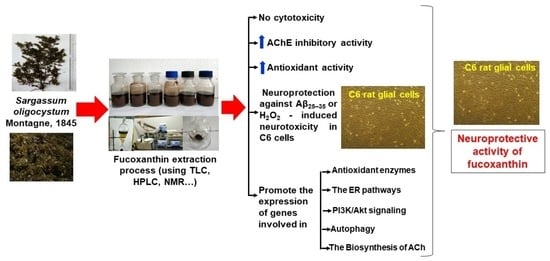
Isolation of Fucoxanthin from Sargassum oligocystum Montagne, 1845 Seaweed in Vietnam and Its Neuroprotective Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Seaweed Samples
2.2. Cell Culture and Treatment
2.3. Chemicals
2.4. Extraction of Fucoxanthin
2.5. Preparation of Methanol Extract
2.6. Isolation of Fucoxanthin
2.7. Column Chromatography Method
2.8. Thin-Layer Chromatography (TLC)
2.9. Determination of Fucoxanthin Content and Purity
2.10. Determination of Fucoxanthin Structure
2.11. DPPH Assay
2.12. AChE Inhibitory Activity Assay
2.13. Cell Culture and Treatment
2.14. MTT Assay
2.15. Measurement of Antioxidant Enzymes
2.16. Real-Time Polymerase Chain Reaction (qPCR)
2.17. Statistical Analysis
3. Results
3.1. Screening Experiment to Identify Species of the Sargassum Genus with the Potential to Accumulate Fucoxanthin
3.2. Purification and Quantification of Fucoxanthin by Column Chromatography and Thin-Layer Chromatography
3.3. Determining the Structure of the Isolated Compound
3.4. Antioxidant Properties of Fucoxanthin
3.5. Acetylcholinesterase (AChE) Inhibitory Activity of Fucoxanthin
3.6. Cytotoxic Effect of Fucoxanthon on C6 Cells
3.7. Neuroprotective Effects of Fucoxanthin against Damage Caused by Oxidative Stress Induced by H2O2 on C6 Cell Lines
3.8. Fucoxanthin Protects C6 Cell Lines against Aβ25–35-Induced Cytotoxicity
3.9. Cytoprotective Effects of Fucoxanthin on C6 Cell Lines against Damage by Oxidative Stress Induced by H2O2
3.10. Fucoxanthin Exhibits a Neuroprotective Effect by Regulating Genes Participating in Multiple Metabolic Pathways in C6 Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ranga Rao, A.; Ravishankar, G.A. Sustainable Global Resources of Seaweeds Volume 1: Bioresources, Cultivation, Trade and Multifarious Applications; Springer Nature: Cham, Switzerland, 2022. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Chopin, T.; Tacon, A.G.J. Importance of seaweeds and extractive species in global aquaculture production. Rev. Fish. Sci. Aquac. 2021, 29, 139–148. [Google Scholar] [CrossRef]
- Alghazwi, M.; Kan, Y.Q.; Zhang, W.; Gai, W.P.; Garson, M.J.; Smid, S. Neuroprotective activities of natural products from marine macroalgae during 1999–2015. J. Appl. Phycol. 2016, 28, 3599–3616. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, W.; Huang, Y.; Wen, Y.; Chu, Y.; Zhao, C. Global seaweed farming and processing in the past 20 years. Food Prod. Process. Nutr. 2022, 4, 28. [Google Scholar] [CrossRef]
- Ranga Rao, A.; Ravishankar, G.A. Sustainable Global Resources of Seaweeds. Volume 2: Food, Pharmaceutical and Health Applications; Springer Nature: Cham, Switzerland, 2022. [Google Scholar]
- Oliyaei, N.; Moosavi-Nasab, M.; Tanideh, N.; Iraji, A. Multiple roles of fucoxanthin and astaxanthin against Alzheimer’s disease: Their pharmacological potential and therapeutic insights. Brain Res. Bull. 2023, 193, 11–21. [Google Scholar] [CrossRef]
- Lakshminarayana, R.; Vijay, K.; Ambedkar, R.; Ranga Rao, A.; Ravishankar, G.A. Biological Activities and Health Benefits of Seaweed Carotenoids with Special Reference to Fucoxanthin. In Sustainable Global Resources of Seaweeds; Ranga Rao, A., Ravishankar, G.A., Eds.; Springer Nature: Cham, Switzerland, 2022; Volume 2, pp. 539–558. [Google Scholar]
