4.8 |
| BNP, pg/mL | 1.0004 (1.0003–1.0005) | <0.0001 | 1.0002 (1.0001–1.0004) | =0.1 | 6.4 |
| eGFR, mL/min | 0.96 (0.95–0.97) | <0.0001 | 0.97 (0.96–0.99) | =0.0006 | 11.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scicchitano, P.; Iacoviello, M.; Massari, A.; De Palo, M.; Potenza, A.; Landriscina, R.; Abruzzese, S.; Tangorra, M.; Guida, P.; Ciccone, M.M.; et al. Anaemia and Congestion in Heart Failure: Correlations and Prognostic Role. Biomedicines 2023, 11, 972. https://doi.org/10.3390/biomedicines11030972
Scicchitano P, Iacoviello M, Massari A, De Palo M, Potenza A, Landriscina R, Abruzzese S, Tangorra M, Guida P, Ciccone MM, et al. Anaemia and Congestion in Heart Failure: Correlations and Prognostic Role. Biomedicines. 2023; 11(3):972. https://doi.org/10.3390/biomedicines11030972
Chicago/Turabian StyleScicchitano, Pietro, Massimo Iacoviello, Antonio Massari, Micaela De Palo, Angela Potenza, Raffaella Landriscina, Silvia Abruzzese, Maria Tangorra, Piero Guida, Marco Matteo Ciccone, and et al. 2023. "Anaemia and Congestion in Heart Failure: Correlations and Prognostic Role" Biomedicines 11, no. 3: 972. https://doi.org/10.3390/biomedicines11030972
APA StyleScicchitano, P., Iacoviello, M., Massari, A., De Palo, M., Potenza, A., Landriscina, R., Abruzzese, S., Tangorra, M., Guida, P., Ciccone, M. M., Caldarola, P., & Massari, F. (2023). Anaemia and Congestion in Heart Failure: Correlations and Prognostic Role. Biomedicines, 11(3), 972. https://doi.org/10.3390/biomedicines11030972