Hesperidin Attenuates Hypothyroidism-Induced Lung Damage in Adult Albino Rats by Modulating Oxidative Stress, Nuclear Factor Kappa-B Pathway, Proliferating Cell Nuclear Antigen and Inflammatory Cytokines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Kits
2.2. Animals
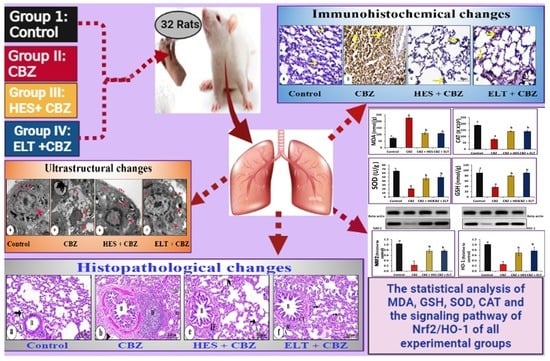
2.3. Experimental Protocol
| Groups | Experimental Design |
| I (normal control) | Given 0.5 mL distilled water orally daily for 90 days |
| II (CBZ) | Given 0.5 mL CBZ (20 mg/kg/day) orally for 90 days |
| III ((HES + CBZ) | Given 0.5 mL of CBZ (20 mg/kg/day) dissolved in distilled water followed by HES (200 mg/kg/day) dissolved in 1% CMC for 90 days |
| IV (ELT + CBZ) | Given 0.5 mL CBZ (20 mg/kg/day) dissolved in distilled water followed by ELT (0.045 mg/kg/day) dissolved in distilled water orally for 90 days |
2.4. Blood Sample Collection and Biochemical Estimations
2.5. Analysis of Lung Homogenates
2.6. Western Blot Assays
2.7. Histopathological and Immunohistochemical Analysis
2.8. Evaluation of Histomorphometry
2.9. Ultrastructural Evaluation
2.10. Statistical Analysis
3. Results
3.1. Biochemical Results
3.2. Histological Changes and Morphometric Findings
3.3. Immunohistochemical Results
3.4. Morphometric Findings of iNOS, NF-κB, and Ki-67 Immunoreactivity
3.5. Ultrastructural Findings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassi, R.; Dhillon, S.; Sharma, S.; Sharma, A.; Tapdiya, M. Effect of thyroid hormone replacement on respiratory function tests in hypothyroid women. Pak. J. Physiol. 2012, 8, 20–23. [Google Scholar]
- Delitala, A.P.; Capobianco, G.; Cherchi, P.L.; Dessole, S.; Delitala, G. Thyroid function and thyroid disorders during pregnancy: A review and care pathway. Arch. Gynecol. Obstet. 2019, 299, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Panait, N.; Michel, F.; D’Ercole, C.; Merrot, T. Esophageal atresia, small omphalocele and ileal prolapse through a patent omphalomesenteric duct: A methamizole embryopathy? J. Pediatr. Surg. 2013, 48, e9–e11. [Google Scholar] [CrossRef] [PubMed]
- Saeed, I.H.; Abdelatif, A.M.; Elnageeb, M.E. Effects of Dose Level of Anti-thyroid Drug Carbimazole on Thermoregulation and Blood Constituents in Male Rabbits (Oryctolagus cuniculus). Adv. Res. 2014, 2, 129–144. [Google Scholar] [CrossRef]
- Sadek, S.H.; Khalifa, W.A.; Azoz, A.M. Pulmonary consequences of hypothyroidism. Ann. Thorac. Med. 2017, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, F.; Kalous, M.; Adorisio, E.; Turturro, N.; Drahota, Z.; Cantatore, P.; Santoro, G. Hypothyroidism leads to a decreased expression of mitochondrial F0F1-ATP synthase in rat liver. J. Bioenerg. Biomembr. 1998, 30, 269. [Google Scholar] [CrossRef] [PubMed]
- Roel, S.; Punyabati, O.; Prasad, L.; Salam, R.; Ningshen, K.; Shimray, A.J.; Sangma, M.A.; Hungyo, H.; Devi, A.N. Assessment of functional lung impairment in hypothyroidism. IOSR J. Dent. Med. Sci. 2014, 13, 04–07. [Google Scholar] [CrossRef]
- McQuade, C.; Skugor, M.; Brennan, D.M.; Hoar, B.; Stevenson, C.; Hoogwerf, B.J. Hypothyroidism and moderate subclinical hypothyroidism are associated with increased all-cause mortality independent of coronary heart disease risk factors: A PreCIS database study. Thyroid 2011, 21, 837–843. [Google Scholar] [CrossRef]