- Robinson, M.; Lee, B.Y.; Hane, F.T. Recent Progress in Alzheimer’s Disease Research, Part 2: Genetics and Epidemiology. J. Alzheimers Dis. 2017, 57, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association Report. 2020 Alzheimer’s disease facts and figures: Alzheimer’s Association Report, Alzheimer’s & dementia. J. Alzheimers Assoc. 2020, 16, 391–460. [Google Scholar]
- Ng, Y.P.; Or, T.C.T.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef]
- Pang, H.; Wu, L.; Tang, Y.; Zhou, G.; Qu, C.; Duan, J.A. Chemical analysis of the herbal medicine Salviae miltiorrhizae Radix et Rhizoma (Danshen). Molecules 2016, 21, 51. [Google Scholar] [CrossRef]
- Aljubiri, S.M.; Elsalam, E.A.; Abd El Hady, F.K.; Radwan, M.O.; Almansour, A.I.; Shaker, K.H. In vitro acetylcholinesterase, tyrosinase inhibitory potentials of secondary metabolites from Euphorbia schimperiana and Euphorbia balsamifera. Z. Naturforsch C J. Biosci. 2022, 78, 209–216. [Google Scholar] [CrossRef]
- Hileman, E.O.; Liu, J.; Albitar, M.; Keating, M.J.; Huang, P. Intrinsic oxidative stress in cancer cells: A biochemical basis for therapeutic selectivity. Cancer Chemother. Pharmacol. 2004, 53, 209–219. [Google Scholar] [CrossRef]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Sosa, S.; Marrelli, M.; Menichini, F.; Statti, G.A.; Uzunov, D.; Tubaro, A.; Menichini, F.; Loggia, R.D. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. J. Ethnopharmacol. 2008, 116, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, H.; Liu, Z.; Sun, X.; Zhang, D.; Wang, S.; Xu, Y.; Zhang, G.; Wang, D. Modulation of gut microbiota by fucoxanthin during alleviation of obesity in high-fat diet-fed mice. J. Agric. Food Chem. 2020, 68, 5118–5128. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Wang, H.D.; Hu, Z.G.; Wang, Q.F.; Yin, H.X. Activation of Nrf2-ARE pathway in brain after traumatic brain injury. Neurosci. Lett. 2008, 431, 150–154. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Jiménez-Moreno, N.; García-Yagüe, A.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rábano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy 2016, 12, 1902–1916. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xing, R.; Liu, S.; Yu, H.; Li, P. Role of fucoxanthin towards cadmium-induced renal impairment with the anti-oxidant and anti-lipid peroxide activities. Bioengineered 2021, 12, 7235–7247. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Piao, M.J.; Kim, K.C.; Yao, C.W.; Cha, J.W.; Hyun, J.W. Fucoxanthin enhances the level of reduced glutathione via the Nrf2-mediated pathway in human keratinocytes. Mar. Drugs 2014, 12, 4214–4230. [Google Scholar] [CrossRef]
- Ryu, J.; Hong, B.H.; Kim, Y.J.; Yang, E.J.; Choi, M.; Kim, H.; Ahn, S.; Baik, T.K.; Woo, R.S.; Kim, H.S. Neuregulin-1 attenuates cognitive function impairments in a transgenic mouse model of Alzheimer’s disease. Cell Death Dis. 2016, 7, e2117. [Google Scholar] [CrossRef]
- Choi, H.; Kol, S.H. Interaction between amyloid beta toxicity and the PI3K pathway in Alzheimer’s disease. J. Alzheimers Dis. 2016, 6, 1–3. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, M.; Guan, Z.; Yu, W. Astrocytic α7 nicotinic receptor activation inhibits amyloid-β aggregation by upregulating endogenous αB-crystallin through the PI3K/Akt signaling pathway. Curr. Alzheimer Res. 2019, 16, 39–48. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C. PI3-kinase/Akt/mTOR signaling: Impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp. Gerontol. 2013, 48, 647–653. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.; Kiely, A.P.; Coakley, M.F.; Manning, S.; Long-Smith, C.M. Insulin and IGF-1 signalling: Longevity, protein homoeostasis and Alzheimer’s disease. Biochem. Soc. Trans. 2012, 40, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Lo, E.H. The role of the PI3K pathway in the regeneration of the damaged brain by neural stem cells after cerebral infarction. J. Clin. Neurol. 2015, 11, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H.; et al. Fucoxanthin, a marine carotenoid, attenuates β-amyloid oligomer-induced neurotoxicity possibly via regulating the PI3K/Akt and the ERK Pathways in SH-SY5Y cells. Oxidative Med. Cell. Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Fan, Y.; Gao, Y.; Li, X.; Hu, Z.; Ding, K.; Wang, Y.; Wang, X. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci. Rep. 2017, 7, 46763. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Efange, S.M.; Garland, E.M.; Staley, J.K.; Khare, A.B.; Mash, D.C. Vesicular acetylcholine transporter density and Alzheimer’s disease. Neurobiol. Aging 1997, 18, 407–413. [Google Scholar] [CrossRef]
- Chen, M.H.; Wang, T.J.; Chen, L.J.; Jiang, M.Y.; Wang, Y.J.; Tseng, G.F.; Chen, J.R. The effects of astaxanthin treatment on a rat model of alzheimer’s disease. Brain Res. Bull. 2021, 172, 151–163. [Google Scholar] [CrossRef]
- Phang, S.W.; Yeong, H.Y.; Ganzon-Fortes, E.T.; Lewmanomont, K.; Prathep, A.; Le, N.H.; Gerung, G.S.; Tan, K.S. Marine algae of the south china sea bordered by Indonesia, Malaysia, Philippines, Singapore, Thailand and Vietnam. Raffles Bull. Zool. 2016, 34, 13–59. [Google Scholar]
- Dang, D.H.; Anh, H.T.L.; Thom, L.T.; Ha, N.C.; Tien, D.D.; Duy, D.A. The Genus Gracilaria in Vietnam. In Taxonomy of Southeast Asian Seaweed III; Monograph Series, 17; Phang, S.-M., Song, S.-L., Lim, P.-E., Eds.; Institute of Ocean & Earth Sciences, University of Malaya Press: Kuala Lumpur, Malaysia, 2019; pp. 29–46. [Google Scholar]
- Dang, D.H.; Ha, N.C. Seaweeds of Vietnam: Opportunities for Commercial Production. In Sustainable Global Resources of Seaweeds Volume 1: Bioresources, Cultivation, Trade and Multifarious Applications; Ranga Rao, A., Ravishankar, G.A., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 109–127. [Google Scholar]
- Phong, N.T.; Tien, H.V. Distribution and species identification of Sargassum genus (Phaeophyta) in Phu Quoc—Kien Giang, Vietnam. Can. Tho Univ. J. Sci. 2019, 55, 57–66. [Google Scholar] [CrossRef]
- Dang, D.H.; Hien, H.M.; Son, P.N. Use of Vietnamese seaweed for functional food, medicine and biofertilizer. J. Appl. Phycol. 2007, 19, 817–826. [Google Scholar]
- Haugan, J.A.; Akermann, T.; Liaaen, J.S. Isolation of fucoxanthin and piridinin. In Carotenoids Part A: Chemistry, Separation, Quatitation and Antioxidants. Methods in Enzymology; Packer, L., Ed.; Academic Press. Inc.: Orlando, FL, USA, 1992; Volume 213, pp. 231–245. [Google Scholar]
- Yan, X.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Hien, H.T.M.; Oanh, H.T.; Quynh, Q.T.; Thu, N.T.H.; Hanh, N.V.; Hong, D.D.; Hoang, M.H. Astaxanthin-loaded nanoparticles enhance its cell uptake, antioxidant and hypolipidemic activities in multiple cell lines. J. Drug Deliv. Sci. Technol. 2023, 80, 104133. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, J.V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, J.A.; Hernández-Aguilar, M.E.; Rojas-Durán, F.; Herrera-Covarrubias, D.; García-Hernández, L.I.; Mestizo-Gutiérrez, S.L.; Aranda-Abreu, G.E. Expression of proteins linked to Alzheimer’s disease in C6 rat glioma cells under the action of lipopolysaccharide (LPS), nimesulide, resveratrol and citalopram. Turk. J. Biochem. 2020, 45, 793–801. [Google Scholar] [CrossRef]
