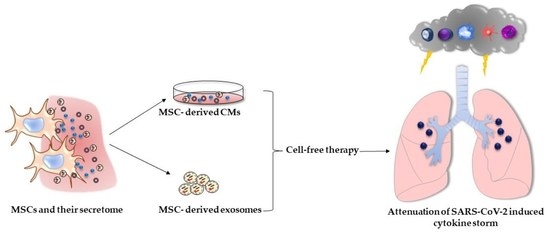
The Immunomodulatory Role of Cell-Free Approaches in SARS-CoV-2-Induced Cytokine Storm—A Powerful Therapeutic Tool for COVID-19 Patients
Abstract
:1. Introduction
2. Immunopathogenesis of COVID-19
3. Immunomodulating Properties of Conditioned Media (CM)
4. Immunomodulating Properties of Extracellular Vesicles
5. The Role of CMs in Cell-Free Therapy of COVID-19
6. The Role of Exosomes in Cell-Free Therapy of COVID-19
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghebreyesus, T.A. WHO Director-General’s Opening Remarks at the Media Briefing—17 March 2023. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing---17-march-2023 (accessed on 2 May 2023).
- Martín Sánchez, F.J.; Martínez-Sellés, M.; Molero García, J.M.; Moreno Guillén, S.; Rodríguez-Artalejo, F.J.; Ruiz-Galiana, J.; Cantón, R.; De Lucas Ramos, P.; García-Botella, A.; García-Lledó, A.; et al. Insights for COVID-19 in 2023. Rev. Esp. Quimioter. 2023, 36, 114–124. [Google Scholar] [CrossRef]
- Charles, J.; Ploplis, V.A. COVID-19 Induces Cytokine Storm and Dysfunctional Hemostasis. Curr. Drug Targets 2022, 23, 1603–1610. [Google Scholar] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e8. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Sanchis-Gomar, F.; Henry, B.M. COVID-19 and its long-term sequelae: What do we know in 2023? Pol. Arch. Intern. Med. 2023, 133, 16402. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Chouw, A.; Milanda, T.; Sartika, C.R.; Kirana, M.N.; Halim, D.; Faried, A. Potency of Mesenchymal Stem Cell and Its Secretome in Treating COVID-19. Regen. Eng. Transl. Med. 2022, 8, 43–54. [Google Scholar] [CrossRef]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef] [Green Version]
- Lai, R.C.; Yeo, R.W.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88. [Google Scholar] [CrossRef]
- Rezakhani, L.; Kelishadrokhi, A.F.; Soleimanizadeh, A.; Rahmati, S. Mesenchymal stem cell (MSC)-derived exosomes as a cell-free therapy for patients Infected with COVID-19: Real opportunities and range of promises. Chem. Phys. Lipids 2021, 234, 105009. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, A.; Zhang, X.; He, H.; Zhou, L.; Naito, Y.; Sugita, S.; Lee, J.W. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin. Biol. Ther. 2020, 20, 125–140. [Google Scholar] [CrossRef]
- Tokhanbigli, S.; Baghaei, K.; Asadirad, A.; Hashemi, S.M.; Asadzadeh-Aghdaei, H.; Zali, M.R. Immunoregulatory impact of human mesenchymal-conditioned media and mesenchymal derived exosomes on monocytes. Mol. Biol. Res. Commun. 2019, 8, 79–89. [Google Scholar] [PubMed]
- Sagulkoo, P.; Plaimas, K.; Suratanee, A.; Colado Simão, A.N.; Vissoci Reiche, E.M.; Maes, M. Immunopathogenesis and Immunogenetic Variants in COVID-19. Curr. Pharm. Des. 2022, 28, 1780–1797. [Google Scholar]
- Perlman, S.; Dandekar, A.A. Immunopathogenesis of coronavirus infections: Implications for SARS. Nat. Rev. Immunol. 2005, 5, 917–927. [Google Scholar] [PubMed] [Green Version]
- Zanza, C.; Romenskaya, T.; Manetti, A.C.; Franceschi, F.; La Russa, R.; Bertozzi, G.; Maiese, A.; Savioli, G.; Volonnino, G.; Longhitano, Y. Cytokine Storm in COVID-19: Immunopathogenesis and Therapy. Medicina 2022, 58, 144. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Mohamed Khosroshahi, L.; Rokni, M.; Mokhtari, T.; Noorbakhsh, F. Immunology, immunopathogenesis and immunotherapeutics of COVID-19; an overview. Int. Immunopharmacol. 2021, 93, 107364. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Liu, J.; Liang, B.; Wang, X.; Wang, H.; Li, W.; Tong, Q.; Yi, J.; Zhao, L.; et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020, 55, 102763. [Google Scholar] [CrossRef]
- Shahgolzari, M.; Yavari, A.; Arjeini, Y.; Miri, S.M.; Darabi, A.; Mozaffari Nejad, A.S.; Keshavarz, M. Immunopathology and Immunopathogenesis of COVID-19, what we know and what we should learn. Gene Rep. 2021, 25, 101417. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Islam, M.S.; Yang, Y.; Hu, Y.; Chen, X. The role of CD4(+)FoxP3(+) regulatory T cells in the immunopathogenesis of COVID-19: Implications for treatment. Int. J. Biol. Sci. 2021, 17, 1507–1520. [Google Scholar] [CrossRef]
- Alahdal, M.; Elkord, E. Exhaustion and over-activation of immune cells in COVID-19: Challenges and therapeutic opportunities. Clin. Immunol. 2022, 245, 109177. [Google Scholar] [CrossRef]
- Yang, M.; Lin, C.; Wang, Y.; Chen, K.; Han, Y.; Zhang, H.; Li, W. Cytokine storm promoting T cell exhaustion in severe COVID-19 revealed by single cell sequencing data analysis. Precis. Clin. Med. 2022, 5, pbac014. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, Ø. Tumor Necrosis Factor and the Tenacious α. JAMA Dermatol. 2016, 152, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, S.; Asgarzadeh, A.; Kianfar, H.; Mohammadnia, A.; Asghariazar, V.; Safarzadeh, E. The role of IL-1 family of cytokines and receptors in pathogenesis of COVID-19. Inflamm. Res. 2022, 71, 923–947. [Google Scholar] [CrossRef] [PubMed]