- Singh, H. Association of Visceral Fat with Pulmonary Function in Hypothyroidism Patients. Sch. J. App. Med. Sci. 2021, 7, 1175–1179. [Google Scholar] [CrossRef]
- Tkaczyk, J.; Vizek, M. Oxidative stress in the lung tissue—Sources of reactive oxygen species and antioxidant defence. Prague Med. Rep. 2007, 108, 105–114. [Google Scholar]
- Torun, A.N.; Kulaksizoglu, S.; Kulaksizoglu, M.; Pamuk, B.O.; Isbilen, E.; Tutuncu, N.B. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. Clin. Endocrinol. 2009, 70, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Raimondo, S.; Di Segni, C.; Persano, M.; Gadotti, G.; Silvestrini, A.; Festa, R.; Tiano, L.; Pontecorvi, A.; Meucci, E. Thyroid hormones and antioxidant systems: Focus on oxidative stress in cardiovascular and pulmonary diseases. Int. J. Mol. Sci. 2013, 14, 23893–23909. [Google Scholar] [CrossRef] [PubMed]
- Farrag, I.M.; Belal, A.; Al Badawi, M.H.; Abdelhady, A.A.; Abou Galala, F.M.; El-Sharkawy, A.; El-Dahshan, A.A.; Mehany, A.B. Antiproliferative, apoptotic effects and suppression of oxidative stress of quercetin against induced toxicity in lung cancer cells of rats: In vitro and in vivo study. J. Cancer 2021, 12, 5249. [Google Scholar] [CrossRef]
- Mikoś, H.; Mikoś, M.; Obara-Moszyńska, M.; Niedziela, M. The role of the immune system and cytokines involved in the pathogenesis of autoimmune thyroid disease (AITD). Endokrynol. Pol. 2014, 65, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.W. Hypertension and the pathogenesis of atherosclerosis: Oxidative stress and the mediation of arterial inflammatory response: A new perspective. Hypertension 1995, 25, 155–161. [Google Scholar] [CrossRef]
- Yamada, A.; Arakaki, R.; Saito, M.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 2195. [Google Scholar] [CrossRef]
- Kimura, H.; Tzou, S.-C.; Rocchi, R.; Kimura, M.; Suzuki, K.; Parlow, A.F.; Rose, N.R.; Caturegli, P. Interleukin (IL)-12-driven primary hypothyroidism: The contrasting roles of two Th1 cytokines (IL-12 and interferon-γ). Endocrinology 2005, 146, 3642–3651. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, G.; Pang, H.; Liu, Q.; Liu, Y. The effect of 131I-induced hypothyroidism on the levels of nitric oxide (NO), interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), total nitric oxide synthase (NOS) activity, and expression of NOS isoforms in rats. Bosn. J. Basic Med. Sci. 2018, 18, 305. [Google Scholar] [CrossRef]
- O’Sullivan, M.J.; Phung, T.-K.N.; Park, J.-A. Bronchoconstriction: A potential missing link in airway remodelling. Open Biol. 2020, 10, 2002. [Google Scholar] [CrossRef]
- Yılmaz, E.E.; Bozdağ, Z.; Ibiloğlu, I.; Arıkanoğlu, Z.; Yazgan, Ü.C.; Kaplan, I.; Gümüş, M.; Atamanalp, S.S. Therapeutic effects of ellagic acid on L-arginin ınduced acute pancreatitis. Acta Cir. Bras. 2016, 31, 396–401. [Google Scholar] [CrossRef]
- Drobnik, J.; Ciosek, J.; Slowinska, D.; Stempniak, B.; Zukowska, D.; Marczynski, A.; Tosik, D.; Bartel, H.; Dabrowski, R.; Szczepanowska, A. Experimental hypothyroidism increases content of collagen and glycosaminoglycans in the heart. Acta Physiol. Pol. 2009, 12, 57. [Google Scholar]