- Lee, A.Y.; Lee, M.H.; Lee, S.; Cho, E.J. Neuroprotective Effect of Alpha-Linolenic Acid against Aβ-Mediated Inflammatory Responses in C6 Glial Cell. J. Agric. Food Chem. 2018, 66, 4853–4861. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. Biomed Res. Int. 2020, 2020, 8210578. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2010, 5, 51–66. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Akpan, N.; Caradonna, K.; Chuenkova, M.V.; PereiraPerrin, M. Chagas’ disease parasite-derived neurotrophic factor activates cholinergic gene expression in neuronal PC12 cells. Brain Res. 2008, 1217, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Altınöz, E.; Ekici, C.; Özyazgan, B.; Çiğremiş, Y. The effects of crocin (active contstituent of saffron) treatment on brain antioxidant enzyme mRNA levels in diabetic rats. Turk. J. Biochem. 2016, 41, 112–117. [Google Scholar]
- Fan, B.; Yu, Y.; Zhang, Y. PI3K-Akt1 expression and its significance in liver tissues with chronic fluorosis. Int. J. Clin. Exp. Pathol. 2015, 8, 1226–1236. [Google Scholar] [PubMed]
- Liao, Z.; Zhou, X.; Li, S.; Jiang, W.; Li, T.; Wang, N.; Xiao, N. Activation of the AKT/GSK-3β/β-catenin pathway via photobiomodulation therapy promotes neural stem cell proliferation in neonatal rat models of hypoxic-ischemic brain damage. Ann. Transl. Med. 2022, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Maruani, D.M.; Spiegel, T.N.; Harris, E.N.; Shachter, A.S.; Unger, H.A.; Herrero-González, S.; Holz, M.K. Estrogenic regulation of S6K1 expression creates a positive regulatory loop in control of breast cancer cell proliferation. Oncogene 2012, 31, 5073–5080. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.G.; Cao, X.Z.; Zhao, Y.S.; Gao, S.Y.; Li, S.D.; Liu, X.Y.; Zhang, Y.; Zhang, K.Q. The molecular mechanism of endoplasmic reticulum stress-induced apoptosis in PC-12 neuronal cells: The protective effect of insulin-like growth factor I. Endocrinology 2009, 150, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, C.; Wu, Z.; Miao, Y.; Muhammad, I.; Ding, L.; Tian, E.; Hu, W.; Ni, H.; Li, R.; et al. A Dual Role of P53 in regulating colistin-induced autophagy in PC-12 Cells. Front. Pharmacol. 2017, 8, 768. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, J.; Huang, X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med. 2018, 15, 873–877. [Google Scholar] [CrossRef]
- Lin, R.; Chen, X.; Li, W.; Han, Y.; Liu, P.; Pi, R. Exposure to metal ions regulates mRNA levels of APP and BACE1 in PC12 cells: Blockage by curcumin. Neurosci. Lett. 2008, 440, 344–347. [Google Scholar] [CrossRef]
- Kumar, V.; Tripathi, V.K.; Jahan, S.; Agrawal, M.; Pandey, A.; Khanna, V.K.; Pant, A.B. Lead intoxication synergies of the ethanol-induced toxic responses in neuronal Cells-PC12. Mol. Neurobiol. 2015, 52, 1504–1520. [Google Scholar] [CrossRef]
- Clementi, M.E.; Lazzarino, G.; Sampaolese, B.; Brancato, A.; Tringali, G. DHA protects PC12 cells against oxidative stress and apoptotic signals through the activation of the NFE2L2/HO-1 axis. Int. J. Mol. Med. 2019, 43, 2523–2531. [Google Scholar] [CrossRef]