- Bittner, Z.A.; Schrader, M.; George, S.E.; Amann, R. Pyroptosis and Its Role in SARS-CoV-2 Infection. Cells 2022, 11, 1717. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chang, W.; Zhang, L.; Zhang, Y. Pyroptotic cell death in SARS-CoV-2 infection: Revealing its roles during the immunopathogenesis of COVID-19. Int. J. Biol. Sci. 2022, 18, 5827–5848. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, J.; Gauba, K.; Gupta, S.; Purohit, P.; Mitra, P.; Garg, M.; Misra, S.; Sharma, P.; Banerjee, M. Interleukin-6 Perpetrator of the COVID-19 Cytokine Storm. Indian J. Clin. Biochem. 2021, 36, 440–450. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in COVID-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Galván-Román, J.M.; Rodríguez-García, S.C.; Roy-Vallejo, E.; Marcos-Jiménez, A.; Sánchez-Alonso, S.; Fernández-Díaz, C.; Alcaraz-Serna, A.; Mateu-Albero, T.; Rodríguez-Cortes, P.; Sánchez-Cerrillo, I.; et al. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study. J. Allergy Clin. Immunol. 2021, 147, 72–80.e78. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Chamberlain, T.C.; Mui, A.L.; Little, J.P. Elevated Interleukin-10 Levels in COVID-19: Potentiation of Pro-Inflammatory Responses or Impaired Anti-Inflammatory Action? Front. Immunol. 2021, 12, 677008. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, H.; Dauphars, D.J.; He, Y.W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2021, 42, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.C.; Shakibakho, S.; Durrer, C.; Simtchouk, S.; Jawanda, K.K.; Cheung, S.T.; Mui, A.L.; Little, J.P. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci. Rep. 2016, 6, 21244. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.K.; Vishnupriyan, K.; Damodar, S.; Gujar, S.; Das, M. IL-6 and IL-10 as predictors of disease severity in COVID-19 patients: Results from meta-analysis and regression. Heliyon 2021, 7, e06155. [Google Scholar] [CrossRef]
- Bönig, H.; Packeisen, J.; Röhne, B.; Hempel, L.; Hannen, M.; Klein-Vehne, A.; Burdach, S.; Körholz, D. Interaction between interleukin 10 and interleukin 6 in human B-cell differentiation. Immunol. Investig. 1998, 27, 267–280. [Google Scholar] [CrossRef]
- Heine, G.; Drozdenko, G.; Grün, J.R.; Chang, H.D.; Radbruch, A.; Worm, M. Autocrine IL-10 promotes human B-cell differentiation into IgM- or IgG-secreting plasmablasts. Eur. J. Immunol. 2014, 44, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Hirano, T. Interleukin 6 in autoimmune and inflammatory diseases: A personal memoir. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 717–730. [Google Scholar] [CrossRef] [Green Version]
- Llorente, L.; Zou, W.; Levy, Y.; Richaud-Patin, Y.; Wijdenes, J.; Alcocer-Varela, J.; Morel-Fourrier, B.; Brouet, J.C.; Alarcon-Segovia, D.; Galanaud, P.; et al. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J. Exp. Med. 1995, 181, 839–844. [Google Scholar] [CrossRef] [Green Version]
- Andreakos, E.; Tsiodras, S. COVID-19: Lambda interferon against viral load and hyperinflammation. EMBO Mol. Med. 2020, 12, e12465. [Google Scholar] [CrossRef]
- Broadbent, L.; Bamford, C.G.G.; Lopez Campos, G.; Manzoor, S.; Courtney, D.; Ali, A.; Touzelet, O.; McCaughey, C.; Mills, K.; Power, U.F. An endogenously activated antiviral state restricts SARS-CoV-2 infection in differentiated primary airway epithelial cells. PLoS ONE 2022, 17, e0266412. [Google Scholar] [CrossRef]
- Shibabaw, T. Inflammatory Cytokine: IL-17A Signaling Pathway in Patients Present with COVID-19 and Current Treatment Strategy. J. Inflamm. Res. 2020, 13, 673–680. [Google Scholar] [CrossRef]
- Maione, F.; Casillo, G.M.; Raucci, F.; Salvatore, C.; Ambrosini, G.; Costa, L.; Scarpa, R.; Caso, F.; Bucci, M. Interleukin-17A (IL-17A): A silent amplifier of COVID-19. Biomed. Pharmacother. 2021, 142, 111980. [Google Scholar] [CrossRef]
- Bunprakob, S.; Hemachudha, P.; Ruchisrisarod, C.; Supharatpariyakorn, T.; Hemachudha, T. IP-10 and complement activation as friend or foe in COVID-19. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221096202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.; Liu, C.; Su, L.; Zhang, D.; Fan, J.; Yang, Y.; Xiao, M.; Xie, J.; Xu, Y.; et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020, 26, 97. [Google Scholar] [CrossRef] [PubMed]
- Mulla, S.; Molla, M.M.A.; Ahmed, S.M.A.; Akhtaruzzaman, A.K.M.; Saleh, A.A.; Anwar, S. Association of interferon gamma inducible protein-10, monocyte chemoattractant protein-1, macrophage inflammatory protein-1 alpha, interleukin-6, and rs12252 single nucleotide polymorphism of interferon-induced transmembrane protein-3 gene with the severity of COVID-19 infection. Egypt J. Intern. Med. 2022, 34, 53. [Google Scholar]
- Guo, Y.; Hu, K.; Li, Y.; Lu, C.; Ling, K.; Cai, C.; Wang, W.; Ye, D. Targeting TNF-α for COVID-19: Recent Advanced and Controversies. Front. Public Health 2022, 10, 833967. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Wang, G.; Xu, J.; Long, J.; Deng, F.; Jiang, W. Role of tumor necrosis factor-α in the mortality of hospitalized patients with severe and critical COVID-19 pneumonia. Aging 2021, 13, 23895–23912. [Google Scholar] [CrossRef]
- Ablamunits, V.; Lepsy, C. Blocking TNF signaling may save lives in COVID-19 infection. Mol. Biol. Rep. 2022, 49, 2303–2309. [Google Scholar] [CrossRef]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef] [Green Version]
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochemistry 2019, 84, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, R.H.; Yu, J.M.; Foskett, A.M.; Peltier, G.; Reneau, J.C.; Bazhanov, N.; Oh, J.Y.; Prockop, D.J. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 16766–16771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.; Abbott, J.; Cheng, L.; Colby, J.K.; Lee, J.W.; Levy, B.D.; Matthay, M.A. Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J. Immunol. 2015, 195, 875–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermudez, M.A.; Sendon-Lago, J.; Seoane, S.; Eiro, N.; Gonzalez, F.; Saa, J.; Vizoso, F.; Perez-Fernandez, R. Anti-inflammatory effect of conditioned medium from human uterine cervical stem cells in uveitis. Exp. Eye Res. 2016, 149, 84–92. [Google Scholar] [CrossRef]