- Yu, G.; Tzouvelekis, A.; Wang, R.; Herazo-Maya, J.D.; Ibarra, G.H.; Srivastava, A.; De Castro, J.P.W.; DeIuliis, G.; Ahangari, F.; Woolard, T. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 2018, 24, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Chiovato, L.; Magri, F.; Carlé, A. Hypothyroidism in context: Where we’ve been and where we’re going. Adv. Ther. 2019, 36, 47–58. [Google Scholar] [CrossRef]
- Tng, E.L. The debate on treating subclinical hypothyroidism. Singap. Med. J. 2016, 57, 539. [Google Scholar] [CrossRef]
- Udovcic, M.; Pena, R.H.; Patham, B.; Tabatabai, L.; Kansara, A. Hypothyroidism and the heart. Methodist DeBakey Cardiovasc. J. 2017, 13, 55. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Kandemir, F.M.; Kucukler, S.; Eldutar, E.; Caglayan, C.; Gülçin, İ. Chrysin protects rat kidney from paracetamol-induced oxidative stress, inflammation, apoptosis, and autophagy: A multi-biomarker approach. Sci. Pharm. 2017, 85, 4. [Google Scholar] [CrossRef]
- Luo, M.; Yan, D.; Sun, Q.; Tao, J.; Xu, L.; Sun, H.; Zhao, H. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J. Cell. Biochem. 2020, 121, 2994–3004. [Google Scholar] [CrossRef]
- Kang, S.R.; Park, K.I.; Park, H.S.; Lee, D.H.; Kim, J.A.; Nagappan, A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Park, M.K. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 2011, 129, 1721–1728. [Google Scholar] [CrossRef]
- Liang, W.; Greven, J.; Fragoulis, A.; Horst, K.; Bläsius, F.; Wruck, C.; Pufe, T.; Kobbe, P.; Hildebrand, F.; Lichte, P. Sulforaphane-dependent up-regulation of NRF2 activity alleviates both systemic inflammatory response and lung injury after hemorrhagic shock/resuscitation in mice. Shock Inj. Inflamm. Sepsis Lab. Clin. Approaches 2022, 57, 221–229. [Google Scholar] [CrossRef]
- Luan, R.; Ding, D.; Yang, J. The protective effect of natural medicines against excessive inflammation and oxidative stress in acute lung injury by regulating the Nrf2 signaling pathway. Front. Pharmacol. 2022, 13, 1039022. [Google Scholar] [CrossRef] [PubMed]
- ALHaithloul, H.A.; Alotaibi, M.F.; Bin-Jumah, M.; Elgebaly, H.; Mahmoud, A.M. Olea europaea leaf extract up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney. Biomed. Pharmacother. 2019, 111, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Mohammed, H.M.; Khadrawy, S.M.; Galaly, S.R. Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem.-Biol. Interact. 2017, 277, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Li, X.; Hu, X.; Wang, J.; Xu, W.; Yi, C.; Ma, R.; Jiang, H. Inhibition of autophagy via activation of PI3K/Akt/mTOR pathway contributes to the protection of hesperidin against myocardial ischemia/reperfusion injury. Int. J. Mol. Med. 2018, 42, 1917–1924. [Google Scholar] [CrossRef]
- Bayomy, N.A.; Elshafey, S.H.; ElBakary, R.H.; Abdelaziz, E.Z. Protective effect of hesperidin against lung injury induced by intestinal ischemia/reperfusion in adult albino rats: Histological, immunohistochemical and biochemical study. Tissue Cell 2014, 46, 304–310. [Google Scholar] [CrossRef]