- Dhami, M.; Raj, K.; Singh, S. Neuroprotective effect of fucoxanthin against intracerebroventricular streptozotocin (ICV-STZ) induced cognitive impairment in experimental rats. Curr. Alzheimer Res. 2021, 18, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Nisa, A.A.; Sedjati, S.; Yudiati, E. Quantitative fucoxanthin extract of tropical Padina sp. and Sargassum sp. (Ocrophyta) and its’ radical scavenging activity. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 584, p. 012044. [Google Scholar]
- Sodik, V.; Tamat, S.; Suwarno, T.; Noviendri, D. Ekstraksi dan purifikasi fukosantin dari rumput laut cokelat Sargassum sp. sebagai antioksidan. J. Ris. Kesehat. Poltekkes Depkes Bdg. 2022, 14, 123–133. [Google Scholar] [CrossRef]
- Terasaki, M.; Kawagoe, C.; Ito, A.; Kumon, H.; Narayan, B.; Hosokawa, M.; Miyashita, K. Spatial and seasonal variations in the biofunctional lipid substances (fucoxanthin and fucosterol) of the laboratory-grown edible Japanese seaweed (Sargassum horneri Turner) cultured in the open sea. Saudi J. Biol. Sci. 2017, 24, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Miyashita, K. Fucoxanthin extractions of brown seaweeds and analysis of their lipid fraction in methanol. Food Sci. Technol. Res. 2012, 18, 251–257. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M. Ultrasound-assisted extraction of fucoxanthin from Sargassum angustifolium and Cystoseira indica brown algae. J. Food Process Preserv. 2021, 45, e15929. [Google Scholar] [CrossRef]
- Ayyad, S.E.N.; Ezmirly, S.T.; Basaif, S.A.; Alarif, W.M.; Badria, A.F.; Badria, F.A. Antioxidant, cytotoxic, antitumor, and protective DNA damage metabolites from the red sea brown alga Sargassum sp. Pharmacol. Res. 2011, 3, 160–165. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A promising medicinal and nutritional ingredient. Evid. Based Complement. Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Ismail, M.; Basri, M.; Yau, S.K.; Khong, N.M.; Chan, K.W.; Ebrahimi, M. Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. J. Biotechnol. 2017, 241, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Hong-Yu, L.; Bin, W.; Chun-Guang, Y.; You-le, Q.; Chuan-ling, S. Evaluation of antioxidant activities of five selected brown seaweeds from China. J. Med. Plant Res. 2010, 4, 2557–2565. [Google Scholar] [CrossRef]
- Chandini, S.K.; Ganesan, P.; Bhaskar, N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008, 107, 707–713. [Google Scholar] [CrossRef]
- Badrinathan, S.; Suneeva, S.C.; Shiju, T.M.; Girish, K.C.; Pragasam, V. Exploration of a novel hydroxyl radical scavenger from Sargassum myriocystum. J. Med. Plant Res. 2011, 5, 1997–2005. [Google Scholar]
- Savira, A.D.R.; Amin, M.N.G.; Alamsjah, M.A. The effect of different type of solvents on the antioxidant activity of fucoxanthin extract from brown seaweed Sargassum duplicatum. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2021; Volume 78, p. 012010. [Google Scholar]
- Kawee-ai, A.; Kuntiya, A.; Kim, S.M. Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Nat. Prod. Commun. 2013, 8, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; Shanmugiahthevar, K.P.; Kasi, P.D. Cholinesterase inhibitors from Sargassum and Gracilaria gracilis: Seaweeds inhabiting South Indian coastal areas (Hare Island, Gulf of Mannar). Nat. Prod. Res. 2009, 23, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Vinutha, B.; Prashanth, D.; Salma, K.; Sreeja, S.L.; Pratiti, D.; Padmaja, R.; Radhika, S.; Amit, A.; Venkateshwarlu, K.; Deepak, M. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2007, 109, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a marine carotenoid, reverses scopolamine-induced cognitive impairments in mice and inhibits acetylcholinesterase in vitro. Mar. Drugs 2016, 14, 67. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, H.; Xiao, C.; Fu, B.D.; Du, C.T. Oroxylin A inhibits H2O2-induced oxidative stress in PC12 cells. Nat. Prod. Res. 2017, 31, 1339–1342. [Google Scholar] [CrossRef]