- McCarthy, S.D.; Horgan, E.; Ali, A.; Masterson, C.; Laffey, J.G.; MacLoughlin, R.; O’Toole, D. Nebulized Mesenchymal Stem Cell Derived Conditioned Medium Retains Anti-bacterial Properties Against Clinical Pathogen Isolates. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 140–152. [Google Scholar] [CrossRef]
- An, S.Y.; Jang, Y.J.; Lim, H.J.; Han, J.; Lee, J.; Lee, G.; Park, J.Y.; Park, S.Y.; Kim, J.H.; Do, B.R.; et al. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology 2017, 152, 1174–1186. [Google Scholar] [CrossRef] [Green Version]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [Green Version]
- Goodarzi, P.; Alavi-Moghadam, S.; Payab, M.; Larijani, B.; Rahim, F.; Gilany, K.; Bana, N.; Tayanloo-Beik, A.; Foroughi Heravani, N.; Hadavandkhani, M.; et al. Metabolomics Analysis of Mesenchymal Stem Cells. Int. J. Mol. Cell. Med. 2019, 8, 30–40. [Google Scholar]
- Ionescu, L.; Byrne, R.N.; van Haaften, T.; Vadivel, A.; Alphonse, R.S.; Rey-Parra, G.J.; Weissmann, G.; Hall, A.; Eaton, F.; Thébaud, B. Stem cell conditioned medium improves acute lung injury in mice: In vivo evidence for stem cell paracrine action. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L967–L977. [Google Scholar] [CrossRef] [Green Version]
- Caneparo, C.; Baratange, C.; Chabaud, S.; Bolduc, S. Conditioned medium produced by fibroblasts cultured in low oxygen pressure allows the formation of highly structured capillary-like networks in fibrin gels. Sci. Rep. 2020, 10, 9291. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Su, Y.; Su, L.; Lan, X.; Wu, D.; Han, S.; Li, J.; Kvederis, L.; Corey, S.; et al. Hypoxia conditioning enhances neuroprotective effects of aged human bone marrow mesenchymal stem cell-derived conditioned medium against cerebral ischemia in vitro. Brain Res. 2019, 1725, 146432. [Google Scholar] [CrossRef]
- Ylöstalo, J.H.; Bartosh, T.J.; Coble, K.; Prockop, D.J. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells 2012, 30, 2283–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waszak, P.; Alphonse, R.; Vadivel, A.; Ionescu, L.; Eaton, F.; Thébaud, B. Pre-conditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 2012, 21, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Zhao, J.; Zhang, Z.; Yang, R.; Xie, J.; Liu, X.; Qi, S. Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE 2014, 9, e96161. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.C.; Jeong, H.J.; Lee, S.K.; Kim, S.J. Hypoxic Conditioned Medium from Human Adipose-Derived Stem Cells Promotes Mouse Liver Regeneration through JAK/STAT3 Signaling. Stem Cells Transl. Med. 2016, 5, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Lin, L.; Fan, Y.; Huselstein, C.; De Isla, N.; He, X.; Chen, Y.; Li, Y. Secretome of Mesenchymal Stem Cells from Consecutive Hypoxic Cultures Promotes Resolution of Lung Inflammation by Reprogramming Anti-inflammatory Macrophages. Int. J. Mol. Sci. 2022, 23, 4333. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xu, Z.; Qu, G.; Wang, H.; Lin, L.; Li, X.; Xie, X.; Lei, Y.; He, X.; Chen, Y.; et al. Hypoxic Pre-conditioning Enhances the Efficacy of Mesenchymal Stem Cells-Derived Conditioned Medium in Switching Microglia toward Anti-inflammatory Polarization in Ischemia/Reperfusion. Cell. Mol. Neurobiol. 2021, 41, 505–524. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their anti-inflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.C.; Whitehead, J.; Falahee, P.C.; Zhou, D.; Simon, S.I.; Leach, J.K. Multifactorial Experimental Design to Optimize the Anti-inflammatory and Proangiogenic Potential of Mesenchymal Stem Cell Spheroids. Stem Cells 2017, 35, 1493–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazrati, A.; Malekpour, K.; Soudi, S.; Hashemi, S.M. CRISPR/Cas9-engineered mesenchymal stromal/stem cells and their extracellular vesicles: A new approach to overcoming cell therapy limitations. Biomed. Pharmacother. 2022, 156, 113943. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goolaerts, A.; Pellan-Randrianarison, N.; Larghero, J.; Vanneaux, V.; Uzunhan, Y.; Gille, T.; Dard, N.; Planès, C.; Matthay, M.A.; Clerici, C. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L975–L985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shologu, N.; Scully, M.; Laffey, J.G.; O’Toole, D. Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury. Int. J. Mol. Sci. 2018, 19, 2996. [Google Scholar] [CrossRef] [Green Version]
- Su, V.Y.; Lin, C.S.; Hung, S.C.; Yang, K.Y. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 2208. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Pinheiro, B.; Anjo, S.I.; Manadas, B.; Da Silva, J.D.; Marote, A.; Behie, L.A.; Teixeira, F.G.; Salgado, A.J. Bone Marrow Mesenchymal Stem Cells’ Secretome Exerts Neuroprotective Effects in a Parkinson’s Disease Rat Model. Front. Bioeng. Biotechnol. 2019, 7, 294. [Google Scholar] [CrossRef] [Green Version]
- Mehrabadi, S.; Motevaseli, E.; Sadr, S.S.; Moradbeygi, K. Hypoxic-conditioned medium from adipose tissue mesenchymal stem cells improved neuroinflammation through alternation of toll like receptor (TLR) 2 and TLR4 expression in model of Alzheimer’s disease rats. Behav. Brain Res. 2020, 379, 112362. [Google Scholar] [CrossRef]