- Kara, S.; Gencer, B.; Karaca, T.; Tufan, H.A.; Arikan, S.; Ersan, I.; Karaboga, I.; Hanci, V. Protective effect of hesperetin and naringenin against apoptosis in ischemia/reperfusion-induced retinal injury in rats. Sci. World. J. 2014, 2014, 797824. [Google Scholar] [CrossRef]
- Wilmsen, P.K.; Spada, D.S.; Salvador, M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J. Agric. Food. Chem. 2005, 53, 4757–4761. [Google Scholar] [CrossRef]
- Abdel Reheim, E.S.; El-Twab, A. Effect of carrot oil supplementation on oxidative stress in experimentally-induced hypothyroidism and hyperthyroidism in albino rats. Egypt. J. Zool. 2014, 62, 229–254. [Google Scholar] [CrossRef]
- Estruel-Amades, S.; Massot-Cladera, M.; Pérez-Cano, F.J.; Franch, À.; Castell, M.; Camps-Bossacoma, M. Hesperidin effects on gut microbiota and gut-associated lymphoid tissue in healthy rats. Nutrients 2019, 11, 324. [Google Scholar] [CrossRef]
- Paget, G.; Barnes, J. Interspecies dosage conversion scheme in evaluation of results and quantitative application in different species. Eval. Drug. Act. Pharmacomet. 1964, 1, 160–162. [Google Scholar]
- Harlow, E.; Lane, D. Using Antibodies: A Laboratory Manual; CSHL Press: Cold Spring Harbor, NY, USA, 1999. [Google Scholar]
- Layton, C.; Bancroft, J. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone: London, UK, 2013; pp. 174–185. [Google Scholar]
- Shi, S.-R.; Guo, J.; Cote, R.J.; Young, L.L.; Hawes, D.; Shi, Y.; Thu, S.; Taylor, C.R. Sensitivity and detection efficiency of a novel two-step detection system (PowerVision) for immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 1999, 7, 201. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics. Int. 2004, 11, 36–42. [Google Scholar]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists; Jones & Bartlett Learning: Burlington, MA, USA, 1999. [Google Scholar]
- Mariia, S. Hypothyroidism and chronic obstructive pulmonary disease. Int. J. Endocrinol. 2020, 16, 643–647. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahhab, K.G.; Mourad, H.H.; Mannaa, F.A.; Morsy, F.A.; Hassan, L.K.; Taher, R.F. Role of ashwagandha methanolic extract in the regulation of thyroid profile in hypothyroidism modeled rats. Mol. Biol. Rep. 2019, 46, 3637–3649. [Google Scholar] [CrossRef] [PubMed]
- Amra, E.A.; El Rehim, S.A.A.; Lashein, F.M.; Shoaeb, H.S. Effect of a bradykinin potentiating factor separated from honey bee venom on thyroid gland and testis in hypothyroid white rats. J. Basic Appl. Zool. 2022, 83, 1. [Google Scholar] [CrossRef]
- Hegazy, W.; Abdel-Moneim, A.; Abdel-Rehiem, E.S.; Salah, M.; Abdul-Hamid, M. The protective effect of hesperidin on the liver of hypothyroid rats mediated by nuclear factor erythroid 2-related factor 2-dependent activation of heme oxygenase 1. J. Mol. Histol. 2022, 53, 543–560. [Google Scholar] [CrossRef]