- Ciofani, G.; Genchi, G.G.; Mazzolai, B.; Mattoli, V. Transcriptional profile of genes involved in oxidative stress and antioxidant defense in PC12 cells following treatment with cerium oxide nanoparticles. Biochim. Biophys. Acta 2014, 1840, 495–506. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J. Fucoxanthin inhibits β-amyloid assembly and attenuates β-amyloid oligomerinduced cognitive impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ1-42) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Kang, H.S.; Kim, J.P.; Kim, S.H.; Lee, K.W.; Cho, M.G.; Jeon, Y.J. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Choi, M.W.; Jung, C.G.; Kim, H.R.; Kim, R.I. Effect of Sargassum serratifolium extracts on β-Amyloid production. Korean J. Fish. Aquat. Sci. 2017, 50, 085–091. [Google Scholar] [CrossRef]
- García-Pérez, E.; Ryu, D.; Lee, C.; Lee, H.J. Ochratoxin A induces oxidative stress in HepG2 cells by impairing the gene expression of anti-oxidant enzymes. Toxins 2021, 13, 271. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Jin, L.; Wu, Z.; Xie, Y.; Zhang, P.; Wang, Q.; Yan, S.; Chen, B.; Liang, H.; Naman, C.B.; et al. PLGA-PEG Nanoparticles Facilitate In Vivo anti-alzheimer’s effects of fucoxanthin, a marine carotenoid derived from edible brown algae. J. Agric. Food Chem. 2021, 69, 9764–9777. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Lin, J.J.; Yu, R.; He, S.; Wang, Q.W.; Cui, W.; Zhang, J.R. Fucoxanthin prevents H2O2-induced neuronal apoptosis via concurrently activating the PI3-K/Akt cascade and inhibiting the ERK pathway. Food Nutr. Res. 2017, 61, 1304678. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, S.; Torres, M.; Vizuete, M. Age-dependent accumulation of soluble amyloid beta (Abeta) oligomer reverses the neuroprotective effect of soluble amyloid precursor protein-alpha (sAPP(alpha)) by modulating phosphatidylinositol 3-kinase (PI3K)/Akt-GSK-3beta pathway in Alzheimer mouse model. J. Biol. Chem. 2011, 286, 18414–18425. [Google Scholar] [PubMed]
- Chong, Y.H.; Shin, Y.J.; Lee, E.O.; Kayed, R.; Glabe, C.G.; Tenner, A.J. ERK1/2 activation mediates Aβ oligomer-induced neurotoxicity via caspase-3 activation and tau cleavage in rat organotypic hippocampal slice cultures. J. Biol. Chem. 2006, 281, 20315–20325. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, J.; Chang, K.T.; Lee, D.S. Peroxiredoxin 5 prevents amyloid-beta oligomer-induced neuronal cell death by inhibiting ERK-Drp1-mediated mitochondrial fragmentation. Free Radic. Biol. Med. 2016, 90, 184–194. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Ferri, K.F.; Kroemer, G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001, 3, E255–E263. [Google Scholar] [CrossRef]
- Gundu, C.; Arruri, V.K.; Sherkhane, B.; Khatri, D.K.; Singh, S.B. GSK2606414 attenuates PERK/p-eIF2α/ATF4/CHOP axis and augments mitochondrial function to mitigate high glucose induced neurotoxicity in N2A cells. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100087. [Google Scholar] [CrossRef]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and function of the cholinergic system in immune cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef]
