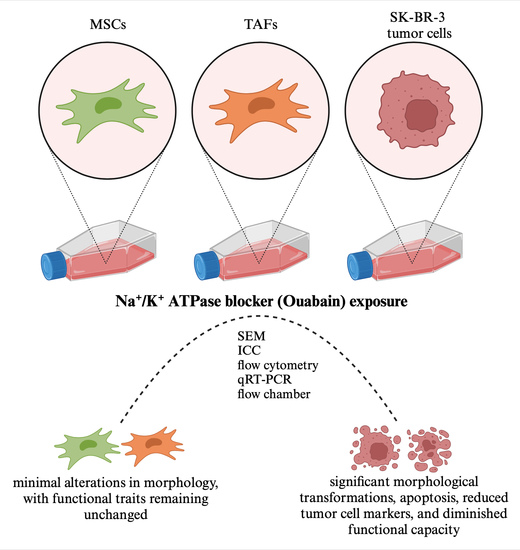
In Vitro Study of the Multimodal Effect of Na+/K+ ATPase Blocker Ouabain on the Tumor Microenvironment and Malignant Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures, Reagents, and Solutions
2.2. Immunocytochemistry
2.3. Flow Cytometry
2.4. Annexin V/PI Assay
2.5. Cell Cycle Test
2.6. Flow Chamber Assay
2.7. RNA Extraction and RT-PCR
2.8. Electron Microscopy
2.9. Statistical Analysis
3. Results
3.1. Morphological Changes of Ouabain-Treated Cells
3.2. Ouabain-Induced Immunophenotypic Changes in Tumor, Tumor-Associated Cells, and MSCs
3.3. Effect of Ouabain on Cellular Viability and Cell Cycle
3.4. Functional Studies—Flow Chamber Assay
3.5. Effects of Ouabain on the Expression of the Na+/K+ Pump Subunits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, J.; Ryu, S.; Ki, I.A.; Woo, H.A.; Lee, K. Some Biological Consequences of the Inhibition of Na,K-ATPase by Translationally Controlled Tumor Protein (TCTP). Int. J. Mol. Sci. 2018, 19, 1657. [Google Scholar] [CrossRef] [Green Version]
- Bommer, U.A.; Thiele, B.J. The translationally controlled tumour protein (TCTP). Int. J. Biochem. Cell Biol. 2004, 36, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Pirkmajer, S.; Chibalin, A.V. Hormonal regulation of Na+-K+-ATPase from the evolutionary perspective. Curr. Top. Membr. 2019, 83, 315–351. [Google Scholar] [CrossRef] [PubMed]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Kumavath, R.; Paul, S.; Pavithran, H.; Paul, M.K.; Ghosh, P.; Barh, D.; Azevedo, V. Emergence of Cardiac Glycosides as Potential Drugs: Current and Future Scope for Cancer Therapeutics. Biomolecules 2021, 11, 1275. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Shimizu, T.; Yamamoto, S.; Funayama, K.; Fujita, K.; Tabuchi, Y.; Ikari, A.; Takeshima, H.; Sakai, H. Crosstalk between Na+,K+-ATPase and a vol-ume-regulated anion channel in membrane microdomains of human cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3792–3804. [Google Scholar] [CrossRef]
- Panizza, E.; Zhang, L.; Fontana, J.M.; Hamada, K.; Svensson, D.; Akkuratov, E.E.; Scott, L.; Mikoshiba, K.; Brismar, H.; Lehtiö, J.; et al. Ouabain-regulated phosphoproteome re-veals molecular mechanisms for Na+, K+-ATPase control of cell adhesion, proliferation, and survival. FASEB J. 2019, 33, 10193–10206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askari, A. The sodium pump and digitalis drugs: Dogmas and fallacies. Pharmacol. Res. Perspect. 2019, 7, e00505. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.M.; Shih, Y.L.; Chen, C.P.; Liu, K.L.; Lee, M.H.; Lee, M.Z.; Hou, H.T.; Huang, H.C.; Lu, H.F.; Peng, S.F.; et al. Ouabain induces apoptotic cell death in human prostate DU 145 cancer cells through DNA damage and TRAIL pathways. Environ. Toxicol. 2019, 34, 1329–1339. [Google Scholar] [CrossRef]