- Silveira, B.M.; Ribeiro, T.O.; Freitas, R.S.; Carreira, A.C.O.; Gonçalves, M.S.; Sogayar, M.; Meyer, R.; Birbrair, A.; Fortuna, V. Secretome from human adipose-derived mesenchymal stem cells promotes blood vessel formation and pericyte coverage in experimental skin repair. PLoS ONE 2022, 17, e0277863. [Google Scholar] [CrossRef]
- Lin, H.; Chen, H.; Zhao, X.; Chen, Z.; Zhang, P.; Tian, Y.; Wang, Y.; Ding, T.; Wang, L.; Shen, Y. Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. J. Transl. Med. 2021, 19, 456. [Google Scholar] [CrossRef]
- Liang, X.; Lin, F.; Ding, Y.; Zhang, Y.; Li, M.; Zhou, X.; Meng, Q.; Ma, X.; Wei, L.; Fan, H.; et al. Conditioned medium from induced pluripotent stem cell-derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis. Stem Cell Res. Ther. 2021, 12, 295. [Google Scholar] [CrossRef]
- Műzes, G.; Sipos, F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Cui, Y.; Hou, Y.; Nie, H. Bone marrow mesenchymal stem cell-conditioned medium facilitates fluid resolution via miR-214-activating epithelial sodium channels. MedComm 2020, 1, 376–385. [Google Scholar] [CrossRef]
- Zhou, Z.; Hua, Y.; Ding, Y.; Hou, Y.; Yu, T.; Cui, Y.; Nie, H. Conditioned Medium of Bone Marrow Mesenchymal Stem Cells Involved in Acute Lung Injury by Regulating Epithelial Sodium Channels via miR-34c. Front. Bioeng. Biotechnol. 2021, 9, 640116. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Ding, F.; Wu, C.; Liu, B. hucMSC Conditioned Medium Ameliorate Lipopolysaccharide-Induced Acute Lung Injury by Suppressing Oxidative Stress and Inflammation via Nrf2/NF-κB Signaling Pathway. Anal. Cell. Pathol. 2021, 2021, 6653681. [Google Scholar] [CrossRef] [PubMed]
- Su, V.Y.; Chiou, S.H.; Chen, W.C.; Yu, W.K.; Wu, H.H.; Chen, H.; Yang, K.Y. Induced Pluripotent Stem Cell-Derived Conditioned Medium Promotes Endogenous Leukemia Inhibitory Factor to Attenuate Endotoxin-Induced Acute Lung Injury. Int. J. Mol. Sci. 2021, 22, 5554. [Google Scholar] [CrossRef]
- Li, L.F.; Liu, Y.Y.; Yang, C.T.; Chien, Y.; Twu, N.F.; Wang, M.L.; Wang, C.Y.; Huang, C.C.; Kao, K.C.; Hsu, H.S.; et al. Improvement of ventilator-induced lung injury by IPS cell-derived conditioned medium via inhibition of PI3K/Akt pathway and IP-10-dependent paracrine regulation. Biomaterials 2013, 34, 78–91. [Google Scholar] [CrossRef]
- Schnabel, L.V.; Abratte, C.M.; Schimenti, J.C.; Felippe, M.J.; Cassano, J.M.; Southard, T.L.; Cross, J.A.; Fortier, L.A. Induced pluripotent stem cells have similar immunogenic and more potent immunomodulatory properties compared with bone marrow-derived stromal cells in vitro. Regen. Med. 2014, 9, 621–635. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zou, Y.; Song, X.; Jiang, A.; Wang, M.; Qin, Q.; Song, Y.; Yue, C.; Yang, D.; Yu, B.; et al. Potential of extracellular vesicles for early prediction of severity and potential risk stratification in critical inflammatory diseases. J. Cell Commun. Signal. 2023, 1–10. [Google Scholar] [CrossRef]
- Rocco, G.D.; Baldari, S.; Toietta, G. Exosomes and other extracellular vesicles-mediated microRNA delivery for cancer therapy. Transl. Cancer Res. 2017, 6, S1321–S1330. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, T.; Saleem, A.; Farooq, N.; Dar, L.A.; Nazir, J.; Saleem, S.; Ismail, S.; Gugjoo, M.B.; Shiekh, P.A.; Ahmad, S.M. Extracellular vesicles derived from mesenchymal stem cells—A novel therapeutic tool in infectious diseases. Inflamm. Regen. 2023, 43, 17. [Google Scholar] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omrani, M.; Beyrampour-Basmenj, H.; Jahanban-Esfahlan, R.; Talebi, M.; Raeisi, M.; Serej, Z.A.; Akbar-Gharalari, N.; Khodakarimi, S.; Wu, J.; Ebrahimi-Kalan, A. Global trend in exosome isolation and application: An update concept in management of diseases. Mol. Cell. Biochem. 2023, 1–13. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.C.; Wu, H.J.; Ugaz, V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab. Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef] [Green Version]
- Blazquez, R.; Sanchez-Margallo, F.M.; de la Rosa, O.; Dalemans, W.; Alvarez, V.; Tarazona, R.; Casado, J.G. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front. Immunol. 2014, 5, 556. [Google Scholar] [CrossRef] [Green Version]
- Basu, J.; Ludlow, J.W. Exosomes for repair, regeneration and rejuvenation. Expert Opin. Biol. Ther. 2016, 16, 489–506. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Asgarpour, K.; Shojaei, Z.; Amiri, F.; Ai, J.; Mahjoubin-Tehran, M.; Ghasemi, F.; ArefNezhad, R.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs derived from mesenchymal stem cells: Cell-to-cell messages. Cell Commun. Signal 2020, 18, 149. [Google Scholar] [CrossRef]
- Ti, D.; Hao, H.; Fu, X.; Han, W. Mesenchymal stem cells-derived exosomal microRNAs contribute to wound inflammation. Sci. China Life Sci. 2016, 59, 1305–1312. [Google Scholar] [CrossRef]