- Lusztajn, J.K.; Berse, B. The cholinergic neuronal phenotype in Alzheimer’s disease. Metab. Brain Dis. 2000, 15, 45–64. [Google Scholar] [CrossRef]
- Orr, M.E.; Oddo, S. Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res. Ther. 2013, 5, 53. [Google Scholar] [CrossRef]






| Number | Scientific Name | Places and Times for Collecting | With Coordinates |
|---|---|---|---|
| 1 | Sargassum mcclurei Setchell, 1933 | Hon Chong, Nha Trang, Khanh Hoa province; 20–21 September 2007, 2008; 27 April 2022 and 19 July 2022. | 12°16′ N 109°12′ E |
| 2 | S. binderi Sonder ex J. Agardh, 1848 | Bau Hamlet, Vinh Nguyen, Nha Trang; 15–20 October 2007 | 12°13′ N 109°14′ E |
| 3 | S. polycystum C. Agardh, 1824 | My Hoa, Ninh Hai, Ninh Thuan province; 15–20 October 2007 | 11°42′ N 109°12′ E |
| 4 | S. duplicatum Bory | Hon Chong, Vinh Phuoc, Nha Trang, Khanh Hoa province; 15–20 October 2007 | 12°16′ N 109°12′ E |
| 5 | S. denticarpum T. Ajusaka, 1994 | My Hoa, Ninh Hai, Ninh Thuan province; 15–20 October 2007 | 11°42′ N 109°12′ E |
| 6 | S. swartzii (Turn.) C. Ag. | Thua Thien Hue province; 15–20 October 2007 | 16°32′ N 107°39′ E |
| 7 | S. microcystum J. Agardh, 1848 | Tri Nguyen Island, Nha Trang, Khanh Hoa province; 20–30 October 2007 | 12°11′ N 109°13′ E |
| 8 | S. crassifolium J. Agardh | Bai Tien, Vinh Hoa, Nha Trang, Khanh Hoa province; 20–30 October 2007 | 12°13′ N 109°12′ E |
| 9 | S. oligocystum Montagne, 1845 | Hon Chong, Nha Trang, Khanh Hoa province; 20–21 September 2007; 2008; 27 April 2022; 19 July 2022 | 12°16′ N 109°12′ E |
| Primer | Sequences (5′-3′) | References | |
|---|---|---|---|
| Forward | Reverse | ||
| CHAT | AGCCCTGCTGTGATCTTTGCTCG | CCTTGGCCCAGTCAGTGGGAA | [48] |
| VAChT | CCCTTAAGCGGGCCTTTCATTGAT | AAAGGCAAACATGACTGTGGAGGC | [48] |
| SOD | GCCTGGATGGCTACGTACA | GGTCCAGCGGATGAAGAG | [49] |
| CAT | AATGAAGACAACGTCACTCAGG | TGTTCTCACACAGGCGTTTC | [49] |
| GPX | GCAATCAGTTCGGACATCAG | CACCGGGTCGGACATACTT | [49] |
| PI3K | AACACAGAAGACCAATACTC | TTCGCCATCTACCACTAC | [50] |
| Akt | GTGGCAAGATGTGTATGAG | CTGGCTGAGTAGGAGAAC | [50] |
| GSK-3β | CATCCTTATCCCTCCTCACGCT | TATTGGTCTGTCCACGGTCTCC | [51] |
| S6K1 | CTCTGAGGATGAGCTGGAGG | TTCTCACAATGTTCCATGCC | [52] |
| CHOP | GGAGAAGGAGCAGGAGAATGA | AGACAGACAGGAGGTGATGC | [53] |
| Atg5 | CCCTGAAGACGGAGAGAAGA | TGCTGATGTGAAGGAAGTTGTC | [54] |
| p62 | CCTATTACCTGGCCTGTGGA | GTTCATCCGTTGTGCATGAG | [54] |
| Caspase-3 | GTGGAACTGACGATGATATGGC | CGCAAAGTGACTGGATGAACC | [55] |
| APP | GGACGACTCCGATGTCTGGT | ACATCAAAGTACCAGCGGGAG | [56] |
| Caspase-9 | AGCCAGATGCTGTCCCATAC | CAGGAGACAAAACCTGGGAA | [57] |
| Beclin | TGGAAATCACTCGTATCTGGAG | CCACCTCTTCTTTGAACTGCT | [54] |
| Bax | GCAGGGAGGATGGCTGGGGAG | TCCAGACAAGCAGCCGCTCACG | [58] |
| β-actin | CTAAGGCCAACCGTGAAAAG | GCCTGGATGGCTACGTACA | [49] |
| No. | Species Name | Place and Time of Samples Collection | Fucoxanthin Content (μg g−1 Dry Weight) |
|---|---|---|---|
| 1 | S. mucclurei | Hon Chong, Nha Trang, Khanh Hoa province, 5–10 December 2007 | 1650.03 ± 7.10 |
| 2 | S. binderi | Vinh Nguyen, Nha Trang, Khanh Hoa province, 15–20 October 2007 | 296.07 ± 5.50 |
| 3 | S. polycystum | My Hao, Ninh Hai, Ninh Thuan province, 15–20 October 2007 | 3.82 ± 0.89 |
| 4 | S. duplicatum | Hon Chong, Nha Trang, Khanh Hoa province, 15–20 October 2008 | 255.03 ± 5.71 |