- Khajah, M.A.; Mathew, P.M.; Luqmani, Y.A. Na+/K+ ATPase activity promotes invasion of endocrine resistant breast cancer cells. PLoS ONE 2018, 13, e0193779. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z. Molecular mechanisms of Na/K-ATPase-mediated signal transduction. Ann. N. Y. Acad. Sci. 2003, 986, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kovacevic, Z.; Peng, Z.; Jin, R.; Wang, P.; Yue, F.; Zheng, M.; Huang, M.L.-H.; Jansson, P.J.; Richardson, V.; et al. The molecular effect of metastasis suppressors on Src signaling and tumorigenesis: New therapeutic targets. Oncotarget 2015, 6, 35522–35541. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Jiang, L.; Chen, F.; Hu, H.; Zhou, M. Cardiac Glycoside Ouabain Exerts Anticancer Activi-ty via Downregulation of STAT3. Front. Oncol. 2021, 11, 684316. [Google Scholar] [CrossRef] [PubMed]
- Menger, L.; Vacchelli, E.; Adjemian, S.; Martins, I.; Ma, Y.; Shen, S.; Yamazaki, T.; Sukkurwala, A.Q.; Michaud, M.; Mignot, G.; et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci. Transl. Med. 2012, 4, 143ra99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paunescu, V.; Bojin, F.M.; Tatu, C.A.; Gavriliuc, O.I.; Rosca, A.; Gruia, A.T.; Tanasie, G.; Bunu, C.; Crisnic, D.; Gherghiceanu, M.; et al. Tumour-associated fibroblasts and mesenchymal stem cells: More similarities than differences. J. Cell. Mol. Med. 2011, 15, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Aperia, A.; Akkuratov, E.E.; Fontana, J.M.; Brismar, H. Na+-K+-ATPase, a new class of plasma membrane receptors. Am. J. Physiol. Cell Physiol. 2016, 310, C491–C495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.; Bai, F.; Chaudhry, M.A.; Pratt, R.; Shapiro, J.I.; Liu, J. The Na/K-ATPase α1 and c-Src form signaling complex under na-tive condition: A crosslinking approach. Sci. Rep. 2020, 10, 6006. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.S.; Xu, Z.W.; Yi, T.L.; Xu, R.C.; Li, J.; Zhang, W.B.; Zhang, S.; Sun, H.T.; Yu, Z.Q.; Xu, H.X.; et al. Ouabain suppresses the growth and migration abilities of glioma U-87MG cells through inhibiting the Akt/mTOR signaling pathway and downregulating the expression of HIF-1α. Mol. Med. Rep. 2018, 17, 5595–5600. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Meng, C.; Lin, J.; Huang, C.; Zhang, X.; Long, Y.; Huang, Y.; Lin, Y. Ouabain targets the Na+/K+-ATPase α3 isoform to inhibit cancer cell proliferation and induce apoptosis. Oncol. Lett. 2017, 14, 6678–6684. [Google Scholar] [CrossRef] [Green Version]
- Ninsontia, C.; Chanvorachote, P. Ouabain mediates integrin switch in human lung cancer cells. Anticancer Res. 2014, 34, 5495–5502. [Google Scholar] [PubMed]
- de Souza, W.F.; Barbosa, L.A.; Liu, L.; de Araujo, W.M.; De-Freitas-Junior, J.C.M.; Fortunato-Miranda, N.; Fontes, C.F.; Morgado-Díaz, J.A. Ouabain-induced alterations of the apical junctional complex involve α1 and β1 Na,K-ATPase downregulation and ERK1/2 activation in-dependent of caveolae in colorectal cancer cells. J. Membr. Biol. 2014, 247, 23–33. [Google Scholar] [CrossRef]
- Guo, W.; Wei, B.; Chen, T.; Xu, X.; Ruan, F.; Xiang, M. The Na+/K+ ATPase inhibitor ouabain attenuates stemness and chemo-resistance of osteosarcoma cells. Med. Sci. Monit. 2019, 25, 9426–9434. [Google Scholar] [CrossRef]