- Song, Y.; Dou, H.; Li, X.; Zhao, X.; Li, Y.; Liu, D.; Ji, J.; Liu, F.; Ding, L.; Ni, Y.; et al. Exosomal miR-146a Contributes to the Enhanced Therapeutic Efficacy of Interleukin-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells 2017, 35, 1208–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Liu, Z.; Hu, L.; Gu, W.; Zhu, L. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp. Cell Res. 2018, 370, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, D.; Yu, Z.; Fu, Z.; Lv, Z.; Meng, L.; Zhao, X. Exosomal miR-150 partially attenuated acute lung injury by mediating microvascular endothelial cells and MAPK pathway. Biosci. Rep. 2022, 42, BSR20203363. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wei, X.; Lv, H.; An, Y.; Li, L.; Lu, P.; Yang, Y.; Zhang, Q.; Yi, H.; Chen, G. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp. Cell Res. 2019, 383, 111454. [Google Scholar] [CrossRef]
- Liu, J.S.; Du, J.; Cheng, X.; Zhang, X.Z.; Li, Y.; Chen, X.L. Exosomal miR-451 from human umbilical cord mesenchymal stem cells attenuates burn-induced acute lung injury. J. Chin. Med. Assoc. 2019, 82, 895–901. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, C.; Cao, L.; Zhang, C.H.; Zhang, Z.H. Exosomal miR-132-3p from mesenchymal stem cells alleviated LPS-induced acute lung injury by repressing TRAF6. Autoimmunity 2021, 54, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Cen, J.; Zhang, X.; Gong, C.; Sun, M.; Meng, W.; Mao, G.; Wan, J.; Hu, B.; He, X.; et al. MiR-146a-5p delivered by hucMSC extracellular vesicles modulates the inflammatory response to sulfur mustard-induced acute lung injury. Stem Cell Res. Ther. 2023, 14, 149. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar]
- Liang, Y.; Duan, L.; Lu, J.; Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 2021, 11, 3183–3195. [Google Scholar] [CrossRef]
- Monsel, A.; Zhu, Y.G.; Gennai, S.; Hao, Q.; Hu, S.; Rouby, J.J.; Rosenzwajg, M.; Matthay, M.A.; Lee, J.W. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am. J. Respir. Crit. Care Med. 2015, 192, 324–336. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Huang, R.; Xu, Q.; Zheng, G.; Qiu, G.; Ge, M.; Shu, Q.; Xu, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles Alleviate Acute Lung Injury Via Transfer of miR-27a-3p. Crit. Care Med. 2020, 48, e599–e610. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Y.; Liu, Q.; Liu, Y.; Chen, L.; Zhao, H.; Guo, H.; Zhu, K.; Zhou, N.; Chai, T.C.; et al. Pyroptosis engagement and bladder urothelial cell-derived exosomes recruit mast cells and induce barrier dysfunction of bladder urothelium after uropathogenic E. coli infection. Am. J. Physiol. Cell Physiol. 2019, 317, C544–C555. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, J.; Li, H.; Li, P.; Xu, C. Serum exosomes derived from Hp-positive gastritis patients inhibit MCP-1 and MIP-1α expression via NLRP12-Notch signaling pathway in intestinal epithelial cells and improve DSS-induced colitis in mice. Int. Immunopharmacol. 2020, 88, 107012. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Zhang, L.; Li, L.; Fan, M.; Hou, L. MiR-339 attenuates LPS-induced intestinal epithelial cells inflammatory responses and apoptosis by targeting TLR4. Genes Genom. 2020, 42, 1097–1105. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Liu, S.; Wu, K.; Zhao, R.; Cao, L.; Wang, H. Prospective application of exosomes derived from adipose-derived stem cells in skin wound healing: A review. J. Cosmet. Dermatol. 2020, 19, 574–581. [Google Scholar] [CrossRef]
- Joorabloo, A.; Liu, T. Engineering exosome-based biomimetic nanovehicles for wound healing. J. Control. Release 2023, 356, 463–480. [Google Scholar] [CrossRef]
- Guo, M.; Yin, Z.; Chen, F.; Lei, P. Mesenchymal stem cell-derived exosome: A promising alternative in the therapy of Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 109. [Google Scholar] [CrossRef]
- Rastogi, S.; Sharma, V.; Bharti, P.S.; Rani, K.; Modi, G.P.; Nikolajeff, F.; Kumar, S. The Evolving Landscape of Exosomes in Neurodegenerative Diseases: Exosomes Characteristics and a Promising Role in Early Diagnosis. Int. J. Mol. Sci. 2021, 22, 440. [Google Scholar] [CrossRef]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, L.; Wu, M.; Cao, K.; Jiang, F.; Chen, D.; Li, N.; Li, W. The significance of exosomes in the development and treatment of hepatocellular carcinoma. Mol. Cancer 2020, 19, 1. [Google Scholar] [CrossRef]
- Wan, T.; Zhong, J.; Pan, Q.; Zhou, T.; Ping, Y.; Liu, X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci. Adv. 2022, 8, eabp9435. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhao, Z.; Meng, Q.; Yu, Y.; Sun, J.; Yang, Z.; Chen, Y.; Li, J.; Ma, T.; et al. Engineered Exosomes with Ischemic Myocardium-Targeting Peptide for Targeted Therapy in Myocardial Infarction. J. Am. Heart Assoc. 2018, 7, e008737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, N.; Zhang, Y.; Zhang, S.; Wang, D.; Ye, H. Exosomes: Cell-Free Therapy for Cardiovascular Diseases. J. Cardiovasc. Transl. Res. 2020, 13, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wu, W.; Jiang, X.; Liu, Y. Mesenchymal stem cell-derived exosomes in cardiovascular and cerebrovascular diseases: From mechanisms to therapy. Biomed. Pharmacother. 2023, 163, 114817. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, C.; Qiao, K.; Li, Z.; Han, J.; Han, X.; Li, K.; Lai, K.; Liu, N.; et al. Biomimetic Immunosuppressive Exosomes that Inhibit Cytokine Storms Contribute to the Alleviation of Sepsis. Adv. Mater. 2022, 34, e2108476. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Bazhanov, N.; Hashimoto, K.; Shimizu, M.; Heathman, T.; Hao, Q.; Nawgiri, R.; Muthukumarana, V.; Lee, J.W.; Prough, D.S.; et al. Mesenchymal stem cell-derived exosomes for treatment of sepsis. Front. Immunol. 2023, 14, 1136964. [Google Scholar] [CrossRef]