- Yisireyili, M.; Wulamu, W.; Aili, A.; Li, Y.; Alimujiang, A.; Aipire, A.; Aizezi, M.; Zhang, W.; Cao, Z.; Mijiti, A. Chronic restraint stress induces esophageal fibrosis with enhanced oxidative stress in a murine model. Exp. Ther. Med. 2019, 18, 1375–1383. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Grotto, D.; Maria, L.S.; Valentini, J.; Paniz, C.; Schmitt, G.; Garcia, S.C.; Pomblum, V.J.; Rocha, J.B.T.; Farina, M. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim. Nova 2009, 32, 169–174. [Google Scholar] [CrossRef]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Bodhankar, S.L.; Mohan, V.; Thakurdesai, P.A. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: Decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem.-Biol. Interact. 2015, 237, 151–165. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Turk, E.; Kandemir, F.M.; Yildirim, S.; Caglayan, C.; Kucukler, S.; Kuzu, M. Protective effect of hesperidin on sodium arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biol. Trace Elem. Res. 2019, 189, 95–108. [Google Scholar] [CrossRef]
- Wang, S.; He, N.; Xing, H.; Sun, Y.; Ding, J.; Liu, L. Function of hesperidin alleviating inflammation and oxidative stress responses in COPD mice might be related to SIRT1/PGC-1α/NF-κB signaling axis. J. Recept. Signal Transduct. 2020, 40, 388–394. [Google Scholar] [CrossRef]
- Yeh, C.-C.; Kao, S.-J.; Lin, C.-C.; Wang, S.-D.; Liu, C.-J.; Kao, S.-T. The immunomodulation of endotoxin-induced acute lung injury by hesperidin in vivo and in vitro. Life Sci. 2007, 80, 1821–1831. [Google Scholar] [CrossRef]
- Kosutova, P.; Mikolka, P.; Kolomaznik, M.; Balentova, S.; Calkovska, A.; Mokra, D. Effects of S-Nitroso-N-Acetyl-Penicillamine (SNAP) on Inflammation, Lung Tissue Apoptosis and iNOS Activity in a Rabbit Model of Acute Lung Injury. In Pulmonary Infection and Inflammation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 13–23. [Google Scholar]
- Li, M.; Li, Y.; Li, J.; Zhao, P.; Bai, Y.; Feng, S.; Liu, X.; Wang, Y.; Bian, Q.; Li, J. Long-term effects of TCM Yangqing Kangxian formula on bleomycin-induced pulmonary fibrosis in rats via regulating nuclear factor-κB signaling. Evid. Based Complement. Altern. Med. 2017, 2017, 2089027. [Google Scholar] [CrossRef]
- Lebe, B.; Pabuççuoglu, U.; Özer, E. The significance of Ki-67 proliferative index and cyclin D1 expression of dysplastic nevi in the biologic spectrum of melanocytic lesions. Appl. Immunohistochem. Mol. Morphol. 2007, 15, 160–164. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Y.; Zhang, J.; Shi, G.; Cheng, L.; Lin, Y.; Li, Y.; Zhang, X.; Zhang, Y.; Chen, X. IL-35 recombinant protein reverses inflammatory bowel disease and psoriasis through regulation of inflammatory cytokines and immune cells. Int. J. Mol. Med. 2018, 22, 1014–1025. [Google Scholar] [CrossRef]
- Hristova, M.; Bekyarova, G.; Tzaneva, M. Heme oxygenase 1 expression and oxidative stress—Related markers in gastric mucosa in skin burns and protection with melatonin. Trakia J. Sci. 2016, 4, 307–313. [Google Scholar] [CrossRef]
- Yang, R.; Jia, Q.; Liu, X.F.; Wang, Y.Y.; Ma, S.F. Effects of hydrogen sulfide on inducible nitric oxide synthase activity and expression of cardiomyocytes in diabetic rats. Mol. Med. Rep. 2017, 16, 5277–5284. [Google Scholar] [CrossRef] [PubMed]