- Saito, S.; Ohtsu, M.; Asano, M.; Ishigami, T. Ouabain signaling in oral squamous cell carcinoma cells. J. Oral Sci. 2019, 61, 498–503. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.M.C.; Azevedo, A.D.N.; Barbosa, R.P.D.S.; Teixeira, M.P.; Vianna, T.A.G.; Fittipaldi, J.; Cabral, V.R.; de Paiva, L.S. Ouabain Decreases Regulatory T Cell Number in Mice by Reducing IL-2 Secretion. Neuroimmunomodulation 2019, 26, 188–197. [Google Scholar] [CrossRef]
- Dang, C.V. MYC on the Path to Cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [Green Version]
- Carroll, P.A.; Freie, B.W.; Mathsyaraja, H.; Eisenman, R.N. The MYC transcription factor network: Balancing metabolism, pro-liferation and oncogenesis. Front. Med. 2018, 12, 412–425. [Google Scholar] [CrossRef] [Green Version]
- Dupont, G.; Combettes, L.; Bird, G.S.; Putney, J.W. Calcium oscillations. Cold Spring Harb. Perspect. Biol. 2011, 3, a004226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santivasi, W.L.; Xia, F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid. Redox Signal. 2014, 21, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, H.; Abdelhady, S.; Segerström, L.; Sveinbjörnsson, B.; Nuriya, M.; Lundgren, T.K.; Desfrere, L.; Miyakawa, A.; Yasui, M.; Kogner, P.; et al. Quiescence and γH2AX in neuroblastoma are regulated by ouabain/Na,K-ATPase. Br. J. Cancer 2012, 106, 1807–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Li, Y.; Su, S.; Wang, N.; Wang, H.; Li, J. Inhibition of cell migration by ouabain in the A549 human lung cancer cell line. Oncol. Lett. 2013, 6, 475–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pongrakhananon, V.; Chunhacha, P.; Chanvorachote, P. Ouabain Suppresses the Migratory Behavior of Lung Cancer Cells. PLoS ONE 2013, 8, e68623. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, K.; Zhang, Y.; Jiang, M.; Lu, R.; Folts, C.J.; Gao, X.; Noble, M.D.; Zhao, T.; Zhou, Z.; et al. Pharmacological targeting of p38 MAP-Kinase 6 (MAP2K6) inhibits the growth of esophageal adenocarcinoma. Cell. Signal. 2018, 51, 222–232. [Google Scholar] [CrossRef]
- Clausen, M.; Hilbers, F.; Poulsen, H. The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol. 2017, 8, 371. [Google Scholar] [CrossRef] [Green Version]
- Mijatovic, T.; Dufrasne, F.; Kiss, R. Na+/K+-ATPase and cancer. Pharm Pat Anal. 2012, 1, 91–106. [Google Scholar] [CrossRef]
- Ribeiro Franco, P.I.; Rodrigues, A.P.; de Menezes, L.B.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Cassim, S.; Pouyssegur, J. Tumor microenvironment: A metabolic player that shapes the immune response. Int. J. Mol. Sci. 2020, 21, 157. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante-Silva, L.H.A.; Lima, É.A.; Carvalho, D.C.M.; de Sales-Neto, J.M.; Alves, A.K.D.A.; Galvão, J.G.F.M.; Silva, J.S.D.F.D.; Rodrigues-Mascarenhas, S. Much more than a cardiotonic steroid: Modulation of inflammation by ouabain. Front. Physiol. 2017, 8, 895. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.-J.; Zhan, Y.-C.; Li, H.-Y.; Wang, Z. Ouabain impairs cancer metabolism and activates AMPK-Src signaling pathway in human cancer cell lines. Acta Pharmacol. Sin. 2020, 41, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z. Na+/K+-ATPase-mediated signal transduction: From protein interaction to cellular function. Mol. Interv. 2003, 3, 157–168. [Google Scholar] [CrossRef]