- Tang, X.D.; Shi, L.; Monsel, A.; Li, X.Y.; Zhu, H.L.; Zhu, Y.G.; Qu, J.M. Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 mRNA. Stem Cells 2017, 35, 1849–1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Zhang, T.; Zhang, C.; Ji, H.; Tong, X.; Xia, R.; Wang, W.; Ma, Z.; Shi, X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care 2021, 25, 356. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, L.; Zhang, Y.; Zheng, L.; Hu, S.; Zhou, W.; Zhang, T.; Xu, W.; Chen, Y.; Zhou, H.; et al. Differential Lung Protective Capacity of Exosomes Derived from Human Adipose Tissue, Bone Marrow, and Umbilical Cord Mesenchymal Stem Cells in Sepsis-Induced Acute Lung Injury. Oxid. Med. Cell. Longev. 2022, 2022, 7837837. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.C.; Gong, C.C.; Wang, Z.; Sun, M.X.; Pei, Z.P.; Meng, W.Q.; Cen, J.F.; He, X.W.; Lu, Y.; Xu, Q.Q.; et al. BMSC-derived exosomes ameliorate sulfur mustard-induced acute lung injury by regulating the GPRC5A-YAP axis. Acta Pharmacol. Sin. 2021, 42, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Qin, S.; Vashi, M.; Predescu, D.N.; Jeganathan, N.; Bardita, C.; Ganesh, B.; diBartolo, S.; Fogg, L.F.; Balk, R.A.; et al. Alk5/Runx1 signaling mediated by extracellular vesicles promotes vascular repair in acute respiratory distress syndrome. Clin. Transl. Med. 2018, 7, 19. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Feng, X.M.; Abbott, J.; Fang, X.H.; Hao, Q.; Monsel, A.; Qu, J.M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Zhang, C.; Lv, N.; Liang, Z.; Ma, T.; Cheng, H.; Xia, Y.; Shi, L. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics 2022, 12, 2928–2947. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2020, 11, 591065. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Neyrinck, A.P.; Matthay, M.A.; Lee, J.W. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J. Biol. Chem. 2010, 285, 26211–26222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, A.; Krasnodembskaya, A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl. Med. 2020, 9, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.I.; Alfarafisa, N.M.; Septiani, P.; Barlian, A.; Firmansyah, M.; Faizal, A.; Melani, L.; Nugrahapraja, H. Potential Cell-Based and Cell-Free Therapy for Patients with COVID-19. Cells 2022, 11, 2319. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. Biomed. Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bari, E.; Ferrarotti, I.; Saracino, L.; Perteghella, S.; Torre, M.L.; Richeldi, L.; Corsico, A.G. Mesenchymal Stromal Cell Secretome for Post-COVID-19 Pulmonary Fibrosis: A New Therapy to Treat the Long-Term Lung Sequelae? Cells 2021, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Pati, S.; Gerber, M.H.; Menge, T.D.; Wataha, K.A.; Zhao, Y.; Baumgartner, J.A.; Zhao, J.; Letourneau, P.A.; Huby, M.P.; Baer, L.A.; et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS ONE 2011, 6, e25171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapuente, J.P.; Blázquez-Martínez, A.; Marco-Brualla, J.; Gómez, G.; Desportes, P.; Sanz, J.; Fernández, P.; García-Gil, M.; Bermejo, F.; San Martín, J.V.; et al. Cytokine Profile and Anti-inflammatory Activity of a Standardized Conditioned Medium Obtained by Co-culture of Monocytes and Mesenchymal Stromal Cells (PRS CK STORM). Biomolecules 2022, 12, 534. [Google Scholar] [CrossRef] [PubMed]
- Gowda, P.; Patrick, S.; Joshi, S.D.; Kumawat, R.K.; Sen, E. Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine 2021, 142, 155496. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Dela Cruz, M.; Subramanyam, D.; Kumar, R.; Markan, S.; Parker, B.; Roy, H.K. Exercise-induced myokines downregulates the ACE2 level in bronchial epithelial cells: Implications for SARS-CoV-2 prevention. PLoS ONE 2022, 17, e0271303. [Google Scholar] [CrossRef]
- Kummarapurugu, A.B.; Hawkridge, A.M.; Ma, J.; Osei, S.; Martin, R.K.; Zheng, S.; Voynow, J.A. Neutrophil Elastase decreases SARS-CoV-2 Spike protein binding to human bronchial epithelia by clipping ACE-2 ectodomian from the epithelial surface. J. Biol. Chem. 2023, 299, 104820. [Google Scholar] [CrossRef]
- Barhoumi, T.; Alghanem, B.; Shaibah, H.; Mansour, F.A.; Alamri, H.S.; Akiel, M.A.; Alroqi, F.; Boudjelal, M. SARS-CoV-2 Coronavirus Spike Protein-Induced Apoptosis, Inflammatory, and Oxidative Stress Responses in THP-1-Like-Macrophages: Potential Role of Angiotensin-Converting Enzyme Inhibitor (Perindopril). Front. Immunol. 2021, 12, 728896. [Google Scholar] [CrossRef]
- Kuate, S.; Cinatl, J.; Doerr, H.W.; Uberla, K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology 2007, 362, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, S.; Vasen, G.; Pablo, M.; Chen, X.; Beutler, N.; Kumar, A.; Tanner, E.; Illouz, S.; Rahgoshay, D.; Burnett, J.; et al. Identification of a therapeutic interfering particle-A single-dose SARS-CoV-2 antiviral intervention with a high barrier to resistance. Cell 2021, 184, 6022–6036.e18. [Google Scholar] [CrossRef]