- Cano-Europa, E.; Blas-Valdivia, V.; Franco-Colin, M.; Gallardo-Casas, C.A.; Ortiz-Butrón, R. Methimazole-induced hypothyroidism causes cellular damage in the spleen, heart, liver, lung and kidney. Acta Histochem. 2011, 113, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Aral, I.; Kilic, N.; Bakan, I.; Aydin, S.; Erman, F. The level of antioxidant enzymes, plasma vitamins C and E in cement plant workers. Clin. Chim. Acta 2004, 341, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Lyamzaev, K.G.; Panteleeva, A.A.; Karpukhina, A.A.; Galkin, I.I.; Popova, E.N.; Pletjushkina, O.Y.; Rieger, B.; Busch, K.B.; Mulkidjanian, A.Y.; Chernyak, B.V. Novel fluorescent mitochondria-targeted probe MitoCLox reports lipid peroxidation in response to oxidative stress in vivo. Oxidative Med. Cell. Longev. 2020, 2020, 3631272. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Dembinski, R.; Maedler, K. Lung surfactant for pulmonary barrier restoration in patients with COVID-19 pneumonia. Front. Med. 2020, 7, 254. [Google Scholar] [CrossRef]
- Zaied, K.; Mohamed, H.; El-Twab, A.; Abdul-Hamid, M. Ameliorative Effects of Panax Ginseng on Lung of Lambada Cyhalotherin-intoxicated Rats. Egypt. J. Histol. 2021, 44, 61–82. [Google Scholar] [CrossRef]
- Salzer, W.L.; Winick, N.J.; Wacker, P.; Lu, X.; Devidas, M.; Shuster, J.J.; Mahoney, D.H.; Lauer, S.J.; Camitta, B.M. Plasma Methotrexate, Red Blood Cell Methotrexate and Red Blood Cell Folate Values and Outcome in Children with Precursor B Acute Lymphoblastic Leukemia: A Report from the Children’s Oncology Group. J. Pediatr. Hematol. Oncol. 2012, 34, e1. [Google Scholar] [CrossRef]
- Robb, C.; Regan, K.; Dorward, D.; Rossi, A. Key Mechanisms Governing Resolution of Lung Inflammation. In Seminars in Immunopathology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 425–448. [Google Scholar]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Larsen, B.T.; Vaszar, L.T.; Colby, T.V.; Tazelaar, H.D. Lymphoid hyperplasia and eosinophilic pneumonia as histologic manifestations of amiodarone-induced lung toxicity. Am. J. Surg. Pathol. 2012, 36, 509–516. [Google Scholar] [CrossRef]
- Kumar, V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- El Ayed, M.; Kadri, S.; Smine, S.; Elkahoui, S.; Limam, F.; Aouani, E. Protective effects of grape seed and skin extract against high-fat-diet-induced lipotoxicity in rat lung. Lipids. Health Dis. 2017, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Romero, F.; Shah, D.; Duong, M.; Penn, R.B.; Fessler, M.B.; Madenspacher, J.; Stafstrom, W.; Kavuru, M.; Lu, B.; Kallen, C.B. A pneumocyte–macrophage paracrine lipid axis drives the lung toward fibrosis. Am. J. Respir. Cell. Mol. Biol. 2015, 53, 74–86. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Q.-Y.; Zhang, X.-J.; Li, X.; Li, W.-T.; Ma, X.-T.; Ma, L.-J. Corilagin attenuates aerosol bleomycin-induced experimental lung injury. Int. J. Mol. Sci. 2014, 15, 9762–9779. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S.; Taguchi, T. Pulmonary fibrosis: Cellular and molecular events. Pathol. Int. 2003, 53, 133–145. [Google Scholar] [CrossRef]
- Wang, G.; Hu, Y.-X.; He, M.-Y.; Xie, Y.-H.; Su, W.; Long, D.; Zhao, R.; Wang, J.; Dai, C.; Li, H. Gut-lung dysbiosis accompanied by diabetes mellitus leads to pulmonary fibrotic change through the NF-κb signaling pathway. Am. J. Pathol. 2021, 191, 838–856. [Google Scholar] [CrossRef]