- Ye, Q.; Lai, F.; Duan, Q.; Li, Z.; Si, A.; Xie, Z. Expression of mutant α1 Na/K-ATPase defective in conformational transition at-tenuates Src-mediated signal transduction. J. Biol. Chem. 2013, 288, 5803–5814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vagin, O.; Dada, L.A.; Tokhtaeva, E.; Sachs, G. The Na-K-ATPase α1β1 heterodimer as a cell adhesion molecule in epithelia. Am. J. Physiol. Cell Physiol. 2012, 302, C1271–C1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Na/K-ATPase alpha 1 | Forward 5′-AAAAACATGGTCCCTCAGCAA-3′ Reverse 5′-CCACAACTTCCTCCGCATTT-3′ | NM_000701.7 (tr. Var 1) 76 bp |
| Na/K-ATPase alpha 2 | Forward 5′-GAATGAGAGGCTCATCAGCATG-3′ Reverse 5′-CAAAGTAGGTGAAGAAGCCACCC-3′ | NM_000702.3 77 bp |
| Na/K-ATPase alpha 3 | Forward 5′-AATGCCTACCTTGAGCTCGG-3′ Reverse 5′-CTCGGGCAGGTAATAATGGC-3′ | NM_152296.3 69 bp |
| Na/K-ATPase alpha 4 | Forward 5′-GATGATCACAAATTAACCTTGGAAGA-3′ Reverse 5′-TTTGCCCTTTGGTGGCTATG-3′ | NM_144699.3 (tr. Var 1) 83 bp |
| Na/K-ATPase beta 1 | Forward 5′-TCAGTGAATTTAAGCCCACATATCA-3′ Reverse 5′-CTTCTGGATCTGAGGAATCTGTGTT-3′ | NM_001677.3 74 bp |
| Na/K-ATPase beta 2 | Forward 5′-CCAGCATGTTCAGAAGCTCAAC-3′ Reverse 5′-GCGGCAGACATCATTCTTTTG-3′ | NM_001678.3 79 bp |
| Na/K-ATPase beta 3 | Forward 5′-CTGGCCGAGTGGAAGCTC-3′ Reverse 5′-GGTGCGCCCCAGGAA-3′ | NM_001679.2 60 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harich, O.-O.; Gavriliuc, O.-I.; Ordodi, V.-L.; Tirziu, A.; Paunescu, V.; Panaitescu, C.; Bojin, M.-F. In Vitro Study of the Multimodal Effect of Na+/K+ ATPase Blocker Ouabain on the Tumor Microenvironment and Malignant Cells. Biomedicines 2023, 11, 2205. https://doi.org/10.3390/biomedicines11082205
Harich O-O, Gavriliuc O-I, Ordodi V-L, Tirziu A, Paunescu V, Panaitescu C, Bojin M-F. In Vitro Study of the Multimodal Effect of Na+/K+ ATPase Blocker Ouabain on the Tumor Microenvironment and Malignant Cells. Biomedicines. 2023; 11(8):2205. https://doi.org/10.3390/biomedicines11082205
Chicago/Turabian StyleHarich, Octavia-Oana, Oana-Isabella Gavriliuc, Valentin-Laurentiu Ordodi, Alexandru Tirziu, Virgil Paunescu, Carmen Panaitescu, and Maria-Florina Bojin. 2023. "In Vitro Study of the Multimodal Effect of Na+/K+ ATPase Blocker Ouabain on the Tumor Microenvironment and Malignant Cells" Biomedicines 11, no. 8: 2205. https://doi.org/10.3390/biomedicines11082205
APA StyleHarich, O. -O., Gavriliuc, O. -I., Ordodi, V. -L., Tirziu, A., Paunescu, V., Panaitescu, C., & Bojin, M. -F. (2023). In Vitro Study of the Multimodal Effect of Na+/K+ ATPase Blocker Ouabain on the Tumor Microenvironment and Malignant Cells. Biomedicines, 11(8), 2205. https://doi.org/10.3390/biomedicines11082205