| 5 | S. denticarpum | My Hoa, Ninh Hai, Ninh Thuan province, 15–20 October 2007 | 24.25 ± 2.72 |
| 6 | S. swartzii | Thua Thien Hue province, 15–25 October 2007 | 217.60 ± 5.04 |
| 7 | S. microcystum | Dao Tri Nguyen, Nha Trang Bay, Khanh Hoa province, 20–30 October 2007 | 161.52 ± 2.90 |
| 8 | S. crassifolium | Bai Tien, Nha Trang, Khanh Hoa province, 20–30 October 2007 | 26.65 ± 2.86 |
| 9 | S. oligocystum | Xom Bau, Vinh Nguyen, Nha Trang, Khanh Hoa province, 17–18 January 2008 | 2927.98 ± 8.01 |
| Sample Collection Time | S. mucclurei (µg g−1 Dry Weight) | S. oligocystum (µg g−1 Dry Weight) |
|---|---|---|
| 21 September 2007 | 697.65 ± 5.78 | 978.22 ± 7.18 |
| 22 October 2007 | 897.82 ± 7.98 | 1362.82 ± 7.92 |
| 20 November 2007 | 1298.67 ± 6.71 | 1872.16 ± 8.62 |
| 20 December 2007 | 1658.81 ± 7.17 | 2276.19 ± 8.68 |
| 20 January 2008 | 1310.72 ± 7.81 | 2986.28 ± 9.01 |
| 20 February 2008 | 1259.17 ± 7.42 | 1968.82 ± 7.62 |
| 15 March 2008 | 1087.16 ± 6.72 | 1276.92 ± 6.73 |
| 17 April 2008 | 962.19 ± 5.17 | 872.19 ± 5.12 |
| 11 May 2008 | 802.29 ± 7.82 | 776.97 ± 5.89 |
| 25 June 2008 | 779.78 ± 5.82 | 672.08 ± 4.82 |
| 20 July 2008 | 563.74 ± 4.17 | 517.81 ± 4.78 |
| 10 August 2008 | 392.81 ± 4.12 | 439.78 ± 3.36 |
| DPPH Scavenging Activity (%) | DPPH Scavenging Activity (%) | ||
|---|---|---|---|
| Concentrations (mg mL−1) | Fucoxanthin | Concentrations (μg mL−1) | Ascorbic Acid |
| 0.1 | 8.34 ± 0.14 | 4 | 32.70 ± 0.52 |
| 0.4 | 13.97 ± 0.10 | 20 | 89.90 ± 1.31 |
| 2 | 31.80 ± 0.84 | 100 | 92.3 ± 1.83 |
| IC50 (mg mL−1) | 3.42 ± 0.15 | IC50 (μg mL−1) | 19.43 ± 0.13 |
| AChE Inhibition Activity (%) | AChE Inhibition Activity (%) | ||
|---|---|---|---|
| Concentrations (µg mL−1) | Fucoxanthin | Concentrations (µg mL−1) | Galantamine |
| 4 | 4.25 ± 0.10 | 0.08 | 9.51 ± 0.52 |
| 20 | 21.35 ± 0.84 | 0.4 | 25.72 ± 1.31 |
| 100 | 46.28 ± 1.05 | 2 | 53.02 ± 1.10 |
| 500 | 75.43 ± 1.46 | 10 | 87.39 ± 2.84 |
| IC50 (µg mL−1) | 130.12 ± 6.65 | IC50 (µg mL−1) | 1.78 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, D.D.; Thom, L.T.; Ha, N.C.; Thu, N.T.H.; Hien, H.T.M.; Tam, L.T.; Dat, N.M.; Duc, T.M.; Tru, N.V.; Hang, N.T.M.; et al. Isolation of Fucoxanthin from Sargassum oligocystum Montagne, 1845 Seaweed in Vietnam and Its Neuroprotective Activity. Biomedicines 2023, 11, 2310. https://doi.org/10.3390/biomedicines11082310
Hong DD, Thom LT, Ha NC, Thu NTH, Hien HTM, Tam LT, Dat NM, Duc TM, Tru NV, Hang NTM, et al. Isolation of Fucoxanthin from Sargassum oligocystum Montagne, 1845 Seaweed in Vietnam and Its Neuroprotective Activity. Biomedicines. 2023; 11(8):2310. https://doi.org/10.3390/biomedicines11082310
Chicago/Turabian StyleHong, Dang Diem, Le Thi Thom, Nguyen Cam Ha, Ngo Thi Hoai Thu, Hoang Thi Minh Hien, Luu Thi Tam, Nguyen Manh Dat, Tran Mai Duc, Nguyen Van Tru, Nguyen Thi Minh Hang, and et al. 2023. "Isolation of Fucoxanthin from Sargassum oligocystum Montagne, 1845 Seaweed in Vietnam and Its Neuroprotective Activity" Biomedicines 11, no. 8: 2310. https://doi.org/10.3390/biomedicines11082310
APA StyleHong, D. D., Thom, L. T., Ha, N. C., Thu, N. T. H., Hien, H. T. M., Tam, L. T., Dat, N. M., Duc, T. M., Tru, N. V., Hang, N. T. M., & Ambati, R. R. (2023). Isolation of Fucoxanthin from Sargassum oligocystum Montagne, 1845 Seaweed in Vietnam and Its Neuroprotective Activity. Biomedicines, 11(8), 2310. https://doi.org/10.3390/biomedicines11082310