- Yao, Z.; Gao, X.; Liu, M.; Chen, Z.; Yang, N.; Jia, Y.-M.; Feng, X.-M.; Xu, Y.; Yang, X.-C.; Wang, G. Diffuse myocardial injuries are present in subclinical hypothyroidism: A clinical study using myocardial T1-mapping quantification. Sci. Rep. 2018, 8, 4999. [Google Scholar] [CrossRef]
- Haddadi, G.H.; Rezaeyan, A.; Mosleh-Shirazi, M.A.; Hosseinzadeh, M.; Fardid, R.; Najafi, M.; Salajegheh, A. Hesperidin as radioprotector against radiation-induced lung damage in rat: A histopathological study. J. Med. Phys. 2017, 42, 25. [Google Scholar] [CrossRef]
- Zhou, Z.; Kandhare, A.D.; Kandhare, A.A.; Bodhankar, S.L. Hesperidin ameliorates bleomycin-induced experimental pulmonary fibrosis via inhibition of TGF-beta1/Smad3/AMPK and IkappaBalpha/NF-kappaB pathways. EXCLI J. 2019, 18, 723. [Google Scholar] [CrossRef]







| Groups | TSH (μiu/ml) | FT3 (pg/mL) | FT4 (ng/dl) | IL-35 (pg/mg) | IL-6 (pg/mg) | TNF-α (pg/mg) |
|---|---|---|---|---|---|---|
| Group 1 (Control) | 0.008 ± 0.00342 b | 5.10 ± 0.462 b | 3.41 ± 0.129 c | 308.1 ± 23.9 c | 65.4 ± 2.7 c | 13.8 ± 0.6 c |
| Group II (CBZ) | 0.011 ± 0.000760 a | 2.96 ± 0.246 a | 2.50 ± 0.162 a | 120.5 ± 1.4 a | 205.8 ± 5.3 a | 81.7 ± 2.7 a |
| Group III (HES + CBZ) | 0.007 ± 0.000258 b | 4.34 ± 0.327 b | 2.95 ± 0.113 b | 169.0 ± 9.7 b | 109.3 ± 4.8 b | 26.1 ± 1.6 b |
| Group IV (ELT + CBZ) | 0.006 ± 0.000654 b | 4.30 ± 0.251 b | 2.75 ± 0.147 ab | 172.8 ± 8.5 b | 107.9 ± 4.3 b | 26.4 ± 2.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegazy, W.; Sakr, H.I.; Abdul Hamid, M.; Abdelaziz, M.A.; Salah, M.; Abdel Rehiem, E.S.; Abdel Moneim, A. Hesperidin Attenuates Hypothyroidism-Induced Lung Damage in Adult Albino Rats by Modulating Oxidative Stress, Nuclear Factor Kappa-B Pathway, Proliferating Cell Nuclear Antigen and Inflammatory Cytokines. Biomedicines 2023, 11, 1570. https://doi.org/10.3390/biomedicines11061570
Hegazy W, Sakr HI, Abdul Hamid M, Abdelaziz MA, Salah M, Abdel Rehiem ES, Abdel Moneim A. Hesperidin Attenuates Hypothyroidism-Induced Lung Damage in Adult Albino Rats by Modulating Oxidative Stress, Nuclear Factor Kappa-B Pathway, Proliferating Cell Nuclear Antigen and Inflammatory Cytokines. Biomedicines. 2023; 11(6):1570. https://doi.org/10.3390/biomedicines11061570
Chicago/Turabian StyleHegazy, Walaa, Hader I. Sakr, Manal Abdul Hamid, Mohamed A. Abdelaziz, Marwa Salah, Eman S. Abdel Rehiem, and Adel Abdel Moneim. 2023. "Hesperidin Attenuates Hypothyroidism-Induced Lung Damage in Adult Albino Rats by Modulating Oxidative Stress, Nuclear Factor Kappa-B Pathway, Proliferating Cell Nuclear Antigen and Inflammatory Cytokines" Biomedicines 11, no. 6: 1570. https://doi.org/10.3390/biomedicines11061570
APA StyleHegazy, W., Sakr, H. I., Abdul Hamid, M., Abdelaziz, M. A., Salah, M., Abdel Rehiem, E. S., & Abdel Moneim, A. (2023). Hesperidin Attenuates Hypothyroidism-Induced Lung Damage in Adult Albino Rats by Modulating Oxidative Stress, Nuclear Factor Kappa-B Pathway, Proliferating Cell Nuclear Antigen and Inflammatory Cytokines. Biomedicines, 11(6), 1570. https://doi.org/10.3390/biomedicines11061570