- Reis, G.; Moreira Silva, E.A.S.; Medeiros Silva, D.C.; Thabane, L.; Campos, V.H.S.; Ferreira, T.S.; Santos, C.V.Q.; Nogueira, A.M.R.; Almeida, A.P.F.G.; Savassi, L.C.M.; et al. Early Treatment with Pegylated Interferon Lambda for COVID-19. N. Engl. J. Med. 2023, 388, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Santer, D.M.; Li, D.; Ghosheh, Y.; Zahoor, M.A.; Prajapati, D.; Hansen, B.E.; Tyrrell, D.L.J.; Feld, J.J.; Gehring, A.J. Interferon-λ treatment accelerates SARS-CoV-2 clearance despite age-related delays in the induction of T cell immunity. Nat. Commun. 2022, 13, 6992. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Reuss, D.; Coey, J.D.; Sukumar, S.; Lang, B.; McLauchlan, J.; Boulant, S.; Stanifer, M.L.; Bamford, C.G.G. Conserved Induction of Distinct Antiviral Signalling Kinetics by Primate Interferon Lambda 4 Proteins. Front. Immunol. 2021, 12, 772588. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Khan, K.; Kim, J.H. Biogenesis, Membrane Trafficking, Functions, and Next Generation Nanotherapeutics Medicine of Extracellular Vesicles. Int. J. Nanomed. 2021, 16, 3357–3383. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef] [Green Version]
- Taghavi-Farahabadi, M.; Mahmoudi, M.; Soudi, S.; Hashemi, S.M. Hypothesis for the management and treatment of the COVID-19-induced acute respiratory distress syndrome and lung injury using mesenchymal stem cell-derived exosomes. Med. Hypotheses 2020, 144, 109865. [Google Scholar] [CrossRef]
- Hum, C.; Loiselle, J.; Ahmed, N.; Shaw, T.A.; Toudic, C.; Pezacki, J.P. MicroRNA Mimics or Inhibitors as Antiviral Therapeutic Approaches Against COVID-19. Drugs 2021, 81, 517–531. [Google Scholar] [CrossRef]
- Bartoszewski, R.; Dabrowski, M.; Jakiela, B.; Matalon, S.; Harrod, K.S.; Sanak, M.; Collawn, J.F. SARS-CoV-2 may regulate cellular responses through depletion of specific host miRNAs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L444–L455. [Google Scholar] [CrossRef]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Winkler, E.S.; Bailey, A.L.; Kafai, N.M.; Nair, S.; McCune, B.T.; Yu, J.; Fox, J.M.; Chen, R.E.; Earnest, J.T.; Keeler, S.P.; et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020, 21, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Yinda, C.K.; Port, J.R.; Bushmaker, T.; Offei Owusu, I.; Purushotham, J.N.; Avanzato, V.A.; Fischer, R.J.; Schulz, J.E.; Holbrook, M.G.; Hebner, M.J.; et al. K18-hACE2 mice develop respiratory disease resembling severe COVID-19. PLoS Pathog. 2021, 17, e1009195. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.T.; Zhang, B.; Teo, J.K.H.; Lai, R.C.; Choo, A.B.H.; Lam, K.P.; Lim, S.K. Mechanism for the attenuation of neutrophil and complement hyperactivity by MSC exosomes. Cytotherapy 2022, 24, 711–719. [Google Scholar] [CrossRef]
- Kaspi, H.; Semo, J.; Abramov, N.; Dekel, C.; Lindborg, S.; Kern, R.; Lebovits, C.; Aricha, R. MSC-NTF (NurOwn®) exosomes: A novel therapeutic modality in the mouse LPS-induced ARDS model. Stem Cell Res. Ther. 2021, 12, 72. [Google Scholar] [CrossRef]
- Fujita, Y.; Kadota, T.; Araya, J.; Ochiya, T.; Kuwano, K. Clinical Application of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapeutics for Inflammatory Lung Diseases. J. Clin. Med. 2018, 7, 355. [Google Scholar] [CrossRef] [Green Version]
- Chu, M.; Wang, H.; Bian, L.; Huang, J.; Wu, D.; Zhang, R.; Fei, F.; Chen, Y.; Xia, J. Nebulization Therapy with Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Pneumonia. Stem Cell Rev. Rep. 2022, 18, 2152–2163. [Google Scholar] [CrossRef]
- Karakaş, N.; Üçüncüoğlu, S.; Uludağ, D.; Karaoğlan, B.S.; Shah, K.; Öztürk, G. Mesenchymal Stem Cell-Based COVID-19 Therapy: Bioengineering Perspectives. Cells 2022, 11, 465. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Zhang, L.; Bao, Q.; Li, L. Mesenchymal stem cell-based cell-free strategies: Safe and effective treatments for liver injury. Stem Cell Res. Ther. 2020, 11, 377. [Google Scholar] [CrossRef]
- Wang, Z.; Popowski, K.D.; Zhu, D.; de Juan Abad, B.L.; Wang, X.; Liu, M.; Lutz, H.; De Naeyer, N.; DeMarco, C.T.; Denny, T.N.; et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat. Biomed. Eng. 2022, 6, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, S.; Liu, L.; Dang, P.; Liu, Y.; Sun, Z.; Qiao, B.; Wang, C. Engineered exosomes from different sources for cancer-targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.A.; Supramaniam, A.; Idris, A.; Cardoso, A.A.; Shrivastava, S.; Kelly, G.; Grepo, N.A.; Soemardy, C.; Ray, R.M.; McMillan, N.A.J.; et al. Engineered extracellular vesicles directed to the spike protein inhibit SARS-CoV-2. Mol. Ther. Methods Clin. Dev. 2022, 24, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Nazerian, Y.; Vakili, K.; Ebrahimi, A.; Niknejad, H. Developing Cytokine Storm-Sensitive Therapeutic Strategy in COVID-19 Using 8P9R Chimeric Peptide and Soluble ACE2. Front. Cell Dev. Biol. 2021, 9, 717587. [Google Scholar] [CrossRef] [PubMed]


| Type of Donor Cell | Animal Model | Induction of Lung Injury | Biological Mechanism | Therapeutic Outcome | Ref. |
|---|---|---|---|---|---|
| MSCs | Wild-type male C57BL/6 mice | Lipopolysaccharide-induced lung injury (ALI) | Enhanced expression of Ym1 and decreased expression of inducible nitric oxide synthase | Attenuation of lung inflammation; promotion of wound healing via M2 alveolar macrophage phenotype activation | [62] |
| MSCs | Wild-type male C57BL/6 mice | Lipopolysaccharide-induced lung injury (ALI) | Enhancement of the neutrophil’s apoptosis and anti-apoptotic molecule (Bcl-xL and Mcl-1) expression reduction; inhibition of the NF-κB pathway | Attenuation of IL-6, macrophage inflammatory protein 2 (MIP-2); reduction in neutrophil accumulation and activity in injured lung tissue | [77] |
| BM-MSCs | Male C57BL/6 mice | Lipopolysaccharide-induced lung injury (ALI) | Increased expression of miR-214, which activates α-epithelial sodium channel in alveolar type 2 epithelial cells (AT2) and H441 cells | Improved alveolar fluid clearance, thus facilitating edema fluid resolution | [84] |
| BM-MSCs | Male C57BL/6 mice | Lipopolysaccharide-induced lung injury (ALI) | Increased protein and miR-34c expression of the γ-epithelial sodium channel in AT2 and H441 cells | Increased viability of AT2 and H441 cells; increased clearance of edema fluid; promotion of repair processes | [85] |
| UC-MSCs | C57BL/6 mice | Lipopolysaccharide-induced lung injury (ALI) | Downregulation of myeloperoxidase activity, IL1β, IL8, and TNFα; increased expression of Arginase-1 and inducible nitric oxide synthase in lung tissue | Attenuation of lung inflammation; promotion of efferocytosis; modulation of anti-inflammatory polarization of lung macrophages | [69] |
| iPSCs | Wild-type male C57BL/6 mice | Lipopolysaccharide-induced lung injury (ALI) | Promotion of endogenous leukemia inhibitory factor (LIF) in the inhibition of neutrophils’ transendothelial migration | Reduction in histopathological changes (pulmonary endothelium permeability and leakage and neutrophil chemotaxis); attenuation of the severity of ALI | [87] |
| iPSCs | Wild-type male C57BL/6 mice | Ventilator-induced lung injury (VILI) | Suppression of PI3K/Akt signaling | Decrease in high-tidal-volume-induced VILI-related inflammatory processes and HMGB1 and PAI-1 production | [88] |
| BM-MSCs | Sprague Dawley neonatal rats | Oxygen-induced lung injury | Enhanced secretion of antioxidant STC-1 | Prevention of pulmonary hypertension; preservation of alveolar growth | [66] |
| Type of Donor Cell Derivate | Animal Model | Induction of Lung Injury | Biological Mechanism | Therapeutic Outcome | Ref. |
|---|---|---|---|---|---|
| BM-MSC-derived microvesicles | Wild-type male C57BL/6 mice | Escherichia coli-induced pneumonia (ALI) | KGF secretion; enhanced monocyte phagocytosis of bacteria; prestimulation of MSCs with Toll-like receptor 3 agonists; decreased TNF secretion and increased IL-10 secretion | Increased survival rate; decreased lung inflammation and protein permeability; elimination of bacteria | [111] |
| MSC-derived EVs | C57BL/6 mice | Lipopolysaccharide-induced lung injury (ALI) | High expression of miR-27a-3p | Alleviation of ALI; promoting M2 macrophage polarization | [112] |
| MSC-derived microvesicles | Wild-type male C57BL/6 mice | Lipopolysaccharide-induced lung injury (ALI) | Overexpression of angiopoietin-1 mRNA; decreased TNF secretion and increased IL-10 secretion | A decreased influx of inflammatory cells in injured alveoli; restoration of pulmonary capillaries permeability; attenuation of histological injury | [130] |
| BM-MSC-derived EVs | Large White–Duroc crossbred pigs | Influenza-virus-induced lung injury (ALI) | Transfer of RNAs from EVs to alveolar cells; increased secretion of IL-10 | Inhibition of virus replication, virus-induced apoptosis, and secretion of proinflammatory cytokines | [136] |
| MSC-derived exosomes | C57BL/6 mice | Lipopolysaccharide-induced lung injury (ALI) | Transfer of stem-cell-derived mitochondria components to alveolar macrophages | Restoration of mitochondrial integrity; shift of alveolar macrophages to anti-inflammatory phenotype; mitigating lung inflammation | [137] |
| BM-MSC-derived exosomes | Male ICR mice | Sulfur-mustard-induced lung injury (ALI) | Upregulation of G protein-coupled receptor family C group 5 type A; facilitation of the expression and relocalization of junction proteins | Protection against pulmonary edema; inhibition of alveolar cell apoptosis; recovery of epithelial barrier | [133] |
| Type of Reaction | Therapeutic Effect |
|---|---|
| Anti-inflammatory | reduction in excessive immune responses reduction in inflammation prevention of CSs |
| Immunomodulatory | regulation leading to a balanced immune response affecting T cells, B cells, and NK cells |
| Regenerative | promotion of tissue regeneration and repair stimulation of proliferation and differentiation of endogenous progenitor cells |
| Anti-viral | inhibition of viral replication via direct anti-viral molecules/properties enhancement of anti-viral defense mechanisms |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csobonyeiova, M.; Smolinska, V.; Harsanyi, S.; Ivantysyn, M.; Klein, M. The Immunomodulatory Role of Cell-Free Approaches in SARS-CoV-2-Induced Cytokine Storm—A Powerful Therapeutic Tool for COVID-19 Patients. Biomedicines 2023, 11, 1736. https://doi.org/10.3390/biomedicines11061736
Csobonyeiova M, Smolinska V, Harsanyi S, Ivantysyn M, Klein M. The Immunomodulatory Role of Cell-Free Approaches in SARS-CoV-2-Induced Cytokine Storm—A Powerful Therapeutic Tool for COVID-19 Patients. Biomedicines. 2023; 11(6):1736. https://doi.org/10.3390/biomedicines11061736
Chicago/Turabian StyleCsobonyeiova, Maria, Veronika Smolinska, Stefan Harsanyi, Michal Ivantysyn, and Martin Klein. 2023. "The Immunomodulatory Role of Cell-Free Approaches in SARS-CoV-2-Induced Cytokine Storm—A Powerful Therapeutic Tool for COVID-19 Patients" Biomedicines 11, no. 6: 1736. https://doi.org/10.3390/biomedicines11061736
APA StyleCsobonyeiova, M., Smolinska, V., Harsanyi, S., Ivantysyn, M., & Klein, M. (2023). The Immunomodulatory Role of Cell-Free Approaches in SARS-CoV-2-Induced Cytokine Storm—A Powerful Therapeutic Tool for COVID-19 Patients. Biomedicines, 11(6), 1736. https://doi.org/10.3390/biomedicines